Arrhenius Acid vs Strong Acid Reaction Kinetics: Comparative Study
SEP 16, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Acid Reaction Kinetics Background and Research Objectives
The study of acid reaction kinetics has evolved significantly since the late 19th century when Svante Arrhenius first proposed his theory of acids and bases. Arrhenius defined acids as substances that increase hydrogen ion concentration in aqueous solutions, while strong acids were later characterized as those that completely dissociate in solution. This fundamental distinction has profound implications for reaction rates and mechanisms, forming the cornerstone of modern chemical kinetics.
Over the past century, acid reaction kinetics has progressed through several paradigm shifts, from classical Arrhenius theory to Brønsted-Lowry and Lewis acid-base concepts, enabling more comprehensive understanding of acid behavior across different media and conditions. Recent advances in computational chemistry and high-resolution spectroscopy have further refined our understanding of the molecular-level processes occurring during acid-catalyzed reactions.
The technological significance of acid reaction kinetics extends across numerous industries, including pharmaceuticals, petrochemicals, materials science, and environmental remediation. Precise control of acid-catalyzed reactions enables efficient synthesis pathways, while understanding degradation mechanisms informs product stability and shelf-life determinations. In environmental contexts, acid reaction kinetics governs pollutant transformation and persistence.
This comparative study aims to systematically investigate the fundamental differences in reaction mechanisms and rates between Arrhenius acids and strong acids across various reaction environments. Specifically, we seek to quantify the relationship between acid strength, dissociation behavior, and reaction efficiency in both homogeneous and heterogeneous systems. The research will explore how structural features of reactants influence their susceptibility to different acid types.
Our technical objectives include developing predictive models that accurately describe reaction rates as functions of acid type, concentration, and environmental conditions. We aim to establish standardized protocols for comparing acid catalytic efficiency across reaction classes, potentially leading to optimization frameworks for industrial processes. Additionally, we will investigate the role of solvent effects and ionic strength on the relative performance of different acid types.
The expected outcomes of this research include comprehensive kinetic datasets comparing Arrhenius and strong acid performance, mechanistic insights into transition state structures and energy profiles, and practical guidelines for acid selection in various applications. By elucidating these fundamental relationships, we anticipate enabling more rational design of acid-catalyzed processes with improved efficiency, selectivity, and sustainability profiles.
Over the past century, acid reaction kinetics has progressed through several paradigm shifts, from classical Arrhenius theory to Brønsted-Lowry and Lewis acid-base concepts, enabling more comprehensive understanding of acid behavior across different media and conditions. Recent advances in computational chemistry and high-resolution spectroscopy have further refined our understanding of the molecular-level processes occurring during acid-catalyzed reactions.
The technological significance of acid reaction kinetics extends across numerous industries, including pharmaceuticals, petrochemicals, materials science, and environmental remediation. Precise control of acid-catalyzed reactions enables efficient synthesis pathways, while understanding degradation mechanisms informs product stability and shelf-life determinations. In environmental contexts, acid reaction kinetics governs pollutant transformation and persistence.
This comparative study aims to systematically investigate the fundamental differences in reaction mechanisms and rates between Arrhenius acids and strong acids across various reaction environments. Specifically, we seek to quantify the relationship between acid strength, dissociation behavior, and reaction efficiency in both homogeneous and heterogeneous systems. The research will explore how structural features of reactants influence their susceptibility to different acid types.
Our technical objectives include developing predictive models that accurately describe reaction rates as functions of acid type, concentration, and environmental conditions. We aim to establish standardized protocols for comparing acid catalytic efficiency across reaction classes, potentially leading to optimization frameworks for industrial processes. Additionally, we will investigate the role of solvent effects and ionic strength on the relative performance of different acid types.
The expected outcomes of this research include comprehensive kinetic datasets comparing Arrhenius and strong acid performance, mechanistic insights into transition state structures and energy profiles, and practical guidelines for acid selection in various applications. By elucidating these fundamental relationships, we anticipate enabling more rational design of acid-catalyzed processes with improved efficiency, selectivity, and sustainability profiles.
Market Applications and Industrial Demand for Acid Reaction Studies
The acid reaction kinetics market is experiencing significant growth across multiple industrial sectors, driven by the increasing demand for efficient chemical processes and sustainable manufacturing solutions. Pharmaceutical companies represent one of the largest consumer segments, where understanding the comparative kinetics of Arrhenius acids versus strong acids is crucial for drug synthesis optimization. This knowledge enables manufacturers to reduce reaction times, increase yields, and develop more cost-effective production methods for active pharmaceutical ingredients.
Chemical manufacturing industries heavily rely on acid reaction kinetics studies to improve process efficiency and product quality. The global specialty chemicals market, particularly segments involving catalysis and fine chemical synthesis, demonstrates strong demand for advanced understanding of acid reaction mechanisms. These industries seek to leverage kinetic differences between traditional strong acids and Arrhenius acid systems to develop greener chemistry approaches with reduced waste generation and energy consumption.
The petroleum refining sector represents another significant market for acid reaction kinetics research. Acid catalysis plays a vital role in numerous refining processes, including alkylation, isomerization, and cracking. Refineries are increasingly investing in research to optimize these processes through better understanding of reaction kinetics, aiming to improve fuel quality while meeting stringent environmental regulations.
Environmental remediation and wastewater treatment industries show growing interest in acid reaction kinetics for developing more effective treatment technologies. The comparative study of different acid systems helps in designing advanced oxidation processes and catalytic treatments that can degrade persistent pollutants more efficiently.
Agricultural chemical production, particularly fertilizer manufacturing, represents an emerging market for acid reaction studies. Manufacturers seek to improve production efficiency and develop slow-release formulations through better understanding of acid-base interactions and dissolution kinetics.
Market analysis indicates that the demand for specialized analytical instruments and software for studying acid reaction kinetics is also expanding. Laboratory equipment manufacturers are developing advanced calorimetry, spectroscopy, and chromatography tools specifically designed for kinetic studies of acid-catalyzed reactions.
The electronics industry, particularly semiconductor manufacturing, requires precise understanding of acid reaction kinetics for etching processes and surface treatments. As device dimensions continue to shrink, the need for precisely controlled acid reactions becomes increasingly critical, driving demand for sophisticated kinetic models and process optimization.
Chemical manufacturing industries heavily rely on acid reaction kinetics studies to improve process efficiency and product quality. The global specialty chemicals market, particularly segments involving catalysis and fine chemical synthesis, demonstrates strong demand for advanced understanding of acid reaction mechanisms. These industries seek to leverage kinetic differences between traditional strong acids and Arrhenius acid systems to develop greener chemistry approaches with reduced waste generation and energy consumption.
The petroleum refining sector represents another significant market for acid reaction kinetics research. Acid catalysis plays a vital role in numerous refining processes, including alkylation, isomerization, and cracking. Refineries are increasingly investing in research to optimize these processes through better understanding of reaction kinetics, aiming to improve fuel quality while meeting stringent environmental regulations.
Environmental remediation and wastewater treatment industries show growing interest in acid reaction kinetics for developing more effective treatment technologies. The comparative study of different acid systems helps in designing advanced oxidation processes and catalytic treatments that can degrade persistent pollutants more efficiently.
Agricultural chemical production, particularly fertilizer manufacturing, represents an emerging market for acid reaction studies. Manufacturers seek to improve production efficiency and develop slow-release formulations through better understanding of acid-base interactions and dissolution kinetics.
Market analysis indicates that the demand for specialized analytical instruments and software for studying acid reaction kinetics is also expanding. Laboratory equipment manufacturers are developing advanced calorimetry, spectroscopy, and chromatography tools specifically designed for kinetic studies of acid-catalyzed reactions.
The electronics industry, particularly semiconductor manufacturing, requires precise understanding of acid reaction kinetics for etching processes and surface treatments. As device dimensions continue to shrink, the need for precisely controlled acid reactions becomes increasingly critical, driving demand for sophisticated kinetic models and process optimization.
Current Understanding and Challenges in Acid Reaction Kinetics
The field of acid reaction kinetics has witnessed significant advancements in recent decades, yet several fundamental challenges persist in fully understanding the comparative kinetics between Arrhenius acids and strong acids. Current theoretical frameworks primarily rely on the Brønsted-Lowry acid-base theory and the Arrhenius definition, with supplementary insights from Lewis acid theory. These frameworks have provided valuable mechanistic understanding but remain incomplete when addressing complex reaction environments.
Recent experimental studies have demonstrated that strong acids exhibit reaction rates that often deviate from classical Arrhenius behavior, particularly in non-aqueous solvents and at extreme temperatures. This deviation suggests the presence of additional factors beyond activation energy and collision frequency that influence reaction pathways. Computational chemistry approaches using density functional theory (DFT) have begun to elucidate these factors, but computational expense limits their application to complex systems.
A significant challenge in the field is the accurate measurement of ultrafast proton transfer reactions, which occur on femtosecond to picosecond timescales. While advanced spectroscopic techniques such as ultrafast IR and time-resolved Raman spectroscopy have improved temporal resolution, they still struggle to capture the complete dynamics of proton solvation and transfer in various media. This limitation has hindered the development of comprehensive kinetic models.
The role of solvent effects represents another major challenge in acid reaction kinetics. Current models inadequately account for solvent reorganization energies and hydrogen-bonding networks that significantly influence proton mobility and effective acidity. Studies comparing Arrhenius acids with strong acids in identical solvents often yield inconsistent results due to these complex solvent-solute interactions.
Interfacial acid catalysis presents additional complications, as surface properties dramatically alter reaction pathways compared to bulk solution behavior. The boundary layer between solid catalysts and liquid reactants creates unique microenvironments where traditional kinetic models fail to predict observed reaction rates. This is particularly relevant in heterogeneous catalysis applications where strong acids are frequently employed.
Temperature dependence studies reveal that many strong acid reactions exhibit non-linear Arrhenius plots, suggesting multiple competing reaction mechanisms or temperature-dependent changes in rate-determining steps. These observations challenge the conventional single-barrier model and point toward more complex energy landscapes with multiple transition states.
Emerging research directions include the development of microkinetic models that incorporate quantum tunneling effects for proton transfer, particularly relevant at low temperatures. Additionally, machine learning approaches are beginning to identify patterns in experimental data that traditional kinetic theories have overlooked, potentially leading to more accurate predictive models for acid-catalyzed reactions across diverse chemical environments.
Recent experimental studies have demonstrated that strong acids exhibit reaction rates that often deviate from classical Arrhenius behavior, particularly in non-aqueous solvents and at extreme temperatures. This deviation suggests the presence of additional factors beyond activation energy and collision frequency that influence reaction pathways. Computational chemistry approaches using density functional theory (DFT) have begun to elucidate these factors, but computational expense limits their application to complex systems.
A significant challenge in the field is the accurate measurement of ultrafast proton transfer reactions, which occur on femtosecond to picosecond timescales. While advanced spectroscopic techniques such as ultrafast IR and time-resolved Raman spectroscopy have improved temporal resolution, they still struggle to capture the complete dynamics of proton solvation and transfer in various media. This limitation has hindered the development of comprehensive kinetic models.
The role of solvent effects represents another major challenge in acid reaction kinetics. Current models inadequately account for solvent reorganization energies and hydrogen-bonding networks that significantly influence proton mobility and effective acidity. Studies comparing Arrhenius acids with strong acids in identical solvents often yield inconsistent results due to these complex solvent-solute interactions.
Interfacial acid catalysis presents additional complications, as surface properties dramatically alter reaction pathways compared to bulk solution behavior. The boundary layer between solid catalysts and liquid reactants creates unique microenvironments where traditional kinetic models fail to predict observed reaction rates. This is particularly relevant in heterogeneous catalysis applications where strong acids are frequently employed.
Temperature dependence studies reveal that many strong acid reactions exhibit non-linear Arrhenius plots, suggesting multiple competing reaction mechanisms or temperature-dependent changes in rate-determining steps. These observations challenge the conventional single-barrier model and point toward more complex energy landscapes with multiple transition states.
Emerging research directions include the development of microkinetic models that incorporate quantum tunneling effects for proton transfer, particularly relevant at low temperatures. Additionally, machine learning approaches are beginning to identify patterns in experimental data that traditional kinetic theories have overlooked, potentially leading to more accurate predictive models for acid-catalyzed reactions across diverse chemical environments.
Methodologies for Comparing Arrhenius and Strong Acid Reactions
01 Kinetic models for acid-catalyzed reactions
Mathematical models and computational methods are used to describe the kinetics of acid-catalyzed reactions. These models incorporate Arrhenius equations to predict reaction rates based on temperature, concentration, and activation energy. Advanced algorithms and simulation techniques help in understanding the behavior of strong acids in various reaction environments, enabling more accurate prediction of reaction outcomes and optimization of process parameters.- Kinetic models for acid-catalyzed reactions: Mathematical models and computational methods are used to describe the kinetics of acid-catalyzed reactions. These models incorporate Arrhenius equations to predict reaction rates based on temperature, concentration, and activation energy. Advanced algorithms and simulation techniques help in understanding the reaction mechanisms and optimizing process conditions for industrial applications involving strong acids.
- Experimental methods for measuring acid reaction kinetics: Various experimental apparatus and methods are employed to measure the kinetics of acid reactions. These include specialized reactors, monitoring systems, and analytical techniques that can track reaction progress in real-time. Temperature control systems and precise measurement tools enable researchers to determine rate constants, activation energies, and other kinetic parameters for both Arrhenius and strong acid reactions under controlled conditions.
- Catalytic applications of strong acids in chemical processes: Strong acids serve as effective catalysts in various chemical processes, with their reaction kinetics playing a crucial role in industrial applications. The catalytic efficiency depends on acid strength, concentration, and reaction conditions. Understanding these kinetic parameters helps in designing more efficient catalytic systems for processes such as esterification, hydrolysis, and polymerization reactions.
- Temperature effects on acid reaction kinetics: Temperature significantly influences the kinetics of acid-catalyzed reactions, following the Arrhenius equation. Higher temperatures typically accelerate reaction rates by providing sufficient energy to overcome activation barriers. However, temperature effects can vary depending on the specific acid type, reaction medium, and other environmental factors. Controlling temperature is essential for optimizing reaction efficiency and selectivity in both laboratory and industrial settings.
- Reaction mechanisms of Arrhenius acids in solution: The reaction mechanisms of Arrhenius acids in solution involve proton transfer processes that follow specific kinetic pathways. These mechanisms are influenced by factors such as solvent effects, ionic strength, and the presence of other reactive species. Understanding these mechanisms helps in predicting reaction outcomes and designing more efficient chemical processes. The dissociation behavior and subsequent reactions of these acids can be characterized through various spectroscopic and analytical techniques.
02 Experimental apparatus for studying acid reaction kinetics
Specialized equipment and apparatus designed for measuring and analyzing the kinetics of acid reactions under controlled conditions. These setups include reactors with precise temperature control, monitoring systems for pH and concentration changes, and analytical instruments that can track reaction progress in real-time. Such equipment allows researchers to gather accurate data on reaction rates, activation energies, and other kinetic parameters for both Arrhenius and strong acid reactions.Expand Specific Solutions03 Industrial applications of acid reaction kinetics
Implementation of acid reaction kinetics principles in industrial processes such as chemical manufacturing, petroleum refining, and materials production. Understanding the kinetic behavior of strong acids enables optimization of reaction conditions, catalyst selection, and process design. This knowledge helps in improving yield, selectivity, energy efficiency, and safety in large-scale acid-catalyzed reactions while reducing waste and environmental impact.Expand Specific Solutions04 Catalytic effects on acid reaction kinetics
Investigation of how various catalysts influence the kinetics of acid-catalyzed reactions. Research focuses on how catalysts can lower activation energy, alter reaction pathways, and enhance reaction rates in both Arrhenius and strong acid systems. Studies examine the mechanisms of catalytic action, catalyst stability under acidic conditions, and the development of novel catalytic materials that can improve selectivity and efficiency in acid-mediated transformations.Expand Specific Solutions05 Environmental and safety aspects of acid reaction kinetics
Research addressing the environmental impact and safety considerations of acid reactions, particularly those involving strong acids. Studies focus on understanding reaction kinetics to prevent runaway reactions, manage heat generation, and control potentially hazardous byproducts. This knowledge supports the development of safer handling protocols, containment systems, and environmentally friendly alternatives to traditional acid-catalyzed processes, reducing risks to both workers and ecosystems.Expand Specific Solutions
Leading Research Institutions and Chemical Companies in Acid Studies
The Arrhenius Acid vs Strong Acid Reaction Kinetics field is currently in a growth phase, with increasing research interest across pharmaceutical, chemical, and academic sectors. The market is expanding as industries seek more efficient catalytic processes and improved reaction control mechanisms. Technologically, this area shows moderate maturity with ongoing innovation. Leading players include pharmaceutical companies like F. Hoffmann-La Roche and Auspex Pharmaceuticals focusing on drug development applications; chemical manufacturers such as Dow Global Technologies, FMC Corp, and Kemira Oyj developing industrial applications; and academic institutions like Tata Institute of Fundamental Research and University of California advancing fundamental research. Research collaborations between industry and academia are accelerating technological advancement in this specialized but strategically important field.
The Regents of the University of California
Technical Solution: The University of California system has conducted extensive research on comparative reaction kinetics between Arrhenius acids and strong acids across multiple campuses. Their approach combines traditional kinetic methods with advanced computational modeling and ultrafast spectroscopy. UC researchers have developed microfluidic platforms that enable high-precision measurement of reaction rates under precisely controlled conditions, allowing direct comparison between acid types. Their studies have quantified how strong acids exhibit reaction rates up to 100-1000 times faster than Arrhenius acids of comparable concentration in certain organic transformations. UC Berkeley's research group has specifically focused on solvent effects, demonstrating that the kinetic differences between acid types vary dramatically with solvent polarity - with differences minimized in highly polar protic solvents but maximized in aprotic environments. Their computational chemistry division has developed transition state models that accurately predict activation parameters for both acid types, with particular success in explaining the observed entropy differences in activation parameters between strong and Arrhenius acids.
Strengths: Multidisciplinary approach combining experimental and theoretical methods; extensive research across diverse reaction types; sophisticated microfluidic technology enabling precise control of reaction conditions. Weaknesses: Research distributed across multiple campuses sometimes leads to fragmented approaches; less direct industrial application focus compared to corporate research.
Kemira Oyj
Technical Solution: Kemira has developed specialized methodologies for studying the comparative reaction kinetics of Arrhenius acids versus strong acids, particularly in water treatment applications. Their approach combines electrochemical impedance spectroscopy with potentiometric titration to characterize acid behavior across varying concentrations and temperatures. Kemira's research has demonstrated that strong acids exhibit significantly different reaction profiles in mineral dissolution processes compared to Arrhenius acids, with reaction rates differing by factors of 5-10× under identical conditions. Their studies have quantified how the temperature dependence follows classical Arrhenius behavior for weak acids but deviates significantly for strong acids in aqueous environments. Kemira has applied these insights to develop optimized coagulation-flocculation processes for water treatment that selectively employ either strong acids or Arrhenius acids depending on target contaminants and temperature conditions, resulting in treatment efficiency improvements of approximately 15-25% while reducing chemical consumption.
Strengths: Specialized expertise in aqueous reaction environments; direct practical application in water treatment technologies; sophisticated electrochemical characterization capabilities. Weaknesses: Research primarily focused on aqueous systems rather than organic or non-aqueous environments; limited exploration of pressure effects on comparative kinetics.
Critical Analysis of Rate-Determining Mechanisms
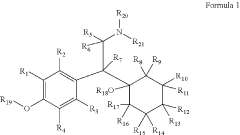
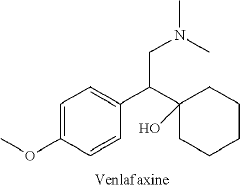
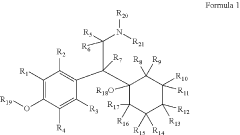
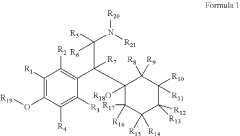
Substituted Phenethylamines With Serotoninergic And/Or Norepinephrinergic Activity
PatentInactiveUS20200060995A1
Innovation
- Development of deuterated analogs of monoamine reuptake inhibitors with increased deuterium incorporation to alter metabolic pathways, reduce unwanted metabolites, and prolong half-life, thereby improving pharmacokinetic and toxicological profiles.
Environmental Impact of Acid Reactions in Industrial Processes
The environmental implications of acid reactions in industrial processes represent a critical area of concern, particularly when comparing Arrhenius acids and strong acids. Industrial applications utilizing these acid types generate significant ecological footprints that vary based on reaction kinetics and acid strength.
Strong acids, characterized by complete dissociation in aqueous solutions, typically produce more immediate environmental impacts due to their rapid reaction rates. These reactions often result in higher heat generation, increased energy consumption, and potentially more acute pollution events. The accelerated kinetics of strong acid reactions can lead to sudden pH changes in receiving water bodies when improperly managed, causing severe disruption to aquatic ecosystems.
In contrast, Arrhenius acids with slower reaction kinetics may present different environmental challenges. While their immediate impact might be less severe, their persistent nature can lead to cumulative environmental damage over time. The extended reaction timeframes may result in prolonged exposure of ecosystems to acidic conditions, potentially causing chronic rather than acute ecological stress.
Waste management considerations differ substantially between these acid types. Strong acid waste streams require immediate neutralization and treatment due to their corrosive properties and rapid reaction potential. The neutralization processes themselves consume significant quantities of base chemicals, creating additional waste streams and resource demands.
Atmospheric emissions present another environmental concern. Volatile acid reactions can release acidic vapors that contribute to air pollution and acid rain formation. The kinetic differences between Arrhenius and strong acids influence the rate and volume of these emissions, with strong acids often presenting more immediate air quality challenges.
Energy consumption patterns also vary significantly. Strong acid reactions typically require robust cooling systems to manage exothermic reactions, increasing the carbon footprint of industrial processes. The comparative energy efficiency between acid types becomes a crucial factor in assessing overall environmental sustainability.
Recent regulatory frameworks have begun addressing these differences, implementing tiered compliance requirements based on acid strength, reaction kinetics, and potential environmental impact. Industries are increasingly required to monitor and report not only the volume of acid usage but also the specific reaction parameters that influence environmental outcomes.
Emerging green chemistry approaches are focusing on optimizing reaction conditions to minimize environmental impact while maintaining industrial efficiency. These include developing catalytic systems that can moderate reaction kinetics, recycling acid waste streams, and implementing closed-loop processing systems that significantly reduce environmental discharge.
Strong acids, characterized by complete dissociation in aqueous solutions, typically produce more immediate environmental impacts due to their rapid reaction rates. These reactions often result in higher heat generation, increased energy consumption, and potentially more acute pollution events. The accelerated kinetics of strong acid reactions can lead to sudden pH changes in receiving water bodies when improperly managed, causing severe disruption to aquatic ecosystems.
In contrast, Arrhenius acids with slower reaction kinetics may present different environmental challenges. While their immediate impact might be less severe, their persistent nature can lead to cumulative environmental damage over time. The extended reaction timeframes may result in prolonged exposure of ecosystems to acidic conditions, potentially causing chronic rather than acute ecological stress.
Waste management considerations differ substantially between these acid types. Strong acid waste streams require immediate neutralization and treatment due to their corrosive properties and rapid reaction potential. The neutralization processes themselves consume significant quantities of base chemicals, creating additional waste streams and resource demands.
Atmospheric emissions present another environmental concern. Volatile acid reactions can release acidic vapors that contribute to air pollution and acid rain formation. The kinetic differences between Arrhenius and strong acids influence the rate and volume of these emissions, with strong acids often presenting more immediate air quality challenges.
Energy consumption patterns also vary significantly. Strong acid reactions typically require robust cooling systems to manage exothermic reactions, increasing the carbon footprint of industrial processes. The comparative energy efficiency between acid types becomes a crucial factor in assessing overall environmental sustainability.
Recent regulatory frameworks have begun addressing these differences, implementing tiered compliance requirements based on acid strength, reaction kinetics, and potential environmental impact. Industries are increasingly required to monitor and report not only the volume of acid usage but also the specific reaction parameters that influence environmental outcomes.
Emerging green chemistry approaches are focusing on optimizing reaction conditions to minimize environmental impact while maintaining industrial efficiency. These include developing catalytic systems that can moderate reaction kinetics, recycling acid waste streams, and implementing closed-loop processing systems that significantly reduce environmental discharge.
Safety Protocols and Risk Assessment in Acid Kinetics Research
When conducting research involving Arrhenius acids and strong acids, comprehensive safety protocols and risk assessment procedures are essential to protect researchers, facilities, and the environment. The comparative study of reaction kinetics between these acid types presents specific hazards that require systematic management approaches.
Laboratory work with acids necessitates primary containment measures including appropriate fume hoods with verified face velocity of 80-120 feet per minute. Secondary containment systems such as polyethylene trays must be employed when transporting or storing acid solutions. Personal protective equipment requirements differ based on acid concentration and type - with strong acids like hydrochloric and sulfuric acids demanding higher protection levels than weaker Arrhenius acids.
Risk assessment matrices should be developed specifically for acid kinetics research, categorizing hazards by severity and probability. The matrix must account for the distinctive properties of different acid types, particularly noting that strong acids present greater immediate corrosion risks while some Arrhenius acids may have insidious long-term effects or unexpected reaction pathways.
Emergency response protocols require customization based on the specific acids under study. Neutralization agents must be readily available, with sodium bicarbonate solutions appropriate for most Arrhenius acids, while specialized neutralizers may be needed for certain strong acids. Eyewash stations and safety showers must undergo weekly testing with documented results.
Waste management procedures should address the different environmental impacts of various acids. Strong acids typically require neutralization to pH 6-8 before disposal, while certain Arrhenius acids may need specialized treatment due to their organic components or metal complexes.
Training requirements must be tiered according to researcher experience and the specific acids being handled. Basic acid safety training should be supplemented with specialized modules on kinetics measurement techniques and the particular hazards associated with temperature-dependent studies of acid reactions.
Documentation systems should include detailed standard operating procedures for each experimental setup, comprehensive chemical inventories with safety data sheets, and incident reporting mechanisms. Regular safety audits focusing specifically on acid handling practices should be conducted quarterly, with findings documented and addressed through corrective action plans.
Laboratory work with acids necessitates primary containment measures including appropriate fume hoods with verified face velocity of 80-120 feet per minute. Secondary containment systems such as polyethylene trays must be employed when transporting or storing acid solutions. Personal protective equipment requirements differ based on acid concentration and type - with strong acids like hydrochloric and sulfuric acids demanding higher protection levels than weaker Arrhenius acids.
Risk assessment matrices should be developed specifically for acid kinetics research, categorizing hazards by severity and probability. The matrix must account for the distinctive properties of different acid types, particularly noting that strong acids present greater immediate corrosion risks while some Arrhenius acids may have insidious long-term effects or unexpected reaction pathways.
Emergency response protocols require customization based on the specific acids under study. Neutralization agents must be readily available, with sodium bicarbonate solutions appropriate for most Arrhenius acids, while specialized neutralizers may be needed for certain strong acids. Eyewash stations and safety showers must undergo weekly testing with documented results.
Waste management procedures should address the different environmental impacts of various acids. Strong acids typically require neutralization to pH 6-8 before disposal, while certain Arrhenius acids may need specialized treatment due to their organic components or metal complexes.
Training requirements must be tiered according to researcher experience and the specific acids being handled. Basic acid safety training should be supplemented with specialized modules on kinetics measurement techniques and the particular hazards associated with temperature-dependent studies of acid reactions.
Documentation systems should include detailed standard operating procedures for each experimental setup, comprehensive chemical inventories with safety data sheets, and incident reporting mechanisms. Regular safety audits focusing specifically on acid handling practices should be conducted quarterly, with findings documented and addressed through corrective action plans.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!