Decane as a Key Solvent in Polymer Synthesis Processes
Decane in Polymer Synthesis: Background and Objectives
Decane has emerged as a crucial solvent in polymer synthesis processes, playing a pivotal role in the development of various polymeric materials. The evolution of decane's application in this field can be traced back to the mid-20th century when researchers began exploring its potential as a non-polar solvent for polymerization reactions. Over the years, its unique properties have made it an indispensable component in numerous polymer synthesis techniques.
The primary objective of utilizing decane in polymer synthesis is to provide an inert and stable medium for polymerization reactions. Its non-polar nature allows for effective dissolution of monomers and initiators while minimizing unwanted side reactions. This characteristic is particularly valuable in free radical polymerization and coordination polymerization processes, where maintaining control over reaction conditions is crucial for achieving desired polymer properties.
As the polymer industry has grown and diversified, the demand for tailored synthesis methods has increased. Decane's role has expanded beyond being a mere solvent to becoming an integral part of advanced polymer engineering. Its low reactivity and high boiling point make it ideal for high-temperature polymerizations, enabling the synthesis of polymers with enhanced thermal and mechanical properties.
The technological trajectory of decane in polymer synthesis has been marked by continuous refinement and optimization. Researchers have focused on understanding the solvent-polymer interactions at a molecular level, leading to more precise control over polymer architecture and morphology. This has resulted in the development of novel polymer structures, including block copolymers, star-shaped polymers, and hyperbranched polymers, all of which have found applications in various industries.
Recent advancements in polymer science have further emphasized the importance of decane as a key solvent. The growing interest in sustainable and environmentally friendly polymer production has led to investigations into decane's potential in green chemistry applications. Its low toxicity and recyclability make it an attractive option for developing eco-friendly polymer synthesis processes.
Looking ahead, the objectives for decane in polymer synthesis are multifaceted. Researchers aim to exploit its properties for the creation of next-generation polymeric materials with enhanced functionalities. This includes the development of smart polymers, self-healing materials, and nanostructured polymers for advanced applications in electronics, healthcare, and energy storage. Additionally, there is a focus on optimizing decane-based synthesis methods to improve efficiency, reduce energy consumption, and minimize waste generation in industrial-scale polymer production.
Market Analysis for Decane-Based Polymer Products
The market for decane-based polymer products has shown significant growth potential in recent years, driven by the increasing demand for high-performance materials across various industries. Decane, as a key solvent in polymer synthesis processes, plays a crucial role in the production of specialty polymers with unique properties and applications.
The global market for decane-based polymers is primarily segmented into automotive, electronics, packaging, and construction industries. In the automotive sector, these polymers are utilized in the manufacturing of lightweight components, contributing to improved fuel efficiency and reduced emissions. The electronics industry employs decane-based polymers in the production of insulation materials and protective coatings for electronic devices.
The packaging industry has witnessed a surge in demand for decane-based polymers due to their excellent barrier properties and chemical resistance. These polymers are extensively used in food packaging, pharmaceutical packaging, and industrial packaging applications. In the construction sector, decane-based polymers find applications in sealants, adhesives, and coatings, offering enhanced durability and weather resistance.
Market analysis indicates that the Asia-Pacific region dominates the decane-based polymer market, followed by North America and Europe. The rapid industrialization and urbanization in emerging economies like China and India have significantly contributed to the market growth in the Asia-Pacific region. Additionally, stringent environmental regulations in developed countries have led to increased adoption of eco-friendly polymer solutions, further driving the market expansion.
The market for decane-based polymers is characterized by intense competition among key players, including multinational chemical companies and specialty polymer manufacturers. These companies are focusing on research and development activities to introduce innovative products and gain a competitive edge in the market. Collaborations and partnerships between raw material suppliers and polymer manufacturers are becoming increasingly common to ensure a stable supply chain and meet the growing market demand.
Future market trends indicate a shift towards sustainable and bio-based polymer solutions, which may impact the demand for traditional decane-based polymers. However, ongoing research in polymer chemistry and material science is expected to lead to the development of novel decane-based polymers with enhanced properties and wider applications, potentially opening new market opportunities.
Current Challenges in Decane Solvent Applications
Despite the widespread use of decane as a solvent in polymer synthesis processes, several challenges persist that hinder its optimal application. One of the primary concerns is the environmental impact associated with decane usage. As a hydrocarbon solvent, decane poses potential risks to ecosystems and human health if not properly managed. Its low biodegradability and tendency to bioaccumulate in aquatic organisms raise concerns about long-term environmental effects.
Another significant challenge is the volatility of decane, which can lead to solvent loss during polymer synthesis processes. This not only increases production costs but also raises safety concerns due to the flammable nature of decane vapors. Implementing effective vapor recovery systems and maintaining stringent safety protocols are essential but can be complex and costly.
The limited solubility of certain polymers in decane presents another hurdle. While decane is an excellent solvent for many non-polar polymers, its effectiveness diminishes with more polar polymer structures. This limitation restricts its applicability across a broader range of polymer synthesis reactions, necessitating the use of alternative solvents or solvent mixtures in some cases.
Temperature sensitivity is another challenge in decane-based polymer synthesis. The relatively low boiling point of decane (174°C) can be a limiting factor in high-temperature polymerization reactions. This constraint may require the use of pressurized systems or alternative high-boiling-point solvents for certain polymer syntheses, adding complexity to the process design.
Purification and recycling of decane after polymer synthesis pose additional challenges. The presence of residual monomers, oligomers, and other reaction by-products can contaminate the solvent, potentially affecting subsequent synthesis batches. Developing efficient and cost-effective purification methods is crucial for maintaining solvent quality and reducing waste.
Regulatory compliance presents an ongoing challenge for industries utilizing decane in polymer synthesis. Stringent environmental regulations and occupational health and safety standards require careful handling, storage, and disposal practices. Meeting these requirements often necessitates significant investments in infrastructure and training.
Lastly, the petrochemical origin of decane raises sustainability concerns. As industries increasingly seek to reduce their carbon footprint and transition towards more sustainable practices, finding renewable alternatives to petroleum-derived solvents like decane becomes a pressing challenge. This drives research into bio-based solvents and green chemistry approaches, which may eventually compete with or replace decane in certain applications.
Existing Decane-Based Polymer Synthesis Methods
01 Synthesis and applications of decane derivatives
Decane and its derivatives are used in various chemical processes and applications. These compounds are synthesized through different methods and can be utilized in the production of polymers, lubricants, and other industrial products. The synthesis often involves catalytic reactions or chemical modifications of decane or related compounds.- Synthesis and applications of decane derivatives: Decane and its derivatives are used in various chemical processes and applications. These compounds are synthesized through different methods and can be utilized in the production of polymers, lubricants, and other industrial products. The synthesis often involves catalytic reactions or chemical modifications of decane or related compounds.
- Decane in pharmaceutical compositions: Decane and its derivatives are used in pharmaceutical formulations. They can serve as solvents, carriers, or active ingredients in various drug compositions. These compounds may enhance the solubility, stability, or bioavailability of certain drugs, making them valuable in pharmaceutical research and development.
- Decane in fuel compositions: Decane is an important component in fuel formulations, particularly in diesel and jet fuels. It contributes to the combustion properties and performance of these fuels. Research focuses on optimizing decane content and its interactions with other fuel components to improve efficiency and reduce emissions.
- Decane in polymer production: Decane and its derivatives play a role in polymer chemistry. They can be used as monomers, co-monomers, or additives in the production of various polymers. These applications may include the synthesis of specialty plastics, elastomers, or polymer composites with specific properties.
- Purification and separation processes involving decane: Decane is involved in various purification and separation processes in chemical engineering. It can be used as a solvent or as a component in extraction, distillation, or chromatography techniques. These processes are important in the production and purification of chemicals, pharmaceuticals, and other industrial products.
02 Decane in pharmaceutical compositions
Decane and its derivatives are used in pharmaceutical formulations. These compounds can serve as solvents, carriers, or active ingredients in various drug compositions. They may enhance the solubility, stability, or delivery of certain drugs, contributing to improved therapeutic efficacy.Expand Specific Solutions03 Decane in fuel and energy applications
Decane is an important component in fuel formulations and energy-related applications. It can be used as a fuel additive or as a model compound for studying combustion processes. Research in this area focuses on improving fuel efficiency and reducing emissions through the use of decane-based formulations.Expand Specific Solutions04 Decane in polymer and material science
Decane and its derivatives play a role in polymer chemistry and material science. They can be used as monomers, chain transfer agents, or additives in polymer synthesis. These compounds contribute to the development of materials with specific properties, such as improved thermal stability or mechanical strength.Expand Specific Solutions05 Purification and analysis of decane
Various methods are employed for the purification and analysis of decane and its related compounds. These techniques may include distillation, chromatography, or spectroscopic methods. Accurate purification and analysis are crucial for ensuring the quality and purity of decane used in different applications.Expand Specific Solutions
Major Companies in Decane and Polymer Industries
The market for decane as a key solvent in polymer synthesis processes is in a growth phase, driven by increasing demand for advanced polymers across various industries. The global market size is expanding, with major players like ExxonMobil Chemical Patents, BASF, and Dow Global Technologies leading the way. Technological maturity varies, with established companies like PetroChina and Johnson Matthey offering traditional solutions, while innovative firms such as Covestro and Nissan Chemical are developing novel applications. Research institutions like Shanghai Institute of Organic Chemistry and University of Florida are contributing to advancements in the field, indicating ongoing potential for technological breakthroughs and market expansion.
ExxonMobil Chemical Patents, Inc.
Asahi Kasei Corp.
Key Patents in Decane-Mediated Polymerization
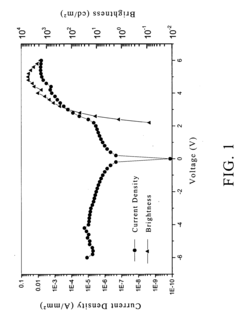
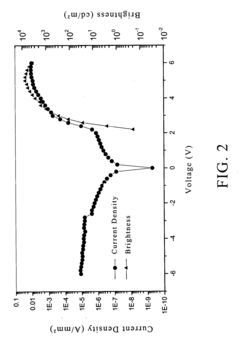
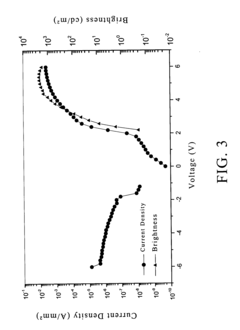
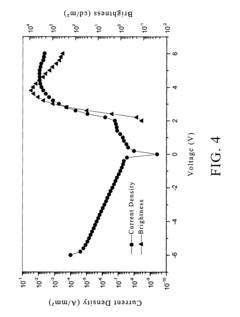
- A composition comprising a fluoropolymer and a fluorinated ionisable block copolymer with specific crystallinity and ionisable group content, combined with a liquid medium, is used to create porous membranes that enhance water permeability while maintaining mechanical strength and chemical resistance, using a method involving film processing and non-solvent induced phase separation.
- A method involving the use of a chelating agent, dissolved in a solvent with the conjugated polymer or its precursor, to chelate residual metal ions, reducing their conductivity and mobility, thereby minimizing leakage current and enhancing material stability.
Environmental Impact of Decane in Polymer Production
The use of decane as a key solvent in polymer synthesis processes has significant environmental implications that warrant careful consideration. Decane, a hydrocarbon solvent, is widely employed in various polymer production methods due to its excellent solvency properties and relatively low toxicity compared to other organic solvents. However, its environmental impact extends across multiple dimensions.
Firstly, the production of decane itself contributes to environmental concerns. As a petroleum-derived compound, its extraction and refinement processes are associated with greenhouse gas emissions and potential ecosystem disruption. The carbon footprint of decane production must be factored into the overall environmental assessment of polymer manufacturing processes that utilize this solvent.
In polymer synthesis, decane's volatility presents both advantages and challenges from an environmental perspective. While its low boiling point facilitates easy removal and recovery, it also increases the risk of atmospheric emissions during handling and processing. These emissions can contribute to the formation of ground-level ozone and photochemical smog, particularly in urban and industrial areas with high polymer production activity.
Water pollution is another critical concern associated with decane use in polymer synthesis. Although decane has low water solubility, accidental spills or improper disposal can lead to contamination of water bodies. This can have detrimental effects on aquatic ecosystems, as decane forms a film on water surfaces, potentially interfering with oxygen transfer and harming marine life.
The persistence of decane in the environment is relatively low compared to some other organic solvents, as it is biodegradable under aerobic conditions. However, its breakdown products and their potential environmental impacts require further study to fully understand the long-term consequences of its use in polymer production.
Efforts to mitigate the environmental impact of decane in polymer synthesis focus on several key areas. Improved solvent recovery and recycling systems are being implemented to reduce emissions and waste. Additionally, research into alternative, more environmentally friendly solvents is ongoing, with bio-based solvents showing promise as potential replacements for petroleum-derived options like decane.
In conclusion, while decane offers valuable properties for polymer synthesis, its environmental impact necessitates ongoing assessment and mitigation strategies. Balancing the benefits of decane as a solvent with its potential environmental risks remains a key challenge for the polymer industry as it strives for more sustainable production methods.
Regulatory Framework for Decane Use in Chemical Processes
The regulatory framework for decane use in chemical processes is a critical aspect of polymer synthesis that manufacturers and researchers must navigate. At the federal level in the United States, the Environmental Protection Agency (EPA) oversees the use of decane under the Toxic Substances Control Act (TSCA). This act requires companies to report new chemical substances before they are manufactured or imported, which includes decane when used in novel polymer synthesis processes.
The Occupational Safety and Health Administration (OSHA) also plays a crucial role in regulating decane use in industrial settings. OSHA has established permissible exposure limits (PELs) for decane and other hydrocarbons to protect worker health. These limits are typically expressed as time-weighted averages over an 8-hour workday. Employers must ensure that their facilities comply with these exposure limits through engineering controls, administrative practices, and personal protective equipment.
At the state level, regulations can vary significantly. Some states, such as California, have more stringent requirements under their Proposition 65 program, which mandates warnings for chemicals known to cause cancer or reproductive harm. Manufacturers using decane in polymer synthesis within California may need to provide specific labeling or warnings depending on the concentration and potential exposure levels.
Internationally, the regulatory landscape for decane use is diverse. The European Union's REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation requires registration of decane and assessment of its safety profile when used in chemical processes. This includes providing detailed information on the substance's properties, potential risks, and safe handling procedures.
In Asia, countries like Japan and South Korea have their own chemical control laws that govern the use of solvents like decane. The Japanese Chemical Substances Control Law (CSCL) and Korea's Act on Registration and Evaluation of Chemicals (K-REACH) both require notification and sometimes registration of new chemical substances, which could apply to novel uses of decane in polymer synthesis.
Compliance with these regulatory frameworks often necessitates extensive documentation, safety data sheets, and sometimes pre-market approval processes. Companies engaged in polymer synthesis using decane must stay abreast of changes in these regulations, as they can impact production processes, labeling requirements, and even market access for their products.