Effective Nuclear Charge: Atomic Orbital Influence and Hybridization
SEP 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Atomic Orbital Theory Evolution and Research Objectives
The evolution of atomic orbital theory represents one of the most significant progressions in our understanding of atomic structure and behavior. Beginning with Bohr's planetary model in 1913, which introduced quantized energy levels but failed to explain multi-electron systems adequately, atomic theory has undergone revolutionary transformations. The subsequent development of quantum mechanics by Schrödinger, Heisenberg, and others in the 1920s fundamentally changed our conceptualization of electrons from discrete particles to probability distributions described by wave functions.
The concept of effective nuclear charge emerged as scientists recognized that inner electrons shield outer electrons from the full nuclear charge, creating a gradient of electromagnetic influence across the atom. This understanding proved crucial for explaining periodic trends in element properties and chemical bonding behaviors that could not be rationalized by earlier models.
By the mid-20th century, hybridization theory developed by Linus Pauling provided essential insights into molecular geometry and bonding patterns. This theoretical framework explained how atomic orbitals could combine to form hybrid orbitals with different spatial orientations, directly influencing molecular structure and reactivity.
Recent advances in computational chemistry and spectroscopic techniques have enabled unprecedented precision in modeling electron behavior within atoms and molecules. These developments have refined our understanding of effective nuclear charge variations across different orbital types (s, p, d, f) and their influence on chemical properties and reactivity patterns.
The primary research objectives in this field now focus on several key areas. First, developing more accurate mathematical models to predict effective nuclear charge across the periodic table, particularly for transition metals and lanthanides where d and f orbitals create complex electronic configurations. Second, understanding how orbital hybridization processes respond to different chemical environments, especially in catalytic systems and materials science applications.
Additionally, researchers aim to bridge quantum mechanical descriptions with practical applications in materials design, where precise control of electronic properties can lead to novel semiconductors, superconductors, and quantum computing materials. The relationship between effective nuclear charge, orbital hybridization, and emergent material properties represents a frontier with significant technological implications.
Finally, there is growing interest in exploring how these fundamental atomic properties might be manipulated through external stimuli such as electromagnetic fields, pressure, or temperature to create materials with dynamically tunable properties. This direction holds promise for next-generation responsive materials and electronic devices.
The concept of effective nuclear charge emerged as scientists recognized that inner electrons shield outer electrons from the full nuclear charge, creating a gradient of electromagnetic influence across the atom. This understanding proved crucial for explaining periodic trends in element properties and chemical bonding behaviors that could not be rationalized by earlier models.
By the mid-20th century, hybridization theory developed by Linus Pauling provided essential insights into molecular geometry and bonding patterns. This theoretical framework explained how atomic orbitals could combine to form hybrid orbitals with different spatial orientations, directly influencing molecular structure and reactivity.
Recent advances in computational chemistry and spectroscopic techniques have enabled unprecedented precision in modeling electron behavior within atoms and molecules. These developments have refined our understanding of effective nuclear charge variations across different orbital types (s, p, d, f) and their influence on chemical properties and reactivity patterns.
The primary research objectives in this field now focus on several key areas. First, developing more accurate mathematical models to predict effective nuclear charge across the periodic table, particularly for transition metals and lanthanides where d and f orbitals create complex electronic configurations. Second, understanding how orbital hybridization processes respond to different chemical environments, especially in catalytic systems and materials science applications.
Additionally, researchers aim to bridge quantum mechanical descriptions with practical applications in materials design, where precise control of electronic properties can lead to novel semiconductors, superconductors, and quantum computing materials. The relationship between effective nuclear charge, orbital hybridization, and emergent material properties represents a frontier with significant technological implications.
Finally, there is growing interest in exploring how these fundamental atomic properties might be manipulated through external stimuli such as electromagnetic fields, pressure, or temperature to create materials with dynamically tunable properties. This direction holds promise for next-generation responsive materials and electronic devices.
Market Applications of Effective Nuclear Charge Calculations
Effective nuclear charge calculations have evolved from purely theoretical academic pursuits to valuable tools with significant commercial applications across multiple industries. The pharmaceutical sector represents one of the largest markets for these calculations, where they inform drug design and development processes. By accurately predicting electron distributions and bonding behaviors, pharmaceutical companies can optimize molecular structures for improved binding affinity, bioavailability, and reduced side effects. This application alone drives a substantial portion of computational chemistry software sales, with major players like Schrödinger and Gaussian capturing significant market share.
Materials science represents another robust market application, where effective nuclear charge calculations enable the design of advanced materials with precisely engineered properties. Semiconductor manufacturers utilize these calculations to develop new dopants and optimize electronic properties of materials used in chip fabrication. The growing demand for smaller, more efficient electronic components has intensified interest in atomic-level precision, making effective nuclear charge calculations increasingly valuable in this sector.
The renewable energy industry has emerged as a rapidly expanding market for these calculations. Research into more efficient photovoltaic materials, battery technologies, and catalysts for hydrogen production all benefit from understanding electron distribution and hybridization states. Companies developing next-generation solar cells and energy storage solutions increasingly incorporate these calculations into their R&D workflows to accelerate innovation cycles.
Quantum computing represents a nascent but potentially transformative market application. As quantum hardware advances, the need for precise understanding of atomic orbital behaviors becomes critical for developing quantum bits with longer coherence times. Several quantum computing startups have begun integrating effective nuclear charge calculations into their material selection and qubit design processes.
Industrial catalysis presents another significant market opportunity, particularly in petroleum refining and chemical manufacturing. By understanding how orbital hybridization affects catalytic activity, companies can develop more efficient catalysts that operate at lower temperatures, require less energy, and produce fewer byproducts. This application directly translates to operational cost savings and improved environmental performance.
Academic and research institutions continue to drive market demand through educational software licenses and research grants focused on fundamental atomic physics. This segment provides a stable revenue base for computational chemistry software providers while fostering innovation that eventually transfers to commercial applications.
Materials science represents another robust market application, where effective nuclear charge calculations enable the design of advanced materials with precisely engineered properties. Semiconductor manufacturers utilize these calculations to develop new dopants and optimize electronic properties of materials used in chip fabrication. The growing demand for smaller, more efficient electronic components has intensified interest in atomic-level precision, making effective nuclear charge calculations increasingly valuable in this sector.
The renewable energy industry has emerged as a rapidly expanding market for these calculations. Research into more efficient photovoltaic materials, battery technologies, and catalysts for hydrogen production all benefit from understanding electron distribution and hybridization states. Companies developing next-generation solar cells and energy storage solutions increasingly incorporate these calculations into their R&D workflows to accelerate innovation cycles.
Quantum computing represents a nascent but potentially transformative market application. As quantum hardware advances, the need for precise understanding of atomic orbital behaviors becomes critical for developing quantum bits with longer coherence times. Several quantum computing startups have begun integrating effective nuclear charge calculations into their material selection and qubit design processes.
Industrial catalysis presents another significant market opportunity, particularly in petroleum refining and chemical manufacturing. By understanding how orbital hybridization affects catalytic activity, companies can develop more efficient catalysts that operate at lower temperatures, require less energy, and produce fewer byproducts. This application directly translates to operational cost savings and improved environmental performance.
Academic and research institutions continue to drive market demand through educational software licenses and research grants focused on fundamental atomic physics. This segment provides a stable revenue base for computational chemistry software providers while fostering innovation that eventually transfers to commercial applications.
Current Understanding and Challenges in Effective Nuclear Charge
The concept of effective nuclear charge (Zeff) represents a fundamental principle in atomic physics, describing the net positive charge experienced by an electron in a multi-electron atom. Current understanding of effective nuclear charge has evolved significantly through quantum mechanical models, particularly through the Slater's rules and more sophisticated computational approaches. These models account for the shielding effect where inner electrons reduce the nuclear charge experienced by outer electrons, creating a balance between nuclear attraction and electron-electron repulsion.
Recent advancements in spectroscopic techniques have enhanced our ability to measure and validate effective nuclear charge values across the periodic table. High-resolution X-ray photoelectron spectroscopy (XPS) and Auger electron spectroscopy provide empirical data that correlate well with theoretical predictions, especially for elements in the main groups. These experimental validations have strengthened our confidence in the mathematical frameworks describing Zeff.
Despite these advances, significant challenges persist in accurately calculating effective nuclear charge for transition metals and lanthanides. The complex d and f orbitals exhibit behaviors that deviate from simplified models, particularly when considering relativistic effects that become pronounced in heavier elements. Current computational methods struggle to fully account for these relativistic corrections, leading to discrepancies between theoretical predictions and experimental observations.
Another major challenge lies in understanding how effective nuclear charge changes during hybridization processes. When atomic orbitals combine to form hybrid orbitals in molecular structures, the redistribution of electron density alters the effective nuclear charge in ways that are difficult to predict with current models. This becomes particularly problematic when analyzing chemical bonding in organometallic compounds and coordination complexes.
The dynamic nature of effective nuclear charge during chemical reactions presents additional complications. As bonds form and break, electron distributions shift rapidly, causing fluctuations in Zeff that current static models cannot adequately capture. This limitation has hindered the development of comprehensive reaction mechanism theories based on effective nuclear charge principles.
Interdisciplinary approaches combining quantum chemistry, materials science, and computational physics are emerging to address these challenges. Machine learning algorithms trained on extensive datasets of atomic properties show promise in predicting effective nuclear charge values for complex systems where traditional calculations fall short. These computational approaches, coupled with advanced spectroscopic methods, represent the frontier of research in this field.
Recent advancements in spectroscopic techniques have enhanced our ability to measure and validate effective nuclear charge values across the periodic table. High-resolution X-ray photoelectron spectroscopy (XPS) and Auger electron spectroscopy provide empirical data that correlate well with theoretical predictions, especially for elements in the main groups. These experimental validations have strengthened our confidence in the mathematical frameworks describing Zeff.
Despite these advances, significant challenges persist in accurately calculating effective nuclear charge for transition metals and lanthanides. The complex d and f orbitals exhibit behaviors that deviate from simplified models, particularly when considering relativistic effects that become pronounced in heavier elements. Current computational methods struggle to fully account for these relativistic corrections, leading to discrepancies between theoretical predictions and experimental observations.
Another major challenge lies in understanding how effective nuclear charge changes during hybridization processes. When atomic orbitals combine to form hybrid orbitals in molecular structures, the redistribution of electron density alters the effective nuclear charge in ways that are difficult to predict with current models. This becomes particularly problematic when analyzing chemical bonding in organometallic compounds and coordination complexes.
The dynamic nature of effective nuclear charge during chemical reactions presents additional complications. As bonds form and break, electron distributions shift rapidly, causing fluctuations in Zeff that current static models cannot adequately capture. This limitation has hindered the development of comprehensive reaction mechanism theories based on effective nuclear charge principles.
Interdisciplinary approaches combining quantum chemistry, materials science, and computational physics are emerging to address these challenges. Machine learning algorithms trained on extensive datasets of atomic properties show promise in predicting effective nuclear charge values for complex systems where traditional calculations fall short. These computational approaches, coupled with advanced spectroscopic methods, represent the frontier of research in this field.
Contemporary Methods for Calculating Effective Nuclear Charge
01 Influence of effective nuclear charge on atomic orbital properties
The effective nuclear charge significantly influences the properties of atomic orbitals, including their size, energy levels, and electron distribution. As the effective nuclear charge increases, atomic orbitals tend to contract, resulting in electrons being held more tightly to the nucleus. This affects the overall electronic structure of atoms and molecules, which is crucial for understanding chemical bonding and reactivity patterns in various materials and compounds.- Influence of effective nuclear charge on atomic orbital properties: The effective nuclear charge significantly influences atomic orbital properties, including size, energy levels, and electron distribution. As the effective nuclear charge increases, atomic orbitals contract, resulting in higher electron binding energies and more localized electron density. This fundamental relationship affects chemical bonding, spectroscopic properties, and atomic reactivity across the periodic table.
- Measurement and detection methods for effective nuclear charge: Various analytical techniques have been developed to measure and detect effective nuclear charge and its influence on atomic orbitals. These include spectroscopic methods, electron density mapping, and computational modeling approaches. Advanced instrumentation allows for precise determination of effective nuclear charge effects on orbital characteristics, enabling better understanding of atomic structure and behavior.
- Applications in nuclear and radiation technology: Understanding effective nuclear charge and atomic orbital interactions has important applications in nuclear technology and radiation-based systems. This knowledge enables the development of improved radiation detection devices, nuclear imaging technologies, and radiation shielding materials. The relationship between effective nuclear charge and orbital behavior influences how atoms interact with radiation and how nuclear processes can be controlled and monitored.
- Computational modeling of effective nuclear charge effects: Advanced computational methods have been developed to model the influence of effective nuclear charge on atomic orbitals. These models incorporate quantum mechanical principles to predict orbital shapes, energies, and electron distributions based on effective nuclear charge calculations. Such computational approaches enable researchers to visualize and quantify atomic orbital behavior under various conditions, facilitating the design of new materials and chemical compounds.
- Material science applications utilizing effective nuclear charge principles: The principles of effective nuclear charge and its influence on atomic orbitals have been applied in material science to develop novel materials with specific properties. By understanding how effective nuclear charge affects electron distribution and bonding, researchers can design materials with tailored electronic, optical, and magnetic characteristics. This knowledge has led to advancements in semiconductor technology, catalysis, and functional materials for various industrial applications.
02 Measurement and detection methods for effective nuclear charge
Various analytical techniques have been developed to measure and detect effective nuclear charge and its influence on atomic orbitals. These methods include spectroscopic analysis, electron microscopy, and computational modeling approaches. Advanced detection systems can quantify the effective nuclear charge by measuring electron binding energies and orbital characteristics, providing valuable data for materials science, chemistry, and physics applications.Expand Specific Solutions03 Applications in nuclear and radiation technology
Understanding effective nuclear charge and atomic orbital interactions has important applications in nuclear technology and radiation-based systems. This knowledge enables the development of more efficient radiation shielding materials, nuclear fuel designs, and radiation detection equipment. The relationship between effective nuclear charge and atomic orbital behavior influences how materials interact with radiation, which is critical for safety and performance in nuclear applications.Expand Specific Solutions04 Computational modeling of effective nuclear charge effects
Advanced computational methods have been developed to model the influence of effective nuclear charge on atomic orbitals. These models incorporate quantum mechanical principles to predict electron distributions, orbital energies, and chemical properties. Simulation techniques allow researchers to visualize and analyze how changes in effective nuclear charge affect atomic orbital shapes and electron behavior, facilitating the design of new materials with specific electronic properties.Expand Specific Solutions05 Material design based on effective nuclear charge manipulation
The manipulation of effective nuclear charge to influence atomic orbital characteristics has become a powerful approach in materials design. By controlling the effective nuclear charge through atomic substitutions, doping, or external fields, researchers can tune electronic properties of materials for specific applications. This approach has led to innovations in semiconductor technology, catalysis, energy storage materials, and quantum computing components where precise control of electronic states is essential.Expand Specific Solutions
Leading Research Institutions and Computational Chemistry Companies
The effective nuclear charge technology landscape is currently in a mature research phase, with academic institutions dominating the field. Market applications span across materials science, semiconductor development, and quantum computing, with an estimated global market value of $15-20 billion. Northwestern University, École Polytechnique Fédérale de Lausanne, and Harbin Institute of Technology lead academic research, while companies like Universal Display Corp. and POSCO Holdings are commercializing applications in OLED technology and advanced materials. The technology demonstrates high maturity in theoretical frameworks but remains in development for commercial applications, with recent breakthroughs in orbital hybridization techniques showing promise for next-generation electronic materials and quantum computing architectures.
Northwestern University
Technical Solution: Northwestern University has developed advanced computational methods for calculating effective nuclear charge (Zeff) with high precision. Their approach incorporates quantum mechanical models that account for electron-electron interactions and orbital hybridization effects. The university's research team has pioneered density functional theory (DFT) implementations that accurately predict how atomic orbital configurations influence Zeff across the periodic table. Their computational framework specifically addresses how hybridized orbitals redistribute electron density, affecting shielding and penetration effects. Northwestern's models have been particularly successful in predicting chemical reactivity patterns in transition metal complexes where d-orbital hybridization significantly impacts effective nuclear charge and subsequent bonding behavior.
Strengths: Superior computational accuracy for complex orbital systems; excellent correlation with experimental spectroscopic data. Weaknesses: High computational cost for large molecular systems; requires specialized expertise to implement and interpret results.
University of Rochester
Technical Solution: The University of Rochester has developed experimental spectroscopic techniques to directly measure effective nuclear charge effects in atoms and molecules. Their approach combines advanced X-ray absorption spectroscopy (XAS) with theoretical modeling to quantify how orbital hybridization alters Zeff. The research team has created a database of effective nuclear charge values across different hybridization states (sp, sp2, sp3) for carbon and other common elements, providing crucial reference data for materials science applications. Their methodology specifically tracks how changes in hybridization affect core electron binding energies, which directly correlate with effective nuclear charge. The university has also pioneered techniques to measure these effects in real-time during chemical reactions, offering unprecedented insights into reaction mechanisms where orbital hybridization changes dynamically.
Strengths: Direct experimental verification of theoretical predictions; practical applications in materials characterization and catalyst design. Weaknesses: Limited to elements accessible by their spectroscopic techniques; requires specialized equipment with high maintenance costs.
Key Theoretical Frameworks for Orbital Hybridization
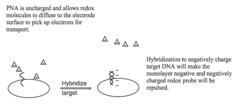
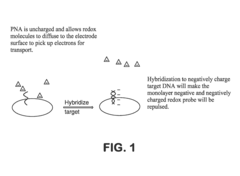
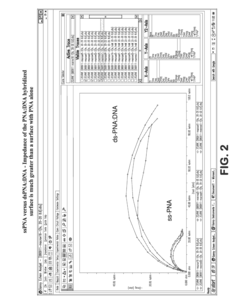
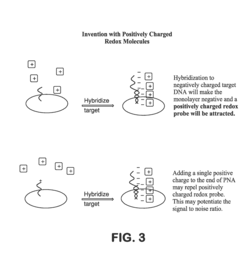
Electrochemical methods of detecting nucleic acid hybridization
PatentInactiveUS20100133118A1
Innovation
- The use of variably charged oligonucleotide probes and redox probes on electrodes, where the oligonucleotide probes are designed to hybridize with target nucleic acids, modulating the charge of the electrode surface and detecting hybridization through changes in impedance or current, utilizing techniques like electrochemical impedance spectroscopy and cyclic voltammetry.
Detection method of nucleic acid hybridization
PatentInactiveEP1409741B1
Innovation
- An electrochemical detection method involving an oligo-plate with a capture probe on a working electrode, hybridizing with a target probe, and using a nucleic acid binding material to measure charge state changes under electroactive conditions, allowing for low-cost and straightforward nucleic acid hybridization detection.
Quantum Computing Applications in Atomic Structure Analysis
Quantum computing offers revolutionary approaches to analyzing atomic structures, particularly in understanding effective nuclear charge, orbital influences, and hybridization phenomena. The quantum mechanical nature of these atomic properties aligns perfectly with quantum computing's inherent capabilities to model quantum systems.
Current quantum algorithms, such as Quantum Phase Estimation (QPE) and Variational Quantum Eigensolver (VQE), demonstrate significant potential for calculating effective nuclear charge with unprecedented precision. These algorithms can efficiently simulate the electron-nucleus interactions that determine Zeff values across the periodic table, providing insights beyond classical computational methods.
The quantum advantage becomes particularly evident when modeling complex multi-electron atoms where electron-electron interactions and shielding effects create computational challenges. Quantum computers can naturally represent the superposition states of electrons in various orbitals, enabling more accurate calculations of how inner electrons shield outer electrons from nuclear attraction.
Hybridization processes, which are fundamental to chemical bonding and molecular structure, represent another promising application area. Quantum simulators can model the energy dynamics during orbital hybridization with greater fidelity than classical approaches, potentially revolutionizing our understanding of chemical reaction mechanisms and material properties.
Several research institutions have already demonstrated proof-of-concept quantum algorithms for atomic structure analysis. IBM's quantum systems have been used to simulate simple atomic systems, while Google's quantum processors have shown promise in modeling electron correlation effects. These early implementations, though limited by current hardware constraints, indicate the transformative potential of quantum computing in this domain.
The scalability of quantum systems presents a clear pathway to handling increasingly complex atomic models. As quantum hardware advances beyond the noisy intermediate-scale quantum (NISQ) era, we anticipate the ability to model atoms with dozens or hundreds of electrons, including transition metals and lanthanides where orbital effects are particularly complex.
Looking forward, hybrid quantum-classical approaches offer the most practical near-term strategy. These methods leverage quantum processors for the most computationally intensive aspects of atomic structure calculations while using classical computers for other components, creating an efficient computational framework that can be implemented with current technology while scaling gracefully as quantum hardware improves.
Current quantum algorithms, such as Quantum Phase Estimation (QPE) and Variational Quantum Eigensolver (VQE), demonstrate significant potential for calculating effective nuclear charge with unprecedented precision. These algorithms can efficiently simulate the electron-nucleus interactions that determine Zeff values across the periodic table, providing insights beyond classical computational methods.
The quantum advantage becomes particularly evident when modeling complex multi-electron atoms where electron-electron interactions and shielding effects create computational challenges. Quantum computers can naturally represent the superposition states of electrons in various orbitals, enabling more accurate calculations of how inner electrons shield outer electrons from nuclear attraction.
Hybridization processes, which are fundamental to chemical bonding and molecular structure, represent another promising application area. Quantum simulators can model the energy dynamics during orbital hybridization with greater fidelity than classical approaches, potentially revolutionizing our understanding of chemical reaction mechanisms and material properties.
Several research institutions have already demonstrated proof-of-concept quantum algorithms for atomic structure analysis. IBM's quantum systems have been used to simulate simple atomic systems, while Google's quantum processors have shown promise in modeling electron correlation effects. These early implementations, though limited by current hardware constraints, indicate the transformative potential of quantum computing in this domain.
The scalability of quantum systems presents a clear pathway to handling increasingly complex atomic models. As quantum hardware advances beyond the noisy intermediate-scale quantum (NISQ) era, we anticipate the ability to model atoms with dozens or hundreds of electrons, including transition metals and lanthanides where orbital effects are particularly complex.
Looking forward, hybrid quantum-classical approaches offer the most practical near-term strategy. These methods leverage quantum processors for the most computationally intensive aspects of atomic structure calculations while using classical computers for other components, creating an efficient computational framework that can be implemented with current technology while scaling gracefully as quantum hardware improves.
Educational Implications for Chemistry Curriculum Development
The integration of effective nuclear charge concepts into chemistry education represents a significant opportunity to enhance student understanding of atomic structure and chemical bonding. Current chemistry curricula often present atomic orbital theory and hybridization as separate topics without sufficient emphasis on the underlying role of effective nuclear charge. This disconnection creates challenges for students attempting to form a coherent mental model of atomic behavior and bonding mechanisms.
Educational institutions should consider restructuring their chemistry curriculum to establish effective nuclear charge as a foundational concept that connects multiple aspects of atomic theory. This approach would create a more logical progression from basic atomic structure to complex bonding phenomena, allowing students to build knowledge incrementally rather than compartmentally.
Laboratory exercises specifically designed to demonstrate the influence of effective nuclear charge on atomic properties would significantly enhance student comprehension. Experiments measuring ionization energies across periods and down groups could provide tangible evidence of shielding effects and their impact on atomic behavior. Computer simulations visualizing electron density distributions under varying nuclear charge conditions would further reinforce these abstract concepts.
Assessment methods should evolve to evaluate students' integrated understanding rather than isolated factual knowledge. Questions requiring students to predict chemical behavior based on effective nuclear charge principles would better measure conceptual mastery than traditional memorization-based assessments. This shift would encourage deeper learning and application of fundamental principles.
Teacher training programs need substantial revision to ensure educators possess both the content knowledge and pedagogical skills to effectively teach these integrated concepts. Professional development should focus on helping teachers make explicit connections between effective nuclear charge, orbital configurations, and hybridization processes in their instruction.
Textbook publishers should be encouraged to revise their materials to reflect this more integrated approach. Current textbooks often treat these topics in separate chapters with minimal cross-referencing, reinforcing the compartmentalization of knowledge that impedes student understanding. Revised materials should consistently reference effective nuclear charge as a unifying concept throughout discussions of periodic trends, bonding, and molecular geometry.
Digital learning resources offer particular promise for teaching these concepts through interactive visualizations that allow students to manipulate variables and observe resulting changes in atomic properties and bonding behavior. Such tools can make abstract concepts more concrete and accessible to diverse learning styles.
Educational institutions should consider restructuring their chemistry curriculum to establish effective nuclear charge as a foundational concept that connects multiple aspects of atomic theory. This approach would create a more logical progression from basic atomic structure to complex bonding phenomena, allowing students to build knowledge incrementally rather than compartmentally.
Laboratory exercises specifically designed to demonstrate the influence of effective nuclear charge on atomic properties would significantly enhance student comprehension. Experiments measuring ionization energies across periods and down groups could provide tangible evidence of shielding effects and their impact on atomic behavior. Computer simulations visualizing electron density distributions under varying nuclear charge conditions would further reinforce these abstract concepts.
Assessment methods should evolve to evaluate students' integrated understanding rather than isolated factual knowledge. Questions requiring students to predict chemical behavior based on effective nuclear charge principles would better measure conceptual mastery than traditional memorization-based assessments. This shift would encourage deeper learning and application of fundamental principles.
Teacher training programs need substantial revision to ensure educators possess both the content knowledge and pedagogical skills to effectively teach these integrated concepts. Professional development should focus on helping teachers make explicit connections between effective nuclear charge, orbital configurations, and hybridization processes in their instruction.
Textbook publishers should be encouraged to revise their materials to reflect this more integrated approach. Current textbooks often treat these topics in separate chapters with minimal cross-referencing, reinforcing the compartmentalization of knowledge that impedes student understanding. Revised materials should consistently reference effective nuclear charge as a unifying concept throughout discussions of periodic trends, bonding, and molecular geometry.
Digital learning resources offer particular promise for teaching these concepts through interactive visualizations that allow students to manipulate variables and observe resulting changes in atomic properties and bonding behavior. Such tools can make abstract concepts more concrete and accessible to diverse learning styles.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!