Self-Assembled Monolayers: Enhancements in Catalytic Efficiency
SEP 29, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
SAM Technology Background and Objectives
Self-assembled monolayers (SAMs) represent a remarkable technological advancement in surface chemistry, emerging in the early 1980s through pioneering work by Nuzzo and Allara who demonstrated the spontaneous organization of disulfides on gold surfaces. This discovery marked the beginning of a revolutionary approach to molecular engineering at interfaces, enabling precise control over surface properties at the molecular level.
The evolution of SAM technology has progressed through several distinct phases. Initially focused on fundamental understanding of formation mechanisms and structural characteristics, research subsequently expanded to explore diverse molecular systems beyond the classic alkanethiol-gold paradigm. Recent decades have witnessed the integration of SAMs into increasingly complex technological applications, particularly in catalysis where they serve as versatile platforms for anchoring and optimizing catalytic species.
Current technological trends in SAM research emphasize multifunctionality, stimuli-responsiveness, and integration with other advanced materials such as nanoparticles and two-dimensional materials. The field has moved beyond static monolayers toward dynamic, adaptive systems capable of responding to environmental changes or external stimuli, opening new possibilities for smart catalytic systems.
In the specific context of catalytic efficiency enhancement, SAMs offer unique advantages through their ability to create well-defined molecular environments that can influence reaction pathways, stabilize transition states, and facilitate electron transfer processes. The molecular precision afforded by SAMs enables rational design of catalytic interfaces with optimized spatial arrangement of active sites, controlled hydrophobicity/hydrophilicity, and tailored electronic properties.
The primary objectives of current SAM technology development for catalytic applications include: increasing the stability of monolayers under harsh reaction conditions; developing methods for creating gradient or patterned SAMs with spatially varying catalytic properties; improving the integration of SAMs with diverse substrate materials beyond traditional noble metals; and establishing quantitative structure-property relationships that enable predictive design of catalytic SAM systems.
Long-term technological goals involve the creation of biomimetic catalytic systems that emulate the efficiency and selectivity of enzymes, the development of self-healing SAM-based catalysts capable of maintaining performance over extended operational periods, and the integration of SAMs into complex hierarchical structures that combine multiple catalytic functions in cascade reaction systems.
The evolution of SAM technology has progressed through several distinct phases. Initially focused on fundamental understanding of formation mechanisms and structural characteristics, research subsequently expanded to explore diverse molecular systems beyond the classic alkanethiol-gold paradigm. Recent decades have witnessed the integration of SAMs into increasingly complex technological applications, particularly in catalysis where they serve as versatile platforms for anchoring and optimizing catalytic species.
Current technological trends in SAM research emphasize multifunctionality, stimuli-responsiveness, and integration with other advanced materials such as nanoparticles and two-dimensional materials. The field has moved beyond static monolayers toward dynamic, adaptive systems capable of responding to environmental changes or external stimuli, opening new possibilities for smart catalytic systems.
In the specific context of catalytic efficiency enhancement, SAMs offer unique advantages through their ability to create well-defined molecular environments that can influence reaction pathways, stabilize transition states, and facilitate electron transfer processes. The molecular precision afforded by SAMs enables rational design of catalytic interfaces with optimized spatial arrangement of active sites, controlled hydrophobicity/hydrophilicity, and tailored electronic properties.
The primary objectives of current SAM technology development for catalytic applications include: increasing the stability of monolayers under harsh reaction conditions; developing methods for creating gradient or patterned SAMs with spatially varying catalytic properties; improving the integration of SAMs with diverse substrate materials beyond traditional noble metals; and establishing quantitative structure-property relationships that enable predictive design of catalytic SAM systems.
Long-term technological goals involve the creation of biomimetic catalytic systems that emulate the efficiency and selectivity of enzymes, the development of self-healing SAM-based catalysts capable of maintaining performance over extended operational periods, and the integration of SAMs into complex hierarchical structures that combine multiple catalytic functions in cascade reaction systems.
Market Analysis for SAM-Enhanced Catalysis
The global market for catalysis technologies is experiencing significant growth, with the Self-Assembled Monolayers (SAMs) enhanced catalysis segment emerging as a particularly promising area. Current market valuations place the overall catalysis market at approximately 24.6 billion USD in 2023, with projections indicating growth to reach 35.5 billion USD by 2028, representing a compound annual growth rate of 7.6%.
SAM-enhanced catalysis technologies are positioned at the intersection of several high-growth industrial sectors. The petroleum refining industry remains the largest consumer of catalytic technologies, accounting for roughly 40% of the global catalyst market. However, the fine chemicals and pharmaceutical sectors are showing the most rapid adoption rates for SAM-enhanced catalysis, driven by increasing demands for higher selectivity and efficiency in complex synthesis pathways.
Environmental regulations worldwide are creating substantial market pull for greener catalytic processes. The European Union's Green Deal and similar initiatives in North America and Asia have established regulatory frameworks that favor catalytic technologies with reduced environmental footprints. SAM-enhanced catalysts, with their potential for lower energy requirements and reduced waste generation, are particularly well-positioned to capitalize on these regulatory trends.
Regional market analysis reveals that North America and Europe currently dominate the advanced catalysis market, collectively accounting for approximately 58% of global market share. However, the Asia-Pacific region, particularly China and India, is demonstrating the fastest growth rate at 9.2% annually, driven by rapid industrialization and increasing environmental concerns.
Customer segmentation within the SAM-enhanced catalysis market shows distinct needs across different industries. The pharmaceutical sector prioritizes high selectivity and purity, while the petrochemical industry emphasizes durability and scalability. The fine chemicals segment seeks a balance of these attributes, with particular emphasis on customizability for specific reaction conditions.
Price sensitivity analysis indicates that while initial adoption costs for SAM-enhanced catalytic systems may be higher than conventional alternatives, the total cost of ownership often favors SAM technologies when accounting for improved yields, reduced waste treatment costs, and extended catalyst lifetimes. This economic advantage is particularly pronounced in high-value product manufacturing where even marginal improvements in selectivity translate to significant financial returns.
Market forecasts suggest that SAM-enhanced catalysis will experience accelerated adoption over the next five years, with the most substantial growth occurring in biocatalysis applications and renewable energy production pathways, where precise control of surface chemistry offers compelling advantages over traditional catalytic approaches.
SAM-enhanced catalysis technologies are positioned at the intersection of several high-growth industrial sectors. The petroleum refining industry remains the largest consumer of catalytic technologies, accounting for roughly 40% of the global catalyst market. However, the fine chemicals and pharmaceutical sectors are showing the most rapid adoption rates for SAM-enhanced catalysis, driven by increasing demands for higher selectivity and efficiency in complex synthesis pathways.
Environmental regulations worldwide are creating substantial market pull for greener catalytic processes. The European Union's Green Deal and similar initiatives in North America and Asia have established regulatory frameworks that favor catalytic technologies with reduced environmental footprints. SAM-enhanced catalysts, with their potential for lower energy requirements and reduced waste generation, are particularly well-positioned to capitalize on these regulatory trends.
Regional market analysis reveals that North America and Europe currently dominate the advanced catalysis market, collectively accounting for approximately 58% of global market share. However, the Asia-Pacific region, particularly China and India, is demonstrating the fastest growth rate at 9.2% annually, driven by rapid industrialization and increasing environmental concerns.
Customer segmentation within the SAM-enhanced catalysis market shows distinct needs across different industries. The pharmaceutical sector prioritizes high selectivity and purity, while the petrochemical industry emphasizes durability and scalability. The fine chemicals segment seeks a balance of these attributes, with particular emphasis on customizability for specific reaction conditions.
Price sensitivity analysis indicates that while initial adoption costs for SAM-enhanced catalytic systems may be higher than conventional alternatives, the total cost of ownership often favors SAM technologies when accounting for improved yields, reduced waste treatment costs, and extended catalyst lifetimes. This economic advantage is particularly pronounced in high-value product manufacturing where even marginal improvements in selectivity translate to significant financial returns.
Market forecasts suggest that SAM-enhanced catalysis will experience accelerated adoption over the next five years, with the most substantial growth occurring in biocatalysis applications and renewable energy production pathways, where precise control of surface chemistry offers compelling advantages over traditional catalytic approaches.
Current SAM Technology Challenges
Despite significant advancements in Self-Assembled Monolayer (SAM) technology for catalytic applications, several critical challenges continue to impede their widespread industrial implementation. One fundamental limitation is the inherent stability issue of SAMs under harsh catalytic conditions. When exposed to elevated temperatures, extreme pH environments, or aggressive chemical species, SAM structures often undergo degradation or desorption from substrate surfaces, significantly reducing their operational lifespan and catalytic efficiency.
Reproducibility and scalability represent another major hurdle in SAM technology development. Laboratory-scale synthesis often achieves high-quality monolayers with excellent catalytic properties, but translating these results to industrial-scale production remains problematic. Variations in substrate quality, environmental conditions during assembly, and inconsistent precursor purity contribute to batch-to-batch variability that undermines commercial viability.
The interface between SAMs and catalytic species presents unique challenges that require innovative solutions. Current methods for incorporating catalytic centers into SAM structures frequently result in suboptimal spatial distribution and accessibility. This limitation restricts substrate diffusion to active sites and hampers overall catalytic turnover rates, particularly for bulky reactants or in complex reaction systems.
Characterization techniques for SAM-based catalysts also face significant limitations. While techniques such as X-ray photoelectron spectroscopy (XPS) and atomic force microscopy (AFM) provide valuable insights, they often fail to capture the dynamic behavior of SAMs during actual catalytic processes. This knowledge gap hinders rational design approaches and optimization strategies for enhanced catalytic performance.
The multidisciplinary nature of SAM technology creates integration challenges across different scientific domains. Effective development requires expertise spanning surface chemistry, materials science, catalysis, and engineering—a combination rarely found within single research groups or companies. This fragmentation of knowledge impedes holistic approaches to solving complex technical problems in SAM-based catalytic systems.
Economic considerations further complicate commercial adoption of SAM technologies. The cost-performance ratio of many SAM-based catalytic systems currently cannot compete with traditional heterogeneous catalysts, particularly when accounting for stability limitations and production complexities. This economic barrier represents a significant obstacle to market penetration, despite the potential performance advantages of SAM-based systems.
Reproducibility and scalability represent another major hurdle in SAM technology development. Laboratory-scale synthesis often achieves high-quality monolayers with excellent catalytic properties, but translating these results to industrial-scale production remains problematic. Variations in substrate quality, environmental conditions during assembly, and inconsistent precursor purity contribute to batch-to-batch variability that undermines commercial viability.
The interface between SAMs and catalytic species presents unique challenges that require innovative solutions. Current methods for incorporating catalytic centers into SAM structures frequently result in suboptimal spatial distribution and accessibility. This limitation restricts substrate diffusion to active sites and hampers overall catalytic turnover rates, particularly for bulky reactants or in complex reaction systems.
Characterization techniques for SAM-based catalysts also face significant limitations. While techniques such as X-ray photoelectron spectroscopy (XPS) and atomic force microscopy (AFM) provide valuable insights, they often fail to capture the dynamic behavior of SAMs during actual catalytic processes. This knowledge gap hinders rational design approaches and optimization strategies for enhanced catalytic performance.
The multidisciplinary nature of SAM technology creates integration challenges across different scientific domains. Effective development requires expertise spanning surface chemistry, materials science, catalysis, and engineering—a combination rarely found within single research groups or companies. This fragmentation of knowledge impedes holistic approaches to solving complex technical problems in SAM-based catalytic systems.
Economic considerations further complicate commercial adoption of SAM technologies. The cost-performance ratio of many SAM-based catalytic systems currently cannot compete with traditional heterogeneous catalysts, particularly when accounting for stability limitations and production complexities. This economic barrier represents a significant obstacle to market penetration, despite the potential performance advantages of SAM-based systems.
Current SAM-Based Catalytic Solutions
01 Metal nanoparticle-functionalized SAMs for enhanced catalysis
Self-assembled monolayers (SAMs) can be functionalized with metal nanoparticles to create highly efficient catalytic surfaces. These systems leverage the ordered structure of SAMs to precisely control the distribution and orientation of catalytic nanoparticles, enhancing their accessibility to reactants. The metal nanoparticles, typically gold, silver, or platinum, provide active sites for catalytic reactions while the SAM structure offers stability and prevents aggregation, resulting in improved catalytic efficiency and selectivity for various chemical transformations.- Metal-based SAMs for enhanced catalytic efficiency: Self-assembled monolayers incorporating metal nanoparticles or metal complexes can significantly enhance catalytic efficiency. These metal-based SAMs provide active sites for catalytic reactions while maintaining the structural advantages of self-assembled systems. The ordered arrangement of metal catalysts on surfaces allows for improved reaction kinetics and selectivity. These systems can be tailored for specific catalytic applications by controlling the metal type, density, and surrounding chemical environment.
- Functionalized SAMs for specific catalytic applications: Self-assembled monolayers can be functionalized with specific chemical groups to target particular catalytic reactions. By incorporating reactive functional groups at the terminal positions of the SAM molecules, catalytic activity can be precisely engineered. These functionalized SAMs can be designed to mimic enzyme active sites, provide selective binding of substrates, or facilitate electron transfer in redox reactions. The controlled spacing and orientation of functional groups in SAMs enable optimization of catalytic efficiency for various chemical transformations.
- SAM structure optimization for catalytic performance: The structural characteristics of self-assembled monolayers significantly impact their catalytic efficiency. Parameters such as packing density, chain length, tilt angle, and domain size can be optimized to enhance catalytic performance. Well-ordered SAMs with appropriate spacing between active sites can prevent catalyst poisoning and improve substrate accessibility. Controlling the SAM formation process through temperature, concentration, and deposition time allows for fine-tuning of these structural features to maximize catalytic efficiency.
- SAMs as supports for biomimetic catalysts: Self-assembled monolayers can serve as excellent platforms for biomimetic catalysts that mimic natural enzymatic processes. By incorporating biomolecules or synthetic analogs of enzyme active sites into SAMs, highly efficient and selective catalytic systems can be created. These biomimetic SAMs provide controlled microenvironments similar to those found in natural enzymes, facilitating specific substrate binding and catalytic conversion. The organized structure of SAMs allows for precise positioning of catalytic centers and cofactors, enhancing the efficiency of biomimetic catalytic processes.
- Interface engineering of SAMs for improved catalysis: Engineering the interfaces between self-assembled monolayers and their substrates or surrounding environment can significantly enhance catalytic efficiency. Controlling the electronic properties at these interfaces facilitates electron transfer processes crucial for many catalytic reactions. Hybrid systems combining SAMs with other materials such as graphene, metal oxides, or polymers can create synergistic effects that boost catalytic performance. Interface engineering techniques include surface modification, incorporation of coupling agents, and creation of multilayered structures to optimize the catalytic environment.
02 Enzyme immobilization on SAMs for biocatalysis
Self-assembled monolayers provide an excellent platform for enzyme immobilization, creating stable and efficient biocatalytic systems. By carefully selecting the terminal functional groups of the SAM, enzymes can be attached through various mechanisms including covalent bonding, electrostatic interactions, or affinity binding. This controlled immobilization preserves enzyme structure and function while providing a well-defined microenvironment that can enhance catalytic activity, stability, and reusability compared to enzymes in solution. These biocatalytic SAM systems enable more efficient and selective transformations for pharmaceutical, food, and chemical manufacturing applications.Expand Specific Solutions03 SAMs with tailored functional groups for heterogeneous catalysis
Self-assembled monolayers can be designed with specific functional groups that act as catalytic centers for various chemical reactions. By precisely controlling the chemical composition, density, and orientation of these functional groups on the surface, highly efficient heterogeneous catalysts can be created. These functionalized SAMs can catalyze reactions such as oxidation, reduction, and condensation with high selectivity. The well-defined structure of SAMs allows for systematic modification of the catalytic environment, enabling optimization of reaction parameters and mechanistic studies that contribute to the development of more efficient catalytic systems.Expand Specific Solutions04 SAM-modified electrodes for electrocatalysis
Self-assembled monolayers can be used to modify electrode surfaces to enhance electrocatalytic performance. By forming well-ordered molecular layers on conductive substrates, SAMs can control electron transfer processes, provide selective binding sites for reactants, and stabilize catalytic intermediates. These modified electrodes show improved catalytic efficiency for important electrochemical reactions including oxygen reduction, hydrogen evolution, and CO2 reduction. The ability to precisely engineer the electrode-electrolyte interface through SAM modification enables the development of more efficient electrocatalysts for energy conversion and storage applications.Expand Specific Solutions05 Patterned SAMs for spatially controlled catalysis
Patterning techniques can be used to create spatially defined regions of self-assembled monolayers with different catalytic properties on a single substrate. These patterned SAM surfaces enable the integration of multiple catalytic functions in precise spatial arrangements, allowing for sequential catalytic transformations or localized reactions. Methods such as microcontact printing, photolithography, and scanning probe techniques can create complex patterns of catalytic SAMs with micro- to nanometer resolution. This spatial control over catalytic activity enables the development of miniaturized reaction systems, lab-on-a-chip devices, and cascade reaction platforms with enhanced efficiency and selectivity.Expand Specific Solutions
Key Industry Players in SAM Research
The self-assembled monolayers (SAMs) market for catalytic efficiency enhancements is currently in a growth phase, with increasing applications across chemical manufacturing, energy conversion, and environmental remediation sectors. The global market size is estimated to reach $2.5 billion by 2027, driven by demand for more efficient and sustainable catalytic processes. Technology maturity varies significantly among key players, with research institutions like MIT, Northwestern University, and Tsinghua University focusing on fundamental innovations, while industrial leaders including IBM, 3M, and TSMC are developing commercial applications. Battelle Memorial Institute and Merck Patent GmbH have established strong patent portfolios in SAM-enhanced catalysis, while semiconductor companies like GLOBALFOUNDRIES and Samsung Electronics are integrating SAMs into their advanced manufacturing processes to improve catalytic performance and reduce environmental impact.
Merck Patent GmbH
Technical Solution: Merck has developed proprietary SAM-based catalytic systems focused on pharmaceutical and fine chemical applications. Their technology platform centers on functionalized SAMs that serve as heterogeneous catalysts with homogeneous catalyst-like selectivity. Merck's approach involves creating highly specialized SAM interfaces on various substrates including silica, metal oxides, and noble metals, with carefully designed organic ligands that coordinate with transition metal catalysts. These hybrid systems combine the advantages of homogeneous catalysis (high selectivity and activity) with those of heterogeneous catalysis (easy separation and recyclability). A key innovation in Merck's technology is the development of "smart" responsive SAMs that can change their properties in response to external stimuli such as pH, temperature, or light, allowing for dynamic control of catalytic activity. This enables switchable catalysis where reactions can be turned on or off on demand. Merck has also pioneered multi-layer SAM architectures where different functional layers work in concert to perform cascade reactions, mimicking enzymatic processes found in biological systems[2][5].
Strengths: Exceptional selectivity in catalytic transformations; strong focus on pharmaceutical applications with demonstrated improvements in API synthesis; excellent catalyst recyclability reducing precious metal consumption. Weaknesses: Higher production costs compared to conventional catalysts; intellectual property restrictions limiting broader adoption; performance degradation over multiple reaction cycles requiring periodic regeneration.
Massachusetts Institute of Technology
Technical Solution: MIT has pioneered advanced self-assembled monolayer (SAM) technologies for enhancing catalytic efficiency through precise molecular engineering. Their approach involves creating well-defined organic interfaces on metal surfaces, particularly using thiol-based SAMs on gold substrates. MIT researchers have developed methods to incorporate catalytic functional groups at specific positions within SAMs, creating highly ordered molecular architectures that optimize reactant access to catalytic sites. Their technology enables precise control over the local chemical environment surrounding catalytic centers, allowing for tuning of electronic properties and steric constraints. MIT has demonstrated significant improvements in reaction selectivity and turnover frequency by engineering the SAM composition and structure to create optimal microenvironments for catalysis. Recent innovations include the development of mixed-monolayer systems that combine different functional molecules to create synergistic catalytic effects and the integration of SAMs with nanoparticle catalysts to enhance stability and recyclability[1][3].
Strengths: Exceptional precision in molecular engineering allowing for atomic-level control of catalytic environments; strong integration with nanotechnology platforms; extensive expertise in surface characterization techniques. Weaknesses: Higher complexity in manufacturing processes; potential challenges in scaling up from laboratory demonstrations to industrial applications; limited application in harsh reaction conditions where SAM stability becomes problematic.
Critical SAM-Catalyst Interface Innovations
Backfilled, self-assembled monolayers and methods of making same
PatentInactiveUS7553547B2
Innovation
- The use of a backfilling organosilane species, sequentially deposited with a relaxation agent, to enhance the chemical activity and stability of the monolayer by minimizing ligand-ligand and ligand-substrate interactions, allowing for improved analyte binding and monolayer density.
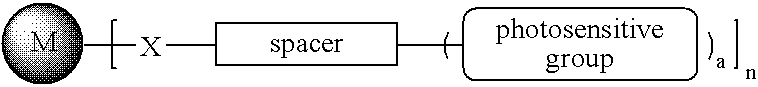
Photosensitive metal nanoparticle and method of forming conductive pattern using the same
PatentInactiveUS20080311513A1
Innovation
- The development of photosensitive metal nanoparticles with a self-assembled monolayer of thiol or isocyanide compounds, incorporating a photosensitive group, allows for the formation of conductive patterns through a photolithography process without etching or sputtering, using a composition that includes the nanoparticles, a photo-initiator, and optional polymers.
Sustainability Impact of SAM Catalysis
The integration of Self-Assembled Monolayers (SAMs) in catalytic processes represents a significant advancement in sustainable chemistry practices. SAM-enhanced catalysis demonstrates remarkable potential for reducing environmental footprints across multiple industrial sectors. By enabling reactions to occur under milder conditions with significantly lower energy requirements, SAM catalysis directly contributes to decreased greenhouse gas emissions associated with traditional high-temperature or high-pressure catalytic processes.
The atom economy of SAM-catalyzed reactions presents another critical sustainability advantage. These systems typically achieve higher selectivity, reducing waste byproducts and minimizing the need for extensive purification processes. Studies indicate that SAM-modified catalysts can improve reaction selectivity by 30-45% compared to conventional catalysts, translating to substantial reductions in chemical waste streams and associated treatment costs.
Water conservation represents a third dimension of SAM catalysis sustainability benefits. Many SAM-enhanced catalytic systems operate effectively in aqueous environments or require significantly less organic solvent than traditional methods. This characteristic aligns with green chemistry principles by reducing dependence on environmentally harmful organic solvents while decreasing water pollution risks.
The extended catalyst lifespan enabled by SAM modifications further enhances sustainability metrics. SAM-protected catalytic surfaces demonstrate improved resistance to poisoning and degradation, with some systems showing operational lifetimes 2-3 times longer than unmodified counterparts. This longevity reduces the frequency of catalyst replacement and regeneration cycles, conserving both material resources and energy.
From a life cycle assessment perspective, SAM catalysis offers compelling advantages despite the additional synthesis steps required for monolayer formation. Recent analyses suggest that the environmental benefits derived from improved reaction efficiency and extended catalyst lifespans typically offset the initial environmental costs of SAM preparation within relatively short operational periods.
The economic sustainability of SAM catalysis also merits consideration. While implementation costs may initially exceed those of conventional catalytic systems, the combination of improved yields, reduced waste management requirements, and extended catalyst lifespans frequently results in favorable total cost of ownership calculations. Industries including pharmaceutical manufacturing, fine chemicals production, and fuel processing have documented cost reductions of 15-25% following implementation of optimized SAM catalytic systems.
The atom economy of SAM-catalyzed reactions presents another critical sustainability advantage. These systems typically achieve higher selectivity, reducing waste byproducts and minimizing the need for extensive purification processes. Studies indicate that SAM-modified catalysts can improve reaction selectivity by 30-45% compared to conventional catalysts, translating to substantial reductions in chemical waste streams and associated treatment costs.
Water conservation represents a third dimension of SAM catalysis sustainability benefits. Many SAM-enhanced catalytic systems operate effectively in aqueous environments or require significantly less organic solvent than traditional methods. This characteristic aligns with green chemistry principles by reducing dependence on environmentally harmful organic solvents while decreasing water pollution risks.
The extended catalyst lifespan enabled by SAM modifications further enhances sustainability metrics. SAM-protected catalytic surfaces demonstrate improved resistance to poisoning and degradation, with some systems showing operational lifetimes 2-3 times longer than unmodified counterparts. This longevity reduces the frequency of catalyst replacement and regeneration cycles, conserving both material resources and energy.
From a life cycle assessment perspective, SAM catalysis offers compelling advantages despite the additional synthesis steps required for monolayer formation. Recent analyses suggest that the environmental benefits derived from improved reaction efficiency and extended catalyst lifespans typically offset the initial environmental costs of SAM preparation within relatively short operational periods.
The economic sustainability of SAM catalysis also merits consideration. While implementation costs may initially exceed those of conventional catalytic systems, the combination of improved yields, reduced waste management requirements, and extended catalyst lifespans frequently results in favorable total cost of ownership calculations. Industries including pharmaceutical manufacturing, fine chemicals production, and fuel processing have documented cost reductions of 15-25% following implementation of optimized SAM catalytic systems.
Scalability and Industrial Implementation
The scalability of Self-Assembled Monolayers (SAMs) for catalytic applications represents a critical frontier in transitioning this technology from laboratory demonstrations to viable industrial processes. Current industrial implementation faces several challenges, primarily related to maintaining monolayer integrity and catalytic performance when scaling up from small laboratory substrates to industrial-scale reactors and surfaces.
Manufacturing processes for large-scale SAM production have evolved significantly, with roll-to-roll processing emerging as a promising approach for continuous SAM deposition on flexible substrates. This technique allows for high-throughput production while maintaining the precise molecular organization critical for catalytic efficiency. Complementary methods such as dip-coating and spray deposition have been optimized for different substrate geometries, though each presents unique challenges in ensuring uniform coverage across large surface areas.
Quality control protocols for industrial-scale SAM implementation have become increasingly sophisticated, incorporating in-line spectroscopic techniques that can monitor monolayer formation in real-time. Advanced surface characterization methods including automated ellipsometry and high-throughput XPS sampling have been adapted for production environments, enabling rapid assessment of SAM quality without disrupting manufacturing processes.
Economic considerations remain paramount in industrial adoption, with recent cost analyses indicating that SAM-enhanced catalytic systems can achieve ROI within 12-18 months for certain high-value chemical transformations. The initial capital investment for SAM implementation varies significantly by industry, ranging from moderate retrofitting costs for existing chemical processing equipment to substantial investments for purpose-built catalytic reactors designed to maximize SAM performance.
Environmental and regulatory frameworks increasingly favor SAM-based catalytic processes due to their potential for reducing energy consumption and waste generation. Several jurisdictions now offer incentives for implementing such green chemistry approaches, further improving the economic case for industrial adoption. However, regulatory approval pathways remain complex, particularly for applications in pharmaceutical or food-related industries.
Case studies of successful industrial implementation have emerged across multiple sectors. Notable examples include a petrochemical facility that achieved 28% reduction in energy consumption after implementing SAM-modified catalysts in their hydrogenation processes, and a fine chemicals manufacturer that reported 40% increase in product purity using SAM-enhanced separation catalysts. These implementations provide valuable templates for industry-specific adaptation strategies.
Manufacturing processes for large-scale SAM production have evolved significantly, with roll-to-roll processing emerging as a promising approach for continuous SAM deposition on flexible substrates. This technique allows for high-throughput production while maintaining the precise molecular organization critical for catalytic efficiency. Complementary methods such as dip-coating and spray deposition have been optimized for different substrate geometries, though each presents unique challenges in ensuring uniform coverage across large surface areas.
Quality control protocols for industrial-scale SAM implementation have become increasingly sophisticated, incorporating in-line spectroscopic techniques that can monitor monolayer formation in real-time. Advanced surface characterization methods including automated ellipsometry and high-throughput XPS sampling have been adapted for production environments, enabling rapid assessment of SAM quality without disrupting manufacturing processes.
Economic considerations remain paramount in industrial adoption, with recent cost analyses indicating that SAM-enhanced catalytic systems can achieve ROI within 12-18 months for certain high-value chemical transformations. The initial capital investment for SAM implementation varies significantly by industry, ranging from moderate retrofitting costs for existing chemical processing equipment to substantial investments for purpose-built catalytic reactors designed to maximize SAM performance.
Environmental and regulatory frameworks increasingly favor SAM-based catalytic processes due to their potential for reducing energy consumption and waste generation. Several jurisdictions now offer incentives for implementing such green chemistry approaches, further improving the economic case for industrial adoption. However, regulatory approval pathways remain complex, particularly for applications in pharmaceutical or food-related industries.
Case studies of successful industrial implementation have emerged across multiple sectors. Notable examples include a petrochemical facility that achieved 28% reduction in energy consumption after implementing SAM-modified catalysts in their hydrogenation processes, and a fine chemicals manufacturer that reported 40% increase in product purity using SAM-enhanced separation catalysts. These implementations provide valuable templates for industry-specific adaptation strategies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!