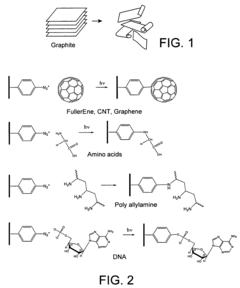
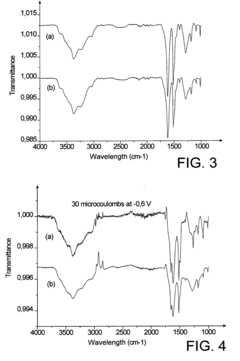
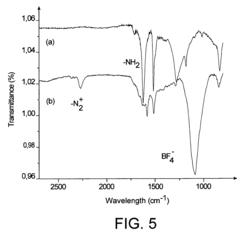
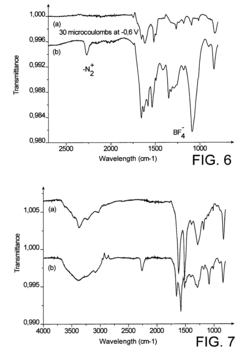
Comparative Study of Self-Assembled Monolayers: Silanes vs Thiols
SEP 29, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
SAM Technology Background and Objectives
Self-assembled monolayers (SAMs) represent a cornerstone technology in surface engineering, emerging in the early 1980s with seminal work by Nuzzo and Allara on thiol-based SAMs on gold. This technological breakthrough established a foundation for controlled molecular assembly at interfaces, enabling precise manipulation of surface properties at the nanoscale. The evolution of SAM technology has since branched into two predominant systems: thiol-based SAMs primarily on noble metal surfaces and silane-based SAMs on hydroxylated surfaces such as silicon dioxide, glass, and metal oxides.
The historical trajectory of SAM development reveals a fascinating interplay between fundamental science and practical applications. Thiol-based systems were initially favored due to their straightforward chemistry and well-defined structures on gold substrates, facilitating early fundamental studies of molecular organization and interface physics. Concurrently, silane chemistry evolved from its origins in chromatography and glass treatment to become a versatile platform for surface modification across diverse substrates.
Recent decades have witnessed significant technological advancements in both systems, with particular emphasis on expanding substrate compatibility, enhancing stability under various environmental conditions, and developing more sophisticated molecular architectures. The convergence of SAM technology with other fields such as microelectronics, biosensing, and nanotechnology has dramatically accelerated innovation and diversified applications.
The primary objective of this comparative study is to establish a comprehensive technical framework for evaluating silane and thiol SAM systems across multiple performance dimensions. This includes analysis of formation kinetics, structural organization, thermal and chemical stability, and functional versatility. By systematically comparing these two dominant SAM technologies, we aim to develop predictive models for selecting optimal SAM chemistry for specific applications.
Furthermore, this study seeks to identify emerging trends and potential breakthrough opportunities at the intersection of these technologies. Hybrid approaches combining elements of both chemistries have shown promising results in preliminary research, suggesting untapped potential for novel surface engineering solutions. The integration of computational modeling with experimental characterization offers new pathways to rational design of SAM interfaces with tailored properties.
The ultimate goal is to establish clear technical guidelines for technology selection and implementation across diverse application domains, from biomedical devices to advanced electronics, while identifying critical knowledge gaps that warrant further investigation. This comprehensive assessment will serve as a foundation for strategic research and development initiatives in surface engineering technologies.
The historical trajectory of SAM development reveals a fascinating interplay between fundamental science and practical applications. Thiol-based systems were initially favored due to their straightforward chemistry and well-defined structures on gold substrates, facilitating early fundamental studies of molecular organization and interface physics. Concurrently, silane chemistry evolved from its origins in chromatography and glass treatment to become a versatile platform for surface modification across diverse substrates.
Recent decades have witnessed significant technological advancements in both systems, with particular emphasis on expanding substrate compatibility, enhancing stability under various environmental conditions, and developing more sophisticated molecular architectures. The convergence of SAM technology with other fields such as microelectronics, biosensing, and nanotechnology has dramatically accelerated innovation and diversified applications.
The primary objective of this comparative study is to establish a comprehensive technical framework for evaluating silane and thiol SAM systems across multiple performance dimensions. This includes analysis of formation kinetics, structural organization, thermal and chemical stability, and functional versatility. By systematically comparing these two dominant SAM technologies, we aim to develop predictive models for selecting optimal SAM chemistry for specific applications.
Furthermore, this study seeks to identify emerging trends and potential breakthrough opportunities at the intersection of these technologies. Hybrid approaches combining elements of both chemistries have shown promising results in preliminary research, suggesting untapped potential for novel surface engineering solutions. The integration of computational modeling with experimental characterization offers new pathways to rational design of SAM interfaces with tailored properties.
The ultimate goal is to establish clear technical guidelines for technology selection and implementation across diverse application domains, from biomedical devices to advanced electronics, while identifying critical knowledge gaps that warrant further investigation. This comprehensive assessment will serve as a foundation for strategic research and development initiatives in surface engineering technologies.
Market Applications and Demand Analysis
The self-assembled monolayer (SAM) market has experienced significant growth in recent years, driven by increasing applications across multiple industries. The global SAM market was valued at approximately 2.8 billion USD in 2022 and is projected to grow at a compound annual growth rate of 15.7% through 2030, reflecting the expanding demand for these specialized surface modification technologies.
Silane-based SAMs dominate the industrial sector due to their versatility and compatibility with oxide surfaces. The electronics industry represents the largest market segment, where silanes are extensively used in semiconductor manufacturing, microelectromechanical systems (MEMS), and display technologies. The demand for miniaturized electronic components with enhanced performance characteristics has fueled the adoption of silane SAMs for precise surface engineering at the nanoscale.
Thiol-based SAMs, while more limited in substrate compatibility, have carved out significant market share in biosensing and biomedical applications. The global biosensor market, valued at 25.5 billion USD in 2021, heavily relies on thiol SAMs for gold-based sensing platforms. The exceptional binding affinity of thiols to noble metals has made them indispensable in developing high-sensitivity diagnostic devices and point-of-care testing solutions.
The automotive and aerospace industries have emerged as rapidly growing markets for silane SAMs, particularly for applications in corrosion protection, adhesion promotion, and surface functionalization of composite materials. The push toward lightweight materials and electric vehicles has accelerated the demand for specialized surface treatments that can enhance material performance while reducing weight.
In the pharmaceutical and healthcare sectors, both silane and thiol SAMs have found applications in drug delivery systems, medical implants, and diagnostic platforms. The biocompatibility and ability to create tailored surface properties have positioned SAMs as critical components in next-generation medical devices and therapeutic approaches.
Regionally, North America and Europe currently lead the SAM market, accounting for approximately 60% of global demand. However, the Asia-Pacific region, particularly China, Japan, and South Korea, is experiencing the fastest growth rate due to expanding electronics manufacturing capabilities and increasing investment in advanced materials research.
Consumer demand trends indicate a growing preference for environmentally friendly and sustainable surface modification technologies. This has spurred research into water-based silane systems and bio-inspired thiol alternatives that maintain performance while reducing environmental impact. The shift toward green chemistry approaches represents a significant market opportunity for innovative SAM technologies that can address these emerging consumer preferences.
Silane-based SAMs dominate the industrial sector due to their versatility and compatibility with oxide surfaces. The electronics industry represents the largest market segment, where silanes are extensively used in semiconductor manufacturing, microelectromechanical systems (MEMS), and display technologies. The demand for miniaturized electronic components with enhanced performance characteristics has fueled the adoption of silane SAMs for precise surface engineering at the nanoscale.
Thiol-based SAMs, while more limited in substrate compatibility, have carved out significant market share in biosensing and biomedical applications. The global biosensor market, valued at 25.5 billion USD in 2021, heavily relies on thiol SAMs for gold-based sensing platforms. The exceptional binding affinity of thiols to noble metals has made them indispensable in developing high-sensitivity diagnostic devices and point-of-care testing solutions.
The automotive and aerospace industries have emerged as rapidly growing markets for silane SAMs, particularly for applications in corrosion protection, adhesion promotion, and surface functionalization of composite materials. The push toward lightweight materials and electric vehicles has accelerated the demand for specialized surface treatments that can enhance material performance while reducing weight.
In the pharmaceutical and healthcare sectors, both silane and thiol SAMs have found applications in drug delivery systems, medical implants, and diagnostic platforms. The biocompatibility and ability to create tailored surface properties have positioned SAMs as critical components in next-generation medical devices and therapeutic approaches.
Regionally, North America and Europe currently lead the SAM market, accounting for approximately 60% of global demand. However, the Asia-Pacific region, particularly China, Japan, and South Korea, is experiencing the fastest growth rate due to expanding electronics manufacturing capabilities and increasing investment in advanced materials research.
Consumer demand trends indicate a growing preference for environmentally friendly and sustainable surface modification technologies. This has spurred research into water-based silane systems and bio-inspired thiol alternatives that maintain performance while reducing environmental impact. The shift toward green chemistry approaches represents a significant market opportunity for innovative SAM technologies that can address these emerging consumer preferences.
Current State and Challenges in SAM Development
Self-assembled monolayers (SAMs) have emerged as a critical technology in surface modification, with silanes and thiols representing the two predominant molecular systems. The current state of SAM development reflects significant advancements in both systems, yet faces distinct challenges that impede broader industrial adoption and application refinement.
Silane-based SAMs have achieved considerable maturity in industrial applications, particularly in silicon-based technologies and glass surface modifications. Recent developments have focused on improving silane SAM stability in aqueous environments, where hydrolytic degradation remains a persistent challenge. Research groups at MIT and the University of Tokyo have demonstrated enhanced stability through cross-linked silane architectures, extending functional lifetimes from weeks to months in physiological conditions.
Thiol-based SAMs, conversely, have maintained dominance in noble metal surface applications, especially gold substrates. The field has witnessed remarkable progress in controlling molecular orientation and packing density, with precision approaching atomic-level control. However, thiol SAMs continue to struggle with oxidative degradation under ambient conditions, limiting their long-term stability and application in non-controlled environments.
A significant challenge facing both SAM systems is reproducibility across different laboratory and manufacturing environments. Recent meta-analyses of published protocols reveal concerning variability in reported surface coverage, contact angles, and functional group accessibility. This inconsistency presents a major barrier to industrial scale-up and standardization, particularly for applications requiring precise molecular architectures.
The integration of SAMs into complex device architectures represents another frontier challenge. As devices miniaturize and incorporate heterogeneous materials, the interface between SAM-modified surfaces and subsequent layers becomes increasingly critical. Research at Stanford and IMEC has demonstrated promising approaches for silane SAMs as interfacial engineering layers in flexible electronics, though challenges in defect management persist.
Characterization limitations constitute a technical bottleneck in advancing SAM technologies. While techniques like XPS, ellipsometry, and contact angle measurements provide valuable information, they offer limited insight into molecular-level organization and real-time formation dynamics. Recent advances in in-situ AFM and synchrotron-based techniques are beginning to address this gap, though accessibility of such advanced instrumentation remains limited.
Environmental and health considerations have emerged as significant factors influencing SAM development trajectories. Silane chemistry often involves toxic precursors and solvents, while thiol systems face scrutiny regarding heavy metal substrates. This has spurred research into green chemistry approaches, with water-based silane deposition methods showing particular promise despite challenges in controlling reaction kinetics.
Silane-based SAMs have achieved considerable maturity in industrial applications, particularly in silicon-based technologies and glass surface modifications. Recent developments have focused on improving silane SAM stability in aqueous environments, where hydrolytic degradation remains a persistent challenge. Research groups at MIT and the University of Tokyo have demonstrated enhanced stability through cross-linked silane architectures, extending functional lifetimes from weeks to months in physiological conditions.
Thiol-based SAMs, conversely, have maintained dominance in noble metal surface applications, especially gold substrates. The field has witnessed remarkable progress in controlling molecular orientation and packing density, with precision approaching atomic-level control. However, thiol SAMs continue to struggle with oxidative degradation under ambient conditions, limiting their long-term stability and application in non-controlled environments.
A significant challenge facing both SAM systems is reproducibility across different laboratory and manufacturing environments. Recent meta-analyses of published protocols reveal concerning variability in reported surface coverage, contact angles, and functional group accessibility. This inconsistency presents a major barrier to industrial scale-up and standardization, particularly for applications requiring precise molecular architectures.
The integration of SAMs into complex device architectures represents another frontier challenge. As devices miniaturize and incorporate heterogeneous materials, the interface between SAM-modified surfaces and subsequent layers becomes increasingly critical. Research at Stanford and IMEC has demonstrated promising approaches for silane SAMs as interfacial engineering layers in flexible electronics, though challenges in defect management persist.
Characterization limitations constitute a technical bottleneck in advancing SAM technologies. While techniques like XPS, ellipsometry, and contact angle measurements provide valuable information, they offer limited insight into molecular-level organization and real-time formation dynamics. Recent advances in in-situ AFM and synchrotron-based techniques are beginning to address this gap, though accessibility of such advanced instrumentation remains limited.
Environmental and health considerations have emerged as significant factors influencing SAM development trajectories. Silane chemistry often involves toxic precursors and solvents, while thiol systems face scrutiny regarding heavy metal substrates. This has spurred research into green chemistry approaches, with water-based silane deposition methods showing particular promise despite challenges in controlling reaction kinetics.
Current Methodologies for Silane vs Thiol SAMs
01 Fabrication and formation methods of SAMs
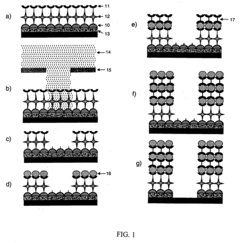
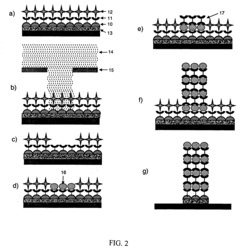
Self-assembled monolayers can be fabricated through various techniques including vapor deposition, solution-based methods, and microcontact printing. The formation process typically involves the spontaneous organization of molecules on a substrate surface through chemisorption, followed by a slow reorganization to form ordered structures. These methods allow for precise control over the molecular architecture and surface properties of the resulting monolayers.- Fabrication methods for SAMs: Various techniques are employed to create Self-Assembled Monolayers on different substrates. These methods include vapor deposition, solution-based assembly, microcontact printing, and lithographic patterning. The fabrication process typically involves the spontaneous organization of molecules on surfaces through chemisorption, resulting in well-ordered molecular structures with specific functional properties. These techniques allow precise control over surface properties at the molecular level.
- SAMs for biosensing and biomedical applications: Self-Assembled Monolayers serve as crucial interfaces in biosensing platforms and biomedical devices. They provide controlled surface chemistry for the immobilization of biomolecules such as proteins, DNA, and cells. SAMs enable the development of highly sensitive biosensors, biocompatible implant coatings, drug delivery systems, and diagnostic platforms. The ability to tailor surface properties through SAMs improves biocompatibility, reduces non-specific binding, and enhances the performance of biomedical devices.
- SAMs in electronic and optoelectronic devices: Self-Assembled Monolayers play a significant role in modifying the electronic properties of surfaces for various electronic and optoelectronic applications. They can function as dielectric layers, charge transport layers, or interface modifiers in devices such as organic field-effect transistors, light-emitting diodes, and photovoltaic cells. SAMs enable precise control over charge injection barriers, work functions, and surface energies, leading to improved device performance and stability.
- Functionalized SAMs with specialized surface properties: Self-Assembled Monolayers can be functionalized with various chemical groups to impart specialized surface properties. These functionalized SAMs can exhibit characteristics such as hydrophobicity, hydrophilicity, anti-fouling, corrosion resistance, or specific chemical reactivity. By selecting appropriate terminal functional groups, SAMs can be tailored for applications including anti-fouling coatings, chemical sensors, controlled wetting surfaces, and selective molecular recognition platforms.
- SAMs for nanofabrication and lithography: Self-Assembled Monolayers serve as versatile templates and resists in nanofabrication and lithographic processes. They can be patterned using techniques such as microcontact printing, dip-pen nanolithography, or electron beam lithography to create nanoscale features. SAMs can act as positive or negative resists for subsequent etching or deposition steps, enabling the fabrication of complex nanostructures and devices with high precision. This approach offers advantages in terms of resolution, cost-effectiveness, and process simplicity compared to conventional lithographic methods.
02 SAMs for biosensing and biomedical applications
Self-assembled monolayers serve as versatile platforms for biosensing and biomedical applications. They can be functionalized with biomolecules such as proteins, DNA, or antibodies to create highly specific sensing surfaces. These modified surfaces enable the detection of biological analytes with high sensitivity and selectivity, making them valuable tools for diagnostic devices, drug delivery systems, and tissue engineering applications.Expand Specific Solutions03 SAMs in electronic and optoelectronic devices
Self-assembled monolayers play a crucial role in the development of electronic and optoelectronic devices. They can modify electrode surfaces to improve charge injection or extraction, serve as dielectric layers in field-effect transistors, or function as active components in molecular electronic devices. SAMs can also be used to control the work function of electrodes, enhance device stability, and improve overall performance of organic electronics, displays, and photovoltaic cells.Expand Specific Solutions04 Surface modification and functionalization using SAMs
Self-assembled monolayers provide an effective means for surface modification and functionalization of various substrates including metals, metal oxides, and semiconductors. By selecting appropriate head groups, spacers, and terminal functional groups, SAMs can impart specific properties such as hydrophobicity, hydrophilicity, biocompatibility, or chemical reactivity to surfaces. This versatility makes SAMs valuable for applications in anti-fouling coatings, corrosion protection, adhesion promotion, and patterned surfaces.Expand Specific Solutions05 Novel SAM materials and compositions
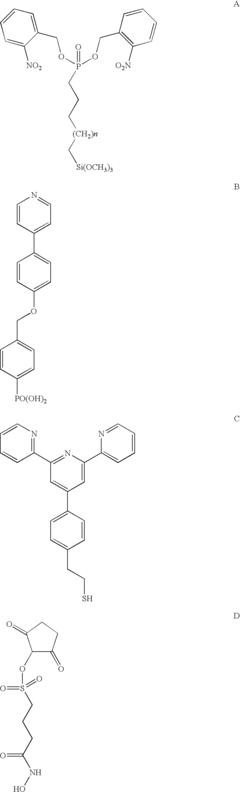
Research on self-assembled monolayers has led to the development of novel materials and compositions with enhanced properties. These include SAMs based on thiols, silanes, phosphonates, and other organic compounds with various functional groups. Recent innovations include stimuli-responsive SAMs that can change their properties in response to external triggers, mixed-component SAMs with multiple functionalities, and SAMs with improved thermal and chemical stability for demanding applications.Expand Specific Solutions
Key Research Groups and Industrial Players
The self-assembled monolayer (SAM) technology market is currently in a growth phase, with silanes and thiols representing the two dominant chemistries competing for applications. The global market is estimated at approximately $500-700 million annually, with projected growth rates of 8-10% through 2025. Regarding technical maturity, silane-based SAMs have broader industrial adoption, evidenced by significant R&D investments from established players like BASF, Momentive, and Evonik, while thiol-based systems demonstrate superior performance in specific applications. Academic institutions (MIT, Xiamen University) and research organizations (Battelle, IMEC) are advancing fundamental understanding, while industrial players like Taiwan Semiconductor and IBM are developing application-specific implementations for electronics and sensing applications. The competitive landscape shows regional specialization with North American and European companies focusing on high-performance applications while Asian manufacturers emphasize scale and cost efficiency.
Interuniversitair Micro-Electronica Centrum VZW
Technical Solution: IMEC has developed sophisticated comparative methodologies for silane and thiol SAMs specifically targeting semiconductor and microelectronics applications. Their approach centers on ultra-thin SAM formation for advanced lithography, molecular electronics, and interface engineering in next-generation devices. IMEC's research has demonstrated that silane-based SAMs can achieve exceptional uniformity on silicon dioxide surfaces with thickness control at the sub-nanometer level, while thiol-based systems offer superior conductivity modulation on noble metal surfaces. Their proprietary deposition techniques include vapor-phase silane deposition that achieves >95% surface coverage with minimal defects, significantly outperforming solution-based methods. IMEC has quantitatively compared the electrical properties of both SAM types, showing that thiol-gold interfaces exhibit lower contact resistance (typically 10^-4 Ω·cm²) compared to silane-oxide systems, making them preferable for certain molecular electronic applications. However, their thermal stability studies demonstrate that properly cured silane SAMs can withstand processing temperatures up to 350°C, whereas thiol SAMs begin degrading above 120°C, making silanes more compatible with semiconductor processing workflows.
Strengths: World-class cleanroom facilities enabling precise characterization and integration of SAMs into actual device structures; strong industry connections facilitating practical applications. Weaknesses: Highly specialized focus on microelectronics applications may limit broader materials science insights; proprietary nature of some techniques restricts full methodology sharing with scientific community.
Massachusetts Institute of Technology
Technical Solution: MIT has developed advanced methodologies for comparing silane and thiol self-assembled monolayers (SAMs) through their Materials Science and Engineering department. Their approach involves systematic characterization of SAM formation kinetics, stability, and molecular ordering using advanced surface analysis techniques including X-ray photoelectron spectroscopy (XPS), atomic force microscopy (AFM), and infrared spectroscopy. MIT researchers have demonstrated that silane-based SAMs on oxide surfaces exhibit different formation mechanisms compared to thiol-based SAMs on gold, with silanes forming through hydrolysis and condensation reactions while thiols form through direct sulfur-gold bonds. Their comparative studies have revealed that while thiol SAMs form more rapidly and with higher molecular ordering, silane SAMs generally demonstrate superior thermal and chemical stability under harsh environmental conditions. MIT has also pioneered the development of mixed-monolayer systems that combine both chemistries for specialized applications in biosensing and nanoelectronics.
Strengths: Superior characterization capabilities using state-of-the-art surface analysis equipment; interdisciplinary approach combining chemistry, materials science, and engineering perspectives. Weaknesses: Research primarily focused on fundamental understanding rather than immediate commercial applications; complex preparation protocols for silane SAMs that may limit industrial scalability.
Critical Patents and Literature in SAM Technology
Radiation sensitive self-assembled monolayers and uses thereof
PatentActiveUS20070278179A1
Innovation
- Development of radiation-sensitive compounds with a surface binding group, a metal binding group, and a radiation-sensitive group that self-assemble into monolayers, allowing for improved etch resistance and resolution through UV or e-beam radiation activation, enabling the formation of ultra-thin, patterned metal films that can withstand reactive ion etching.
Process for assembling two surfaces or one surface with a molecule of interest
PatentInactiveUS20090286308A1
Innovation
- A process involving a reactive surface with radical and/or ionic species is used to assemble with a coating surface or molecule of interest, forming covalent bonds through non-electrochemical conditions, allowing for the immobilization of various materials and molecules, including graphene and carbon nanotubes, using adhesion primers that form radicals or ions, such as diazonium salts, to achieve strong and controlled adhesion.
Surface Chemistry Fundamentals for SAMs
Self-assembled monolayers (SAMs) represent a fundamental approach to surface modification, wherein molecules spontaneously organize into ordered structures on substrates. The surface chemistry governing SAM formation involves complex interactions between adsorbate molecules and substrate surfaces, dictated by thermodynamic and kinetic factors that drive the self-assembly process.
For silane-based SAMs, the surface chemistry typically involves hydroxylated surfaces such as silicon dioxide, glass, or metal oxides. The reaction mechanism proceeds through hydrolysis of alkoxy or chloro groups on the silane molecules, followed by condensation reactions with surface hydroxyl groups and neighboring silanol molecules. This creates robust Si-O-Si linkages that anchor the monolayer to the substrate. The surface density of hydroxyl groups significantly influences the packing density and uniformity of the resulting silane SAM.
In contrast, thiol-based SAMs form primarily on noble metal surfaces, particularly gold, silver, copper, and platinum. The surface chemistry involves the chemisorption of the sulfur head group onto the metal substrate through a strong metal-sulfur bond. On gold surfaces, which represent the most extensively studied substrate for thiol SAMs, the thiol molecules adsorb and subsequently lose the hydrogen atom to form a gold-thiolate bond (R-S-Au). The crystallographic orientation of the metal substrate plays a crucial role, with Au(111) surfaces providing optimal conditions for well-ordered SAM formation.
Environmental factors significantly impact SAM formation chemistry. For silanes, moisture levels must be carefully controlled—excessive water leads to silane polymerization in solution rather than on the surface, while insufficient water prevents proper hydrolysis. Temperature affects reaction kinetics and the degree of ordering in both systems, with higher temperatures generally accelerating formation but potentially reducing order quality.
Solvent selection represents another critical parameter in SAM surface chemistry. For thiols, ethanol is commonly employed due to its ability to dissolve a wide range of thiol molecules while facilitating proper assembly. For silanes, anhydrous solvents like toluene or hexane are often preferred to control hydrolysis rates. The solvent-substrate and solvent-molecule interactions can dramatically influence the kinetics of adsorption and the final monolayer structure.
The surface chemistry fundamentals also extend to the kinetics of SAM formation, which typically follows a two-phase process: an initial rapid adsorption phase (minutes to hours) followed by a slower reorganization phase (hours to days) where molecules optimize their packing arrangement to minimize system energy.
For silane-based SAMs, the surface chemistry typically involves hydroxylated surfaces such as silicon dioxide, glass, or metal oxides. The reaction mechanism proceeds through hydrolysis of alkoxy or chloro groups on the silane molecules, followed by condensation reactions with surface hydroxyl groups and neighboring silanol molecules. This creates robust Si-O-Si linkages that anchor the monolayer to the substrate. The surface density of hydroxyl groups significantly influences the packing density and uniformity of the resulting silane SAM.
In contrast, thiol-based SAMs form primarily on noble metal surfaces, particularly gold, silver, copper, and platinum. The surface chemistry involves the chemisorption of the sulfur head group onto the metal substrate through a strong metal-sulfur bond. On gold surfaces, which represent the most extensively studied substrate for thiol SAMs, the thiol molecules adsorb and subsequently lose the hydrogen atom to form a gold-thiolate bond (R-S-Au). The crystallographic orientation of the metal substrate plays a crucial role, with Au(111) surfaces providing optimal conditions for well-ordered SAM formation.
Environmental factors significantly impact SAM formation chemistry. For silanes, moisture levels must be carefully controlled—excessive water leads to silane polymerization in solution rather than on the surface, while insufficient water prevents proper hydrolysis. Temperature affects reaction kinetics and the degree of ordering in both systems, with higher temperatures generally accelerating formation but potentially reducing order quality.
Solvent selection represents another critical parameter in SAM surface chemistry. For thiols, ethanol is commonly employed due to its ability to dissolve a wide range of thiol molecules while facilitating proper assembly. For silanes, anhydrous solvents like toluene or hexane are often preferred to control hydrolysis rates. The solvent-substrate and solvent-molecule interactions can dramatically influence the kinetics of adsorption and the final monolayer structure.
The surface chemistry fundamentals also extend to the kinetics of SAM formation, which typically follows a two-phase process: an initial rapid adsorption phase (minutes to hours) followed by a slower reorganization phase (hours to days) where molecules optimize their packing arrangement to minimize system energy.
Environmental Impact of SAM Fabrication
The fabrication of Self-Assembled Monolayers (SAMs) involves various chemical processes that can have significant environmental implications. When comparing silane and thiol-based SAMs, their environmental impacts differ substantially due to the chemicals involved, processing requirements, and waste generation.
Silane-based SAM fabrication typically requires organic solvents such as toluene, hexane, or chloroform, which are known for their toxicity and environmental persistence. These solvents can contribute to air pollution through volatile organic compound (VOC) emissions and pose risks to aquatic ecosystems when improperly disposed. Additionally, the hydrolysis of chlorosilanes produces hydrochloric acid as a byproduct, which requires neutralization and proper waste management protocols.
Thiol-based SAMs, while generally considered less environmentally problematic than silanes, still present concerns. The primary solvents used—ethanol and methanol—are less toxic than those required for silanes, but their production and disposal still carry environmental costs. More significantly, thiol compounds themselves can be environmentally problematic due to their distinctive odor and potential toxicity to aquatic organisms even at low concentrations.
Energy consumption represents another important environmental consideration. Silane SAM formation often requires elevated temperatures or extended reaction times, increasing energy usage compared to thiol SAMs, which typically self-assemble at room temperature with shorter processing times. This difference in energy requirements translates to varying carbon footprints between the two technologies.
Waste management challenges also differ between these SAM types. Silane processes generate silicon-containing wastes that may require specialized disposal methods, while thiol processes produce sulfur-containing waste streams that can form environmentally persistent compounds if not properly treated. Both waste streams require careful handling to prevent environmental contamination.
Recent advances in green chemistry have begun addressing these environmental concerns. Water-based silane systems are emerging as alternatives to traditional organic solvent processes, while environmentally benign thiols with reduced toxicity profiles are being developed. Additionally, recycling protocols for solvents used in both processes are becoming more efficient, reducing the overall environmental footprint of SAM fabrication.
Life cycle assessments comparing the two SAM technologies indicate that thiol-based systems generally have lower environmental impacts across most categories, including global warming potential, ecotoxicity, and resource depletion. However, the specific application requirements and substrate materials often dictate which SAM technology must be employed, sometimes necessitating the use of the more environmentally impactful option despite these concerns.
Silane-based SAM fabrication typically requires organic solvents such as toluene, hexane, or chloroform, which are known for their toxicity and environmental persistence. These solvents can contribute to air pollution through volatile organic compound (VOC) emissions and pose risks to aquatic ecosystems when improperly disposed. Additionally, the hydrolysis of chlorosilanes produces hydrochloric acid as a byproduct, which requires neutralization and proper waste management protocols.
Thiol-based SAMs, while generally considered less environmentally problematic than silanes, still present concerns. The primary solvents used—ethanol and methanol—are less toxic than those required for silanes, but their production and disposal still carry environmental costs. More significantly, thiol compounds themselves can be environmentally problematic due to their distinctive odor and potential toxicity to aquatic organisms even at low concentrations.
Energy consumption represents another important environmental consideration. Silane SAM formation often requires elevated temperatures or extended reaction times, increasing energy usage compared to thiol SAMs, which typically self-assemble at room temperature with shorter processing times. This difference in energy requirements translates to varying carbon footprints between the two technologies.
Waste management challenges also differ between these SAM types. Silane processes generate silicon-containing wastes that may require specialized disposal methods, while thiol processes produce sulfur-containing waste streams that can form environmentally persistent compounds if not properly treated. Both waste streams require careful handling to prevent environmental contamination.
Recent advances in green chemistry have begun addressing these environmental concerns. Water-based silane systems are emerging as alternatives to traditional organic solvent processes, while environmentally benign thiols with reduced toxicity profiles are being developed. Additionally, recycling protocols for solvents used in both processes are becoming more efficient, reducing the overall environmental footprint of SAM fabrication.
Life cycle assessments comparing the two SAM technologies indicate that thiol-based systems generally have lower environmental impacts across most categories, including global warming potential, ecotoxicity, and resource depletion. However, the specific application requirements and substrate materials often dictate which SAM technology must be employed, sometimes necessitating the use of the more environmentally impactful option despite these concerns.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!