Biomedical Applications of Flexible Piezoelectric Sensors
JUL 17, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Piezoelectric Sensors in Biomedicine: Background and Objectives
Piezoelectric sensors have emerged as a groundbreaking technology in the field of biomedicine, offering unprecedented opportunities for real-time, non-invasive monitoring of physiological parameters. The development of these sensors can be traced back to the discovery of the piezoelectric effect by Jacques and Pierre Curie in 1880. Since then, the technology has evolved significantly, with flexible piezoelectric sensors becoming a focal point of research in recent years.
The primary objective of implementing flexible piezoelectric sensors in biomedical applications is to enhance patient care through continuous, accurate, and comfortable monitoring of vital signs and biomechanical movements. These sensors aim to overcome the limitations of traditional rigid sensors by conforming to the complex contours of the human body, thereby improving signal quality and patient comfort.
The evolution of piezoelectric materials has been crucial in advancing this technology. From early ceramic-based sensors to modern polymer-based flexible sensors, the field has witnessed a paradigm shift in material science and engineering. This progression has enabled the development of sensors that can be seamlessly integrated into wearable devices, implants, and even smart textiles.
In the biomedical context, flexible piezoelectric sensors are being explored for a wide range of applications. These include continuous blood pressure monitoring, respiratory rate detection, gait analysis, and even early detection of neurodegenerative disorders through subtle movement analysis. The potential of these sensors extends to personalized medicine, where real-time data collection can inform tailored treatment strategies.
The current technological landscape is driven by the need for miniaturization, improved sensitivity, and biocompatibility. Researchers are focusing on developing sensors that can operate reliably in diverse physiological environments while maintaining long-term stability. Additionally, there is a growing emphasis on creating self-powered sensors that can harness energy from body movements, addressing the challenge of power supply in long-term implantable devices.
As we look towards the future, the integration of flexible piezoelectric sensors with artificial intelligence and big data analytics presents exciting possibilities. This convergence could lead to predictive healthcare models, early disease detection, and more efficient clinical decision-making processes. The ultimate goal is to create a seamless interface between the human body and digital health systems, paving the way for more proactive and personalized healthcare solutions.
The primary objective of implementing flexible piezoelectric sensors in biomedical applications is to enhance patient care through continuous, accurate, and comfortable monitoring of vital signs and biomechanical movements. These sensors aim to overcome the limitations of traditional rigid sensors by conforming to the complex contours of the human body, thereby improving signal quality and patient comfort.
The evolution of piezoelectric materials has been crucial in advancing this technology. From early ceramic-based sensors to modern polymer-based flexible sensors, the field has witnessed a paradigm shift in material science and engineering. This progression has enabled the development of sensors that can be seamlessly integrated into wearable devices, implants, and even smart textiles.
In the biomedical context, flexible piezoelectric sensors are being explored for a wide range of applications. These include continuous blood pressure monitoring, respiratory rate detection, gait analysis, and even early detection of neurodegenerative disorders through subtle movement analysis. The potential of these sensors extends to personalized medicine, where real-time data collection can inform tailored treatment strategies.
The current technological landscape is driven by the need for miniaturization, improved sensitivity, and biocompatibility. Researchers are focusing on developing sensors that can operate reliably in diverse physiological environments while maintaining long-term stability. Additionally, there is a growing emphasis on creating self-powered sensors that can harness energy from body movements, addressing the challenge of power supply in long-term implantable devices.
As we look towards the future, the integration of flexible piezoelectric sensors with artificial intelligence and big data analytics presents exciting possibilities. This convergence could lead to predictive healthcare models, early disease detection, and more efficient clinical decision-making processes. The ultimate goal is to create a seamless interface between the human body and digital health systems, paving the way for more proactive and personalized healthcare solutions.
Market Analysis for Flexible Biomedical Sensors
The market for flexible biomedical sensors, particularly those utilizing piezoelectric technology, is experiencing rapid growth and transformation. This expansion is driven by increasing demand for wearable health monitoring devices, advancements in materials science, and the growing emphasis on personalized healthcare. The global market for flexible sensors in biomedical applications is projected to reach significant value in the coming years, with a compound annual growth rate (CAGR) outpacing many other segments in the healthcare technology sector.
Several factors contribute to the rising demand for flexible piezoelectric sensors in biomedical applications. Firstly, the aging population in many developed countries has led to a greater need for continuous health monitoring solutions that are non-invasive and comfortable for long-term use. Flexible sensors meet these requirements by conforming to body contours and providing reliable data without causing discomfort.
Additionally, the trend towards preventive healthcare and remote patient monitoring has created new opportunities for flexible sensor technologies. These sensors enable the collection of real-time physiological data outside traditional clinical settings, allowing for early detection of health issues and more personalized treatment plans. The COVID-19 pandemic has further accelerated this trend, highlighting the importance of remote monitoring capabilities in healthcare systems.
The market for flexible biomedical sensors is also benefiting from advancements in materials science and manufacturing techniques. Innovations in nanomaterials and flexible electronics have led to the development of sensors with improved sensitivity, durability, and biocompatibility. These technological improvements are expanding the range of potential applications and driving market growth.
In terms of application areas, the market for flexible piezoelectric sensors in biomedicine spans various segments. Wearable fitness trackers and smartwatches represent a significant portion of the current market, with continuous glucose monitoring devices and smart textiles for health monitoring showing strong growth potential. Emerging applications include flexible sensors for prosthetics, rehabilitation devices, and implantable medical devices.
Geographically, North America and Europe currently lead the market for flexible biomedical sensors, owing to their advanced healthcare infrastructure and high adoption rates of new technologies. However, the Asia-Pacific region is expected to show the fastest growth in the coming years, driven by increasing healthcare expenditure, growing awareness of preventive healthcare, and rapid technological advancements in countries like China and Japan.
Despite the positive outlook, the market faces challenges such as regulatory hurdles, data privacy concerns, and the need for standardization in sensor technologies. Overcoming these obstacles will be crucial for realizing the full potential of flexible piezoelectric sensors in biomedical applications and sustaining long-term market growth.
Several factors contribute to the rising demand for flexible piezoelectric sensors in biomedical applications. Firstly, the aging population in many developed countries has led to a greater need for continuous health monitoring solutions that are non-invasive and comfortable for long-term use. Flexible sensors meet these requirements by conforming to body contours and providing reliable data without causing discomfort.
Additionally, the trend towards preventive healthcare and remote patient monitoring has created new opportunities for flexible sensor technologies. These sensors enable the collection of real-time physiological data outside traditional clinical settings, allowing for early detection of health issues and more personalized treatment plans. The COVID-19 pandemic has further accelerated this trend, highlighting the importance of remote monitoring capabilities in healthcare systems.
The market for flexible biomedical sensors is also benefiting from advancements in materials science and manufacturing techniques. Innovations in nanomaterials and flexible electronics have led to the development of sensors with improved sensitivity, durability, and biocompatibility. These technological improvements are expanding the range of potential applications and driving market growth.
In terms of application areas, the market for flexible piezoelectric sensors in biomedicine spans various segments. Wearable fitness trackers and smartwatches represent a significant portion of the current market, with continuous glucose monitoring devices and smart textiles for health monitoring showing strong growth potential. Emerging applications include flexible sensors for prosthetics, rehabilitation devices, and implantable medical devices.
Geographically, North America and Europe currently lead the market for flexible biomedical sensors, owing to their advanced healthcare infrastructure and high adoption rates of new technologies. However, the Asia-Pacific region is expected to show the fastest growth in the coming years, driven by increasing healthcare expenditure, growing awareness of preventive healthcare, and rapid technological advancements in countries like China and Japan.
Despite the positive outlook, the market faces challenges such as regulatory hurdles, data privacy concerns, and the need for standardization in sensor technologies. Overcoming these obstacles will be crucial for realizing the full potential of flexible piezoelectric sensors in biomedical applications and sustaining long-term market growth.
Current Challenges in Flexible Piezoelectric Sensor Technology
Despite the significant advancements in flexible piezoelectric sensor technology, several challenges persist in their biomedical applications. One of the primary obstacles is achieving consistent and reliable performance under dynamic physiological conditions. The human body's complex and ever-changing environment, including variations in temperature, humidity, and pH levels, can significantly affect sensor sensitivity and accuracy.
Material limitations pose another significant challenge. While flexibility is crucial for biocompatibility and comfort, many current piezoelectric materials struggle to maintain their electrical properties when subjected to repeated bending or stretching. This can lead to reduced sensor lifespan and compromised data integrity over time, particularly in long-term monitoring applications.
Biocompatibility and long-term stability remain ongoing concerns. Ensuring that flexible piezoelectric sensors do not elicit adverse biological responses or degrade when exposed to bodily fluids is critical for their successful integration into medical devices. Additionally, the potential for material leaching or degradation products must be carefully evaluated to prevent toxicity risks.
Signal-to-noise ratio (SNR) optimization presents a persistent challenge, especially in implantable or wearable devices. Ambient electromagnetic interference, motion artifacts, and biological noise can significantly impact the quality of sensor readings. Developing effective noise cancellation techniques without compromising the sensor's flexibility or increasing its power consumption is a complex engineering task.
Power management is another critical issue, particularly for wireless and implantable sensors. Balancing the need for continuous monitoring with limited battery capacity requires innovative energy harvesting and low-power circuit designs. The challenge lies in miniaturizing these components while maintaining sensor performance and flexibility.
Scalability and manufacturing consistency pose significant hurdles in transitioning from laboratory prototypes to mass-produced devices. Ensuring uniform sensor characteristics across large production batches while keeping costs competitive is essential for widespread adoption in the biomedical field.
Lastly, data integration and interpretation present challenges on the software side. Developing robust algorithms to process and interpret the vast amounts of data generated by flexible piezoelectric sensors, especially in real-time applications, requires advanced signal processing and machine learning techniques. Ensuring the reliability and clinical relevance of these interpretations is crucial for their acceptance in medical diagnostics and treatment monitoring.
Material limitations pose another significant challenge. While flexibility is crucial for biocompatibility and comfort, many current piezoelectric materials struggle to maintain their electrical properties when subjected to repeated bending or stretching. This can lead to reduced sensor lifespan and compromised data integrity over time, particularly in long-term monitoring applications.
Biocompatibility and long-term stability remain ongoing concerns. Ensuring that flexible piezoelectric sensors do not elicit adverse biological responses or degrade when exposed to bodily fluids is critical for their successful integration into medical devices. Additionally, the potential for material leaching or degradation products must be carefully evaluated to prevent toxicity risks.
Signal-to-noise ratio (SNR) optimization presents a persistent challenge, especially in implantable or wearable devices. Ambient electromagnetic interference, motion artifacts, and biological noise can significantly impact the quality of sensor readings. Developing effective noise cancellation techniques without compromising the sensor's flexibility or increasing its power consumption is a complex engineering task.
Power management is another critical issue, particularly for wireless and implantable sensors. Balancing the need for continuous monitoring with limited battery capacity requires innovative energy harvesting and low-power circuit designs. The challenge lies in miniaturizing these components while maintaining sensor performance and flexibility.
Scalability and manufacturing consistency pose significant hurdles in transitioning from laboratory prototypes to mass-produced devices. Ensuring uniform sensor characteristics across large production batches while keeping costs competitive is essential for widespread adoption in the biomedical field.
Lastly, data integration and interpretation present challenges on the software side. Developing robust algorithms to process and interpret the vast amounts of data generated by flexible piezoelectric sensors, especially in real-time applications, requires advanced signal processing and machine learning techniques. Ensuring the reliability and clinical relevance of these interpretations is crucial for their acceptance in medical diagnostics and treatment monitoring.
Existing Flexible Piezoelectric Sensor Solutions
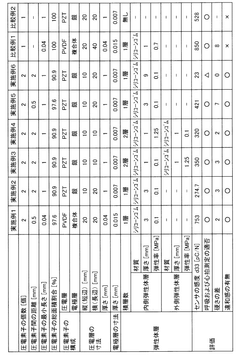
01 Flexible piezoelectric materials for sensors
Flexible piezoelectric materials are used to create sensors that can bend and conform to various shapes. These materials, such as polymers or thin films, maintain their piezoelectric properties while being flexible, allowing for the development of sensors that can be integrated into wearable devices or applied to curved surfaces.- Flexible piezoelectric materials: Development of flexible piezoelectric materials that can maintain their piezoelectric properties while being bent or stretched. These materials often include polymers or composites that combine piezoelectric elements with flexible substrates, allowing for the creation of sensors that can conform to curved surfaces or withstand deformation.
- Nanostructured piezoelectric sensors: Utilization of nanostructures such as nanowires, nanofibers, or nanoparticles to enhance the flexibility and sensitivity of piezoelectric sensors. These nanostructures can be incorporated into flexible matrices or deposited on bendable substrates to create highly responsive and adaptable sensing devices.
- Flexible substrate integration: Integration of piezoelectric elements onto flexible substrates such as polymers, textiles, or thin films. This approach allows for the creation of conformable sensors that can be easily applied to various surfaces or incorporated into wearable devices while maintaining high sensitivity to mechanical stimuli.
- Multilayer and composite structures: Development of multilayer or composite structures that combine different materials to achieve both flexibility and piezoelectric functionality. These structures may include alternating layers of piezoelectric materials and flexible polymers or the incorporation of piezoelectric particles into a flexible matrix to create sensors with tailored mechanical and electrical properties.
- Fabrication techniques for flexible sensors: Innovative fabrication techniques such as printing, roll-to-roll processing, or solution-based deposition methods that enable the production of large-area, flexible piezoelectric sensors. These techniques focus on maintaining the piezoelectric properties of the materials while ensuring compatibility with flexible substrates and scalable manufacturing processes.
02 Nanostructures for enhanced flexibility
Incorporating nanostructures, such as nanowires or nanofibers, into piezoelectric sensors improves their flexibility while maintaining or enhancing their sensing capabilities. These nanostructures allow for greater deformation without compromising the sensor's performance, making them suitable for applications requiring high flexibility.Expand Specific Solutions03 Substrate selection for flexible sensors
The choice of substrate plays a crucial role in developing flexible piezoelectric sensors. Flexible substrates, such as polymers or thin metal foils, are used to support the piezoelectric material while allowing the entire sensor structure to remain flexible. This enables the creation of bendable and stretchable sensor devices.Expand Specific Solutions04 Composite materials for improved flexibility
Composite materials combining piezoelectric elements with flexible matrices are developed to enhance the overall flexibility of the sensors. These composites can include piezoelectric particles or fibers embedded in a flexible polymer matrix, resulting in sensors that can withstand significant deformation while maintaining their sensing capabilities.Expand Specific Solutions05 Structural design for flexibility
Innovative structural designs are employed to improve the flexibility of piezoelectric sensors. These designs may include serpentine patterns, mesh structures, or segmented layouts that allow for greater deformation and bending of the sensor without compromising its functionality. Such designs enable the sensors to conform to complex shapes and dynamic surfaces.Expand Specific Solutions
Key Players in Biomedical Piezoelectric Sensor Industry
The biomedical applications of flexible piezoelectric sensors market is in a growth phase, driven by increasing demand for wearable health monitoring devices and advancements in materials science. The market size is expanding rapidly, with projections indicating significant growth in the coming years. Technologically, the field is progressing from early-stage development to more mature applications, with companies like Massachusetts Institute of Technology, Shanghai Jiao Tong University, and Wuhan University leading research efforts. Industry players such as Sumitomo Riko Co. Ltd. and Mitsui Chemicals, Inc. are actively developing commercial applications, while startups like Patientech LLC are introducing innovative solutions. The competitive landscape is diverse, with academic institutions, established corporations, and emerging companies all contributing to technological advancements in this promising field.
Massachusetts Institute of Technology
Technical Solution: MIT has developed a flexible piezoelectric sensor for biomedical applications using a novel nanofiber-based approach. Their technology utilizes electrospun piezoelectric polymer nanofibers to create highly sensitive and conformable sensors. These sensors can detect minute mechanical deformations and convert them into electrical signals, making them ideal for various biomedical monitoring applications. The nanofiber structure allows for enhanced flexibility and sensitivity compared to traditional bulk piezoelectric materials[1]. MIT's sensors have demonstrated excellent performance in measuring physiological parameters such as pulse rate, respiration, and muscle movements[2]. The fabrication process involves electrospinning of poly(vinylidene fluoride-trifluoroethylene) (PVDF-TrFE) nanofibers, followed by integration with flexible electrodes and encapsulation[3].
Strengths: High sensitivity, excellent flexibility, and biocompatibility. Weaknesses: Potential challenges in large-scale manufacturing and long-term stability in biological environments.
Shanghai Jiao Tong University
Technical Solution: Shanghai Jiao Tong University has developed innovative flexible piezoelectric sensors for biomedical applications, focusing on self-powered devices. Their approach utilizes nanostructured piezoelectric materials, such as zinc oxide nanowires and barium titanate nanoparticles, to create sensors that can harvest energy from body movements while simultaneously measuring physiological signals. The university's technology involves a unique fabrication process that combines electrospinning and hydrothermal growth methods to create hierarchical nanostructures with enhanced piezoelectric properties[12]. These sensors have demonstrated high sensitivity in detecting subtle body movements, including heartbeats and muscle contractions. SJTU researchers have also developed a novel encapsulation technique using biocompatible polymers to protect the sensors from bodily fluids while maintaining flexibility[13]. The self-powered nature of these sensors makes them particularly suitable for long-term health monitoring applications, as they do not require external power sources or frequent battery replacements[14].
Strengths: Self-powered operation, high sensitivity to subtle movements. Weaknesses: Complexity in fabrication process, potential variability in energy harvesting efficiency.
Core Innovations in Flexible Piezoelectric Sensing
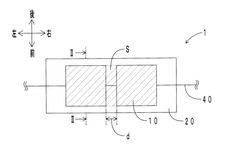
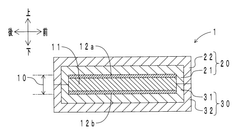
Piezoelectric sensor
PatentWO2020066157A1
Innovation
- A flexible piezoelectric sensor design featuring a piezoelectric layer with spaced-apart elements covered by an elastic layer with an elastic modulus of 10 MPa or less, allowing for reduced discomfort and enhanced sensitivity by using hard ceramics or resins without feeling hard, and enabling precise detection of weak vibrations.
Biocompatibility and Safety Considerations
The biocompatibility and safety of flexible piezoelectric sensors are paramount considerations for their successful implementation in biomedical applications. These sensors, designed to interface directly with biological tissues, must meet stringent requirements to ensure patient safety and long-term functionality.
Biocompatibility is a critical factor in the development of flexible piezoelectric sensors for biomedical use. The materials used in sensor construction must not elicit adverse reactions when in contact with living tissues. Commonly used piezoelectric materials, such as polyvinylidene fluoride (PVDF) and its copolymers, have shown promising biocompatibility profiles. However, comprehensive in vitro and in vivo studies are necessary to evaluate potential cytotoxicity, inflammatory responses, and long-term tissue interactions.
The encapsulation of flexible piezoelectric sensors plays a crucial role in enhancing biocompatibility and safety. Advanced encapsulation techniques using biocompatible polymers, such as parylene or polyimide, can effectively isolate the sensor components from biological fluids and tissues. This encapsulation not only protects the sensor from degradation but also prevents the leaching of potentially harmful substances into the surrounding environment.
Mechanical compatibility is another essential aspect of safety considerations. Flexible piezoelectric sensors must match the mechanical properties of the target tissues to minimize stress and strain at the interface. This compatibility reduces the risk of tissue damage and improves the overall comfort for patients in long-term monitoring applications.
Electrical safety is of utmost importance, particularly for implantable or wearable piezoelectric sensors. Proper insulation and grounding techniques must be employed to prevent electrical leakage and potential tissue damage. Additionally, the integration of these sensors with other electronic components and power sources must adhere to strict safety standards to mitigate risks associated with electromagnetic interference and electrical malfunction.
Sterilization compatibility is a crucial factor for sensors intended for use in sterile medical environments. The materials and construction of flexible piezoelectric sensors must withstand common sterilization methods, such as ethylene oxide treatment or gamma irradiation, without compromising their performance or biocompatibility.
Long-term stability and degradation characteristics of flexible piezoelectric sensors must be thoroughly evaluated to ensure sustained safety and efficacy. This includes assessing the potential for material breakdown, ion leaching, and changes in sensor performance over extended periods of use in physiological conditions.
Regulatory compliance is a critical aspect of bringing flexible piezoelectric sensors to market for biomedical applications. Manufacturers must navigate complex regulatory frameworks, such as FDA guidelines in the United States or CE marking in Europe, to demonstrate the safety and efficacy of their devices through rigorous testing and documentation.
Biocompatibility is a critical factor in the development of flexible piezoelectric sensors for biomedical use. The materials used in sensor construction must not elicit adverse reactions when in contact with living tissues. Commonly used piezoelectric materials, such as polyvinylidene fluoride (PVDF) and its copolymers, have shown promising biocompatibility profiles. However, comprehensive in vitro and in vivo studies are necessary to evaluate potential cytotoxicity, inflammatory responses, and long-term tissue interactions.
The encapsulation of flexible piezoelectric sensors plays a crucial role in enhancing biocompatibility and safety. Advanced encapsulation techniques using biocompatible polymers, such as parylene or polyimide, can effectively isolate the sensor components from biological fluids and tissues. This encapsulation not only protects the sensor from degradation but also prevents the leaching of potentially harmful substances into the surrounding environment.
Mechanical compatibility is another essential aspect of safety considerations. Flexible piezoelectric sensors must match the mechanical properties of the target tissues to minimize stress and strain at the interface. This compatibility reduces the risk of tissue damage and improves the overall comfort for patients in long-term monitoring applications.
Electrical safety is of utmost importance, particularly for implantable or wearable piezoelectric sensors. Proper insulation and grounding techniques must be employed to prevent electrical leakage and potential tissue damage. Additionally, the integration of these sensors with other electronic components and power sources must adhere to strict safety standards to mitigate risks associated with electromagnetic interference and electrical malfunction.
Sterilization compatibility is a crucial factor for sensors intended for use in sterile medical environments. The materials and construction of flexible piezoelectric sensors must withstand common sterilization methods, such as ethylene oxide treatment or gamma irradiation, without compromising their performance or biocompatibility.
Long-term stability and degradation characteristics of flexible piezoelectric sensors must be thoroughly evaluated to ensure sustained safety and efficacy. This includes assessing the potential for material breakdown, ion leaching, and changes in sensor performance over extended periods of use in physiological conditions.
Regulatory compliance is a critical aspect of bringing flexible piezoelectric sensors to market for biomedical applications. Manufacturers must navigate complex regulatory frameworks, such as FDA guidelines in the United States or CE marking in Europe, to demonstrate the safety and efficacy of their devices through rigorous testing and documentation.
Regulatory Landscape for Medical Piezoelectric Devices
The regulatory landscape for medical piezoelectric devices is complex and evolving, reflecting the unique characteristics and potential risks associated with these innovative technologies. In the United States, the Food and Drug Administration (FDA) plays a central role in regulating medical devices, including those incorporating piezoelectric sensors. These devices typically fall under Class II or Class III classifications, depending on their intended use and potential risks.
For Class II devices, manufacturers must submit a 510(k) premarket notification, demonstrating that their device is substantially equivalent to a legally marketed predicate device. This process involves providing detailed information on the device's design, performance, and safety features. Class III devices, which pose a higher risk, require a more rigorous premarket approval (PMA) process, including clinical trials to demonstrate safety and efficacy.
In the European Union, medical piezoelectric devices are regulated under the Medical Device Regulation (MDR), which came into full effect in May 2021. The MDR introduces stricter requirements for clinical evidence, post-market surveillance, and traceability. Manufacturers must obtain CE marking to market their devices in the EU, which involves a conformity assessment procedure and, in some cases, review by a notified body.
Japan's regulatory framework for medical devices is overseen by the Pharmaceuticals and Medical Devices Agency (PMDA). The approval process for piezoelectric sensors in medical applications typically involves submitting a premarket approval application, which includes data on the device's safety, efficacy, and quality.
Globally, there is an increasing focus on harmonizing regulatory approaches through initiatives like the International Medical Device Regulators Forum (IMDRF). This aims to streamline the approval process for innovative medical technologies across different markets, potentially reducing time-to-market for piezoelectric sensor-based devices.
Key regulatory considerations for piezoelectric sensors in medical applications include biocompatibility, electrical safety, electromagnetic compatibility, and long-term stability. Manufacturers must demonstrate that their devices meet these requirements through extensive testing and documentation. Additionally, as these sensors often involve data collection and processing, compliance with data protection regulations such as GDPR in the EU is crucial.
The regulatory landscape is continually adapting to keep pace with technological advancements. Emerging trends include the development of specific guidance for wearable medical devices and the integration of artificial intelligence in medical device software. These developments may have significant implications for the regulation of flexible piezoelectric sensors in biomedical applications, potentially leading to more tailored regulatory pathways for these innovative technologies.
For Class II devices, manufacturers must submit a 510(k) premarket notification, demonstrating that their device is substantially equivalent to a legally marketed predicate device. This process involves providing detailed information on the device's design, performance, and safety features. Class III devices, which pose a higher risk, require a more rigorous premarket approval (PMA) process, including clinical trials to demonstrate safety and efficacy.
In the European Union, medical piezoelectric devices are regulated under the Medical Device Regulation (MDR), which came into full effect in May 2021. The MDR introduces stricter requirements for clinical evidence, post-market surveillance, and traceability. Manufacturers must obtain CE marking to market their devices in the EU, which involves a conformity assessment procedure and, in some cases, review by a notified body.
Japan's regulatory framework for medical devices is overseen by the Pharmaceuticals and Medical Devices Agency (PMDA). The approval process for piezoelectric sensors in medical applications typically involves submitting a premarket approval application, which includes data on the device's safety, efficacy, and quality.
Globally, there is an increasing focus on harmonizing regulatory approaches through initiatives like the International Medical Device Regulators Forum (IMDRF). This aims to streamline the approval process for innovative medical technologies across different markets, potentially reducing time-to-market for piezoelectric sensor-based devices.
Key regulatory considerations for piezoelectric sensors in medical applications include biocompatibility, electrical safety, electromagnetic compatibility, and long-term stability. Manufacturers must demonstrate that their devices meet these requirements through extensive testing and documentation. Additionally, as these sensors often involve data collection and processing, compliance with data protection regulations such as GDPR in the EU is crucial.
The regulatory landscape is continually adapting to keep pace with technological advancements. Emerging trends include the development of specific guidance for wearable medical devices and the integration of artificial intelligence in medical device software. These developments may have significant implications for the regulation of flexible piezoelectric sensors in biomedical applications, potentially leading to more tailored regulatory pathways for these innovative technologies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!