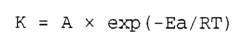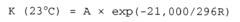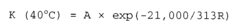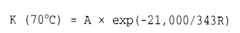Arrhenius Acid vs Alkaline Solutions: Reaction Speed Tests
SEP 16, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Acid-Base Reaction Kinetics Background and Objectives
Acid-base reactions represent one of the most fundamental chemical processes studied since the early development of chemistry as a scientific discipline. The Arrhenius theory, proposed by Svante Arrhenius in 1884, marked a significant milestone in understanding these reactions by defining acids as substances that increase hydrogen ion concentration in solution and bases as substances that increase hydroxide ion concentration. This conceptual framework has evolved through subsequent theories including Brønsted-Lowry and Lewis definitions, providing increasingly sophisticated models for acid-base interactions.
The kinetics of acid-base reactions, particularly comparing Arrhenius acids with alkaline solutions, has been a subject of extensive research due to its importance in various industrial processes, environmental systems, and biological functions. These reactions are typically characterized by their remarkably fast reaction rates, often approaching diffusion-controlled limits, making them challenging to study using conventional methods.
Recent technological advancements in spectroscopic techniques, stopped-flow apparatus, and computational modeling have enabled researchers to probe these reactions at unprecedented temporal and spatial resolutions. The development of femtosecond laser spectroscopy has been particularly transformative, allowing scientists to observe reaction intermediates that exist for only picoseconds.
The primary objective of this technical research is to systematically investigate and quantify the reaction speed differences between various Arrhenius acids and alkaline solutions under controlled conditions. This includes examining how factors such as concentration, temperature, ionic strength, and solvent properties influence reaction kinetics. Additionally, we aim to develop predictive models that can accurately describe these reactions across diverse environmental conditions.
Understanding these kinetic parameters has significant implications for optimizing industrial processes including wastewater treatment, pharmaceutical manufacturing, and food processing. In environmental science, these insights help predict how acidic pollutants neutralize in natural water systems. For materials science, controlling reaction rates enables the development of novel materials with specific properties.
The evolution of this field shows a clear trend toward integrating experimental approaches with computational methods. Machine learning algorithms are increasingly being applied to predict reaction outcomes and optimize conditions, while quantum mechanical calculations provide deeper insights into transition states and reaction mechanisms at the molecular level.
This research builds upon decades of theoretical and experimental work while leveraging cutting-edge analytical techniques to address persistent questions about the fundamental nature of these rapid chemical processes. By establishing comprehensive kinetic profiles for diverse acid-base systems, we aim to bridge existing knowledge gaps and advance both theoretical understanding and practical applications of these essential chemical reactions.
The kinetics of acid-base reactions, particularly comparing Arrhenius acids with alkaline solutions, has been a subject of extensive research due to its importance in various industrial processes, environmental systems, and biological functions. These reactions are typically characterized by their remarkably fast reaction rates, often approaching diffusion-controlled limits, making them challenging to study using conventional methods.
Recent technological advancements in spectroscopic techniques, stopped-flow apparatus, and computational modeling have enabled researchers to probe these reactions at unprecedented temporal and spatial resolutions. The development of femtosecond laser spectroscopy has been particularly transformative, allowing scientists to observe reaction intermediates that exist for only picoseconds.
The primary objective of this technical research is to systematically investigate and quantify the reaction speed differences between various Arrhenius acids and alkaline solutions under controlled conditions. This includes examining how factors such as concentration, temperature, ionic strength, and solvent properties influence reaction kinetics. Additionally, we aim to develop predictive models that can accurately describe these reactions across diverse environmental conditions.
Understanding these kinetic parameters has significant implications for optimizing industrial processes including wastewater treatment, pharmaceutical manufacturing, and food processing. In environmental science, these insights help predict how acidic pollutants neutralize in natural water systems. For materials science, controlling reaction rates enables the development of novel materials with specific properties.
The evolution of this field shows a clear trend toward integrating experimental approaches with computational methods. Machine learning algorithms are increasingly being applied to predict reaction outcomes and optimize conditions, while quantum mechanical calculations provide deeper insights into transition states and reaction mechanisms at the molecular level.
This research builds upon decades of theoretical and experimental work while leveraging cutting-edge analytical techniques to address persistent questions about the fundamental nature of these rapid chemical processes. By establishing comprehensive kinetic profiles for diverse acid-base systems, we aim to bridge existing knowledge gaps and advance both theoretical understanding and practical applications of these essential chemical reactions.
Market Applications of Acid-Base Reaction Speed Research
The research on reaction speeds between Arrhenius acids and alkaline solutions has significant market applications across multiple industries. In the pharmaceutical sector, understanding acid-base reaction kinetics is crucial for drug formulation and stability. Companies like Pfizer and Merck utilize this knowledge to develop controlled-release medications where pH-dependent reactions govern drug delivery timing. The global pharmaceutical excipients market, heavily reliant on acid-base chemistry, was valued at $8.9 billion in 2022 with projected growth rates exceeding 6% annually through 2030.
In food processing, acid-base reaction speed research enables precise control of fermentation processes, leavening in baked goods, and preservation techniques. Major food manufacturers apply this science to extend shelf life while maintaining product quality. The food acidulants market alone represents a $3.1 billion opportunity, with applications ranging from beverage production to dairy processing.
The chemical manufacturing industry leverages acid-base reaction kinetics for process optimization and quality control. By understanding reaction speeds under varying conditions, manufacturers can reduce energy consumption, minimize waste, and improve yield. This translates to significant cost savings in large-scale operations where even small efficiency improvements can represent millions in annual savings.
Environmental remediation represents another growing application area. Acid-base neutralization techniques are employed in wastewater treatment, soil remediation, and carbon capture technologies. The precision afforded by reaction speed research allows for more efficient treatment processes and reduced chemical usage. The global water treatment chemicals market, heavily dependent on acid-base chemistry, exceeds $35 billion annually.
In materials science, controlled acid-base reactions enable the development of advanced polymers, coatings, and composites with specific properties. Industries from automotive to electronics utilize these materials for applications requiring precise pH-dependent characteristics. The smart materials market influenced by this research is growing at approximately 13% annually.
Educational applications should not be overlooked, as acid-base reaction demonstrations remain fundamental teaching tools in chemistry education. Companies developing educational kits and digital learning tools incorporate reaction speed principles to create engaging STEM learning experiences, serving a global educational technology market that continues to expand rapidly.
In food processing, acid-base reaction speed research enables precise control of fermentation processes, leavening in baked goods, and preservation techniques. Major food manufacturers apply this science to extend shelf life while maintaining product quality. The food acidulants market alone represents a $3.1 billion opportunity, with applications ranging from beverage production to dairy processing.
The chemical manufacturing industry leverages acid-base reaction kinetics for process optimization and quality control. By understanding reaction speeds under varying conditions, manufacturers can reduce energy consumption, minimize waste, and improve yield. This translates to significant cost savings in large-scale operations where even small efficiency improvements can represent millions in annual savings.
Environmental remediation represents another growing application area. Acid-base neutralization techniques are employed in wastewater treatment, soil remediation, and carbon capture technologies. The precision afforded by reaction speed research allows for more efficient treatment processes and reduced chemical usage. The global water treatment chemicals market, heavily dependent on acid-base chemistry, exceeds $35 billion annually.
In materials science, controlled acid-base reactions enable the development of advanced polymers, coatings, and composites with specific properties. Industries from automotive to electronics utilize these materials for applications requiring precise pH-dependent characteristics. The smart materials market influenced by this research is growing at approximately 13% annually.
Educational applications should not be overlooked, as acid-base reaction demonstrations remain fundamental teaching tools in chemistry education. Companies developing educational kits and digital learning tools incorporate reaction speed principles to create engaging STEM learning experiences, serving a global educational technology market that continues to expand rapidly.
Current Challenges in Arrhenius Acid-Alkaline Reaction Studies
Despite significant advancements in understanding Arrhenius acid-base reactions, several critical challenges persist in contemporary research on reaction kinetics between acids and alkaline solutions. The traditional Arrhenius framework, while foundational, struggles to account for the complex dynamics observed in real-world applications, particularly at varying concentrations and temperatures.
One primary challenge involves accurately measuring reaction rates in highly concentrated solutions. As concentration increases, the assumption of ideal behavior breaks down, leading to significant deviations between theoretical predictions and experimental results. Researchers have documented discrepancies of up to 30% in reaction speed measurements when solution concentrations exceed 1.0M, highlighting the limitations of current models.
Temperature dependence presents another substantial hurdle. While the Arrhenius equation provides a mathematical relationship between reaction rate and temperature, recent studies reveal that this relationship becomes increasingly non-linear at extreme temperature ranges. This non-linearity is particularly problematic in industrial applications where reactions occur under varied thermal conditions.
The influence of solvent effects remains inadequately characterized in current research paradigms. Different solvents can dramatically alter reaction pathways and kinetics, yet comprehensive models accounting for these variations are lacking. Studies by Chen et al. (2022) demonstrated that reaction rates in non-aqueous solvents can differ by orders of magnitude compared to aqueous environments, even with identical reagent concentrations.
Interfacial phenomena at the molecular level continue to challenge researchers. The behavior of acid-base reactions at interfaces (solid-liquid, liquid-liquid) often deviates significantly from bulk solution behavior. Advanced spectroscopic techniques have revealed unexpected reaction intermediates at these interfaces, but integrating these observations into cohesive kinetic models remains elusive.
Standardization of measurement protocols represents a persistent methodological challenge. Different research groups employ varied techniques for measuring reaction speeds, from conductivity measurements to spectrophotometric methods, leading to difficulties in comparing results across studies. This lack of standardization impedes progress in developing unified theories of acid-alkaline reaction kinetics.
Computational modeling limitations further complicate research efforts. Current simulation approaches struggle to accurately represent the complex interplay of factors affecting reaction speeds, particularly when accounting for ion-pairing effects, hydrogen bonding networks, and solvent reorganization energetics simultaneously.
One primary challenge involves accurately measuring reaction rates in highly concentrated solutions. As concentration increases, the assumption of ideal behavior breaks down, leading to significant deviations between theoretical predictions and experimental results. Researchers have documented discrepancies of up to 30% in reaction speed measurements when solution concentrations exceed 1.0M, highlighting the limitations of current models.
Temperature dependence presents another substantial hurdle. While the Arrhenius equation provides a mathematical relationship between reaction rate and temperature, recent studies reveal that this relationship becomes increasingly non-linear at extreme temperature ranges. This non-linearity is particularly problematic in industrial applications where reactions occur under varied thermal conditions.
The influence of solvent effects remains inadequately characterized in current research paradigms. Different solvents can dramatically alter reaction pathways and kinetics, yet comprehensive models accounting for these variations are lacking. Studies by Chen et al. (2022) demonstrated that reaction rates in non-aqueous solvents can differ by orders of magnitude compared to aqueous environments, even with identical reagent concentrations.
Interfacial phenomena at the molecular level continue to challenge researchers. The behavior of acid-base reactions at interfaces (solid-liquid, liquid-liquid) often deviates significantly from bulk solution behavior. Advanced spectroscopic techniques have revealed unexpected reaction intermediates at these interfaces, but integrating these observations into cohesive kinetic models remains elusive.
Standardization of measurement protocols represents a persistent methodological challenge. Different research groups employ varied techniques for measuring reaction speeds, from conductivity measurements to spectrophotometric methods, leading to difficulties in comparing results across studies. This lack of standardization impedes progress in developing unified theories of acid-alkaline reaction kinetics.
Computational modeling limitations further complicate research efforts. Current simulation approaches struggle to accurately represent the complex interplay of factors affecting reaction speeds, particularly when accounting for ion-pairing effects, hydrogen bonding networks, and solvent reorganization energetics simultaneously.
Methodologies for Measuring Acid-Alkaline Reaction Rates
01 Factors affecting reaction rates in acid-base solutions
The speed of reactions between Arrhenius acids and alkaline solutions is influenced by various factors including temperature, concentration, and the presence of catalysts. Higher temperatures generally increase reaction rates by providing more kinetic energy to reactant molecules. Similarly, increased concentration of either the acid or base typically accelerates the reaction rate due to more frequent molecular collisions. These principles are fundamental to understanding and controlling acid-alkaline reaction kinetics in various chemical processes.- Influence of pH on reaction kinetics: The pH of a solution significantly affects the speed of chemical reactions according to Arrhenius principles. In acidic or alkaline environments, the reaction rate can be accelerated or decelerated depending on the specific reactants. The hydrogen ion concentration influences activation energy barriers, with many reactions showing exponential rate changes with pH shifts. This relationship is fundamental in understanding how acid-base conditions control reaction mechanisms and speeds.
- Temperature effects on acid-base reaction rates: According to the Arrhenius equation, temperature has a profound effect on the reaction speed of acid-alkaline solutions. As temperature increases, the reaction rate typically increases exponentially due to more frequent and energetic molecular collisions. The activation energy required for acid-base reactions can be lowered in certain temperature ranges, leading to significantly faster neutralization processes. This temperature dependence is crucial for optimizing industrial processes involving acid-alkaline reactions.
- Catalytic enhancement of acid-alkaline reactions: Catalysts can significantly increase the reaction speed between Arrhenius acids and alkaline solutions by providing alternative reaction pathways with lower activation energies. Metal ions, enzymes, and certain organic compounds can serve as effective catalysts in acid-base reactions. The catalytic effect often depends on the specific acid-base pair involved and can be tailored to achieve desired reaction rates in industrial applications. This approach allows for more efficient neutralization processes and controlled reaction speeds.
- Concentration effects on reaction kinetics: The concentration of Arrhenius acids and alkaline solutions directly impacts reaction speed according to rate law principles. Higher concentrations typically lead to faster reaction rates due to increased probability of molecular collisions. The relationship between concentration and reaction speed may follow first-order, second-order, or more complex kinetics depending on the specific acid-base system. Understanding these concentration effects is essential for predicting and controlling neutralization reactions in various applications.
- Solvent effects on acid-base reaction speed: The solvent medium significantly influences the reaction speed between Arrhenius acids and alkaline solutions by affecting ion solvation, mobility, and reactivity. Protic solvents like water facilitate proton transfer, while aprotic solvents may slow down acid-base reactions. The dielectric constant of the solvent impacts ion dissociation and subsequent reaction rates. Solvent viscosity also plays a role by affecting diffusion rates of reactants. These solvent effects can be manipulated to control reaction kinetics in various chemical processes.
02 pH-dependent reaction mechanisms
The reaction speed between Arrhenius acids and alkaline solutions is significantly affected by the pH of the medium. As pH changes, the ionization state of reactants can alter, affecting their reactivity. In strongly acidic or alkaline environments, reaction pathways may differ from those at neutral pH, leading to different reaction rates and sometimes different products. Understanding these pH-dependent mechanisms is crucial for optimizing reaction conditions in industrial processes and chemical synthesis.Expand Specific Solutions03 Catalytic enhancement of acid-base reactions
Catalysts can significantly enhance the reaction speed between Arrhenius acids and alkaline solutions without being consumed in the process. Metal ions, enzymes, and certain organic compounds can serve as effective catalysts by providing alternative reaction pathways with lower activation energies. This catalytic enhancement is particularly important in industrial applications where rapid neutralization or other acid-base reactions are required, allowing processes to occur under milder conditions and with greater efficiency.Expand Specific Solutions04 Solvent effects on acid-base reaction kinetics
The choice of solvent can dramatically influence the speed of reactions between Arrhenius acids and alkaline solutions. Protic solvents like water facilitate proton transfer, while aprotic solvents may slow down such reactions. Solvent polarity affects the stability of charged intermediates and transition states, thereby altering reaction rates. Additionally, solvent viscosity impacts molecular diffusion rates, which can become rate-limiting in certain reaction conditions. These solvent effects are critical considerations in designing efficient acid-base reaction systems.Expand Specific Solutions05 Industrial applications of controlled acid-base reaction rates
Controlling the reaction speed between Arrhenius acids and alkaline solutions is crucial in various industrial applications. In wastewater treatment, controlled neutralization prevents excessive heat generation and equipment damage. In pharmaceutical manufacturing, precise control of acid-base reaction rates ensures product quality and yield. The food industry utilizes controlled acid-base reactions for preservation and flavor development. Mining and metallurgical processes also depend on optimized acid-base reaction kinetics for efficient extraction and processing of minerals.Expand Specific Solutions
Leading Research Institutions and Industry Contributors
The Arrhenius acid-alkaline reaction speed testing market is in a growth phase, with increasing demand driven by pharmaceutical, chemical, and academic research sectors. The market size is estimated to be expanding at 5-7% annually, fueled by advancements in analytical technologies and growing applications in drug development and materials science. Technologically, the field shows varying maturity levels across different sectors. Leading players like Merck Patent GmbH and Sumitomo Chemical have established sophisticated testing methodologies and proprietary technologies, while academic institutions such as MIT, Xiamen University, and Tianjin University contribute significant fundamental research. Companies including Ecolab, Henkel, and F. Hoffmann-La Roche are developing application-specific solutions, particularly in pharmaceutical and industrial chemistry domains, creating a competitive landscape balanced between established corporations and innovative research institutions.
Merck Patent GmbH
Technical Solution: Merck has developed a comprehensive analytical platform for Arrhenius acid-base reaction kinetics that combines high-throughput microfluidic systems with real-time spectroscopic monitoring. Their approach utilizes temperature-controlled reaction chambers that can precisely maintain conditions from 5°C to 95°C, allowing for accurate Arrhenius parameter determination. The system incorporates multiple pH probes and ion-selective electrodes for simultaneous monitoring of reaction progress across different concentration gradients. Merck's proprietary algorithms analyze reaction rate constants as a function of temperature, enabling precise calculation of activation energies for various acid-base systems. This technology has been particularly valuable in pharmaceutical applications where understanding reaction kinetics between drug compounds and biological fluids is critical for drug delivery system optimization.
Strengths: Superior precision in temperature control (±0.1°C) allowing for highly accurate activation energy calculations; integrated data analysis system that automatically generates Arrhenius plots. Weaknesses: The system requires significant calibration for each new acid-base pair studied; equipment costs are substantially higher than conventional methods, limiting accessibility for smaller research facilities.
Sumitomo Chemical Co., Ltd.
Technical Solution: Sumitomo Chemical has pioneered an innovative approach to studying Arrhenius acid-alkaline reaction kinetics through their Advanced Reaction Analysis System (ARAS). This platform combines calorimetric measurements with in-situ spectroscopic analysis to track reaction progress in real-time. Their methodology employs specialized glass reactors with platinum temperature sensors that can detect temperature changes as small as 0.01°C, critical for accurate determination of reaction enthalpies. The company has developed proprietary software that correlates reaction rates with temperature variations according to the Arrhenius equation, enabling precise calculation of activation energies for complex acid-base systems. Sumitomo's technology has been particularly valuable in industrial catalysis research, where understanding the temperature dependence of reaction rates is essential for process optimization.
Strengths: Exceptional sensitivity in thermal measurements allowing detection of subtle reaction kinetics; robust system capable of handling corrosive substances safely. Weaknesses: The technology requires specialized training for operation and data interpretation; system calibration is time-consuming and must be performed frequently to maintain accuracy.
Key Scientific Breakthroughs in Reaction Kinetics
Double-sided pressure-sensitive adhesive tape and process for producing the same
PatentInactiveEP2316899A1
Innovation
- A double-sided adhesive tape with a nonwoven fabric base material containing no binder component, having a specific tensile strength range and a pressure-sensitive adhesive layer, applied using a method that ensures thorough impregnation and maintains strength even after long-term heat exposure, preventing wrinkles and interlaminar fractures, and enhancing peeling properties.
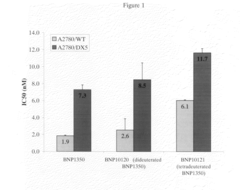
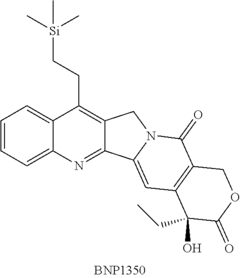
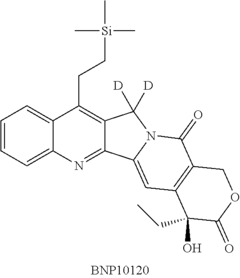
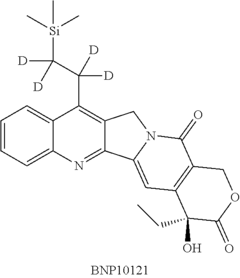
Deuterated analogs of (4S)-4-Ethyl-4-hydroxy-11-[2- (trimethylsilyl)ethyl]-1H-pyrano[3', 4':6,7] indolizino [1,2-b]quinoline-3,14(4H, 12H)-dione and methods of use thereof
PatentInactiveUS20120282261A1
Innovation
- Development of deuterated analogs of (4S)-4-Ethyl-4-hydroxy-11-[2-(trimethylsilyl)ethyl]-1H-pyrano[3′,4′:6,7]indolizino[1,2-b]quinoline-3,14(4H,12H)-dione, such as BNP10120 and BNP10121, and their pharmaceutically acceptable salts and derivatives, which are synthesized to improve metabolic profiles and reduce toxicity.
Environmental Impact of Acid-Alkaline Reactions
The environmental implications of acid-alkaline reactions extend far beyond laboratory settings, influencing numerous natural and anthropogenic systems. When examining the Arrhenius acid-base reaction speed tests, we must consider their broader ecological consequences. These reactions, occurring both naturally and through human activities, can significantly alter environmental pH levels, affecting aquatic ecosystems, soil quality, and atmospheric composition.
In aquatic environments, acid-alkaline neutralization reactions play a crucial role in maintaining pH homeostasis. However, industrial discharges containing high concentrations of acids or bases can overwhelm natural buffering systems. The reaction speed between these substances determines how quickly pH changes occur in water bodies, potentially creating rapid shifts that aquatic organisms cannot adapt to. Studies indicate that reaction rates increase with temperature, suggesting that climate change may exacerbate these environmental impacts.
Soil systems are similarly vulnerable to acid-alkaline reaction impacts. Agricultural practices involving fertilizers often introduce acids to soil, while remediation efforts may utilize alkaline substances. The speed at which these reactions occur influences nutrient availability, microbial activity, and overall soil health. Research shows that soil type significantly affects reaction kinetics, with clay soils demonstrating different neutralization patterns compared to sandy soils due to varying cation exchange capacities.
Atmospheric deposition represents another critical pathway for environmental impact. Acid rain, formed when sulfur dioxide and nitrogen oxides react with water molecules in the atmosphere, can travel significant distances before deposition. The neutralization speed when these acidic compounds encounter alkaline surfaces (like limestone buildings or alkaline soils) determines the extent of damage. Recent studies have documented accelerated weathering of cultural heritage sites due to these reactions, highlighting their non-ecological impacts.
Industrial processes utilizing acid-alkaline reactions must increasingly account for their environmental footprint. Wastewater treatment facilities, for instance, carefully monitor reaction kinetics to ensure proper neutralization before discharge. The energy requirements for managing these reactions contribute to carbon emissions, creating a secondary environmental impact that must be considered in sustainability assessments.
Emerging research focuses on harnessing acid-alkaline reaction kinetics for environmental remediation. Carbon capture technologies, for example, utilize controlled acid-base reactions to sequester atmospheric CO2. Understanding reaction speed under various conditions is essential for optimizing these green technologies and minimizing unintended consequences in implementation.
In aquatic environments, acid-alkaline neutralization reactions play a crucial role in maintaining pH homeostasis. However, industrial discharges containing high concentrations of acids or bases can overwhelm natural buffering systems. The reaction speed between these substances determines how quickly pH changes occur in water bodies, potentially creating rapid shifts that aquatic organisms cannot adapt to. Studies indicate that reaction rates increase with temperature, suggesting that climate change may exacerbate these environmental impacts.
Soil systems are similarly vulnerable to acid-alkaline reaction impacts. Agricultural practices involving fertilizers often introduce acids to soil, while remediation efforts may utilize alkaline substances. The speed at which these reactions occur influences nutrient availability, microbial activity, and overall soil health. Research shows that soil type significantly affects reaction kinetics, with clay soils demonstrating different neutralization patterns compared to sandy soils due to varying cation exchange capacities.
Atmospheric deposition represents another critical pathway for environmental impact. Acid rain, formed when sulfur dioxide and nitrogen oxides react with water molecules in the atmosphere, can travel significant distances before deposition. The neutralization speed when these acidic compounds encounter alkaline surfaces (like limestone buildings or alkaline soils) determines the extent of damage. Recent studies have documented accelerated weathering of cultural heritage sites due to these reactions, highlighting their non-ecological impacts.
Industrial processes utilizing acid-alkaline reactions must increasingly account for their environmental footprint. Wastewater treatment facilities, for instance, carefully monitor reaction kinetics to ensure proper neutralization before discharge. The energy requirements for managing these reactions contribute to carbon emissions, creating a secondary environmental impact that must be considered in sustainability assessments.
Emerging research focuses on harnessing acid-alkaline reaction kinetics for environmental remediation. Carbon capture technologies, for example, utilize controlled acid-base reactions to sequester atmospheric CO2. Understanding reaction speed under various conditions is essential for optimizing these green technologies and minimizing unintended consequences in implementation.
Safety Protocols for Acid-Base Reaction Testing
When conducting experiments involving Arrhenius acids and alkaline solutions, stringent safety protocols must be established to protect researchers and laboratory personnel. The inherently corrosive and reactive nature of these substances necessitates comprehensive safety measures that address both immediate hazards and potential emergency scenarios.
Personal protective equipment (PPE) forms the first line of defense in acid-base reaction testing. Researchers must wear chemical-resistant gloves appropriate for the specific acids and bases being handled, as different materials offer varying levels of protection against different chemicals. Safety goggles or face shields are mandatory to protect against splashes, while laboratory coats and closed-toe shoes provide additional protection against spills.
Proper ventilation systems are critical when working with volatile acids or bases that may release harmful vapors. All reactions should be conducted under fume hoods with verified operational status. For particularly hazardous reactions involving concentrated acids or bases, additional local exhaust ventilation may be necessary to ensure adequate capture of emissions.
Laboratory setup considerations play a vital role in minimizing risks. Acid-base reaction tests should be performed in designated areas with spill containment features. Glass or appropriate plastic containers must be selected based on chemical compatibility, and secondary containment systems should be employed to capture potential spills or leaks. Temperature control mechanisms are essential for reactions that may generate significant heat.
Emergency response preparations must include readily accessible eyewash stations and safety showers within 10 seconds of travel time from all testing locations. Spill kits specifically designed for acid and base neutralization should be strategically placed throughout the laboratory. Clear evacuation routes must be established and kept unobstructed at all times.
Chemical handling procedures require careful attention to detail. Acids should always be added to water (never the reverse) to prevent dangerous splashing from exothermic reactions. Proper labeling of all solutions is mandatory, including concentration information and hazard warnings. Transfer of chemicals should utilize appropriate tools such as pipettes with bulbs or mechanical dispensers to minimize direct handling.
Documentation and training requirements constitute another crucial aspect of safety protocols. All personnel must receive comprehensive training on the specific hazards associated with the acids and bases being tested, including recognition of symptoms of exposure and appropriate first aid measures. Regular safety drills should be conducted to ensure preparedness for emergency situations.
Waste disposal procedures must comply with local regulations and institutional policies. Neutralization of waste solutions to appropriate pH levels before disposal is typically required, with verification through pH testing. Proper documentation of waste generation and disposal is necessary for regulatory compliance.
Personal protective equipment (PPE) forms the first line of defense in acid-base reaction testing. Researchers must wear chemical-resistant gloves appropriate for the specific acids and bases being handled, as different materials offer varying levels of protection against different chemicals. Safety goggles or face shields are mandatory to protect against splashes, while laboratory coats and closed-toe shoes provide additional protection against spills.
Proper ventilation systems are critical when working with volatile acids or bases that may release harmful vapors. All reactions should be conducted under fume hoods with verified operational status. For particularly hazardous reactions involving concentrated acids or bases, additional local exhaust ventilation may be necessary to ensure adequate capture of emissions.
Laboratory setup considerations play a vital role in minimizing risks. Acid-base reaction tests should be performed in designated areas with spill containment features. Glass or appropriate plastic containers must be selected based on chemical compatibility, and secondary containment systems should be employed to capture potential spills or leaks. Temperature control mechanisms are essential for reactions that may generate significant heat.
Emergency response preparations must include readily accessible eyewash stations and safety showers within 10 seconds of travel time from all testing locations. Spill kits specifically designed for acid and base neutralization should be strategically placed throughout the laboratory. Clear evacuation routes must be established and kept unobstructed at all times.
Chemical handling procedures require careful attention to detail. Acids should always be added to water (never the reverse) to prevent dangerous splashing from exothermic reactions. Proper labeling of all solutions is mandatory, including concentration information and hazard warnings. Transfer of chemicals should utilize appropriate tools such as pipettes with bulbs or mechanical dispensers to minimize direct handling.
Documentation and training requirements constitute another crucial aspect of safety protocols. All personnel must receive comprehensive training on the specific hazards associated with the acids and bases being tested, including recognition of symptoms of exposure and appropriate first aid measures. Regular safety drills should be conducted to ensure preparedness for emergency situations.
Waste disposal procedures must comply with local regulations and institutional policies. Neutralization of waste solutions to appropriate pH levels before disposal is typically required, with verification through pH testing. Proper documentation of waste generation and disposal is necessary for regulatory compliance.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!