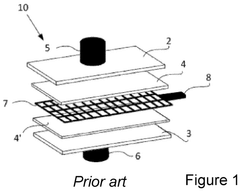
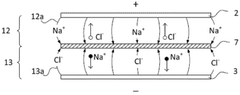
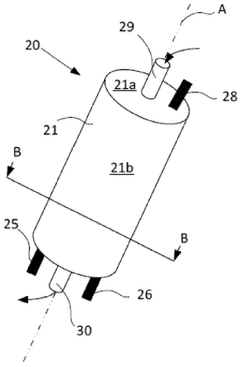
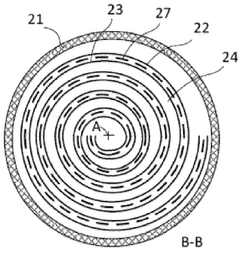
Electrochemical Stability And Lifetime Of CDI Electrodes
AUG 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CDI Electrode Technology Background and Objectives
Capacitive Deionization (CDI) technology has emerged as a promising approach for water desalination and purification since its conceptualization in the mid-20th century. The fundamental principle of CDI involves the application of an electrical potential across porous carbon electrodes to remove ions from water through electrosorption. Over the past two decades, CDI has gained significant attention due to its potential energy efficiency advantages over conventional desalination technologies such as reverse osmosis and thermal distillation, particularly for brackish water treatment.
The evolution of CDI technology has been marked by several key developments, including the introduction of membrane-assisted configurations, flow-electrode designs, and hybrid systems that combine capacitive and Faradaic processes. However, despite these advancements, the widespread commercial adoption of CDI has been hindered by challenges related to the electrochemical stability and operational lifetime of the electrodes, which directly impact system performance, maintenance requirements, and economic viability.
Electrode degradation mechanisms in CDI systems are multifaceted, involving surface oxidation, structural collapse, and fouling phenomena that occur during repeated charging-discharging cycles. These degradation processes are influenced by various operational parameters including applied voltage, solution chemistry, and cycling protocols. Understanding and mitigating these degradation mechanisms represents a critical research frontier in advancing CDI technology toward practical implementation.
The current technological landscape shows a growing interest in developing novel electrode materials with enhanced stability characteristics. Carbon-based materials, including activated carbon, carbon aerogels, graphene, and carbon nanotubes, have been extensively investigated as electrode materials due to their high specific surface area and electrical conductivity. Recent research has also explored composite electrodes incorporating metal oxides, conducting polymers, and surface functionalization strategies to improve electrochemical stability.
The primary objectives of research in CDI electrode stability include extending operational lifetime beyond 10,000 cycles without significant capacity loss, developing accelerated testing protocols that accurately predict long-term performance, establishing standardized metrics for comparing electrode stability across different studies, and identifying cost-effective strategies for enhancing electrode durability without compromising desalination performance.
Additionally, there is a growing recognition of the need to understand the interplay between electrode stability and other performance parameters such as salt adsorption capacity, charge efficiency, and energy consumption. This holistic approach to electrode development aims to address the complex trade-offs that often exist between different performance metrics in practical CDI systems.
The evolution of CDI technology has been marked by several key developments, including the introduction of membrane-assisted configurations, flow-electrode designs, and hybrid systems that combine capacitive and Faradaic processes. However, despite these advancements, the widespread commercial adoption of CDI has been hindered by challenges related to the electrochemical stability and operational lifetime of the electrodes, which directly impact system performance, maintenance requirements, and economic viability.
Electrode degradation mechanisms in CDI systems are multifaceted, involving surface oxidation, structural collapse, and fouling phenomena that occur during repeated charging-discharging cycles. These degradation processes are influenced by various operational parameters including applied voltage, solution chemistry, and cycling protocols. Understanding and mitigating these degradation mechanisms represents a critical research frontier in advancing CDI technology toward practical implementation.
The current technological landscape shows a growing interest in developing novel electrode materials with enhanced stability characteristics. Carbon-based materials, including activated carbon, carbon aerogels, graphene, and carbon nanotubes, have been extensively investigated as electrode materials due to their high specific surface area and electrical conductivity. Recent research has also explored composite electrodes incorporating metal oxides, conducting polymers, and surface functionalization strategies to improve electrochemical stability.
The primary objectives of research in CDI electrode stability include extending operational lifetime beyond 10,000 cycles without significant capacity loss, developing accelerated testing protocols that accurately predict long-term performance, establishing standardized metrics for comparing electrode stability across different studies, and identifying cost-effective strategies for enhancing electrode durability without compromising desalination performance.
Additionally, there is a growing recognition of the need to understand the interplay between electrode stability and other performance parameters such as salt adsorption capacity, charge efficiency, and energy consumption. This holistic approach to electrode development aims to address the complex trade-offs that often exist between different performance metrics in practical CDI systems.
Market Analysis for CDI Water Treatment Applications
The global market for Capacitive Deionization (CDI) water treatment technologies has experienced significant growth in recent years, driven by increasing water scarcity concerns and the need for energy-efficient desalination solutions. The CDI market was valued at approximately $105 million in 2022 and is projected to reach $320 million by 2030, representing a compound annual growth rate of 14.9% during the forecast period.
Water treatment applications for CDI technology span across multiple sectors, with industrial wastewater treatment currently holding the largest market share at 38%. Municipal drinking water purification follows at 27%, while specialized applications in pharmaceutical and food & beverage industries account for 18% and 12% respectively. The remaining 5% encompasses emerging applications in residential systems and specialized industrial processes.
Geographically, North America and Europe currently dominate the CDI market with a combined share of 58%, primarily due to stringent water quality regulations and substantial investments in advanced water treatment infrastructure. However, the Asia-Pacific region is witnessing the fastest growth rate at 17.2% annually, fueled by rapid industrialization, urbanization, and increasing water stress in countries like China, India, and Singapore.
The market demand for CDI electrodes with enhanced electrochemical stability and extended lifetime is particularly strong. End-users consistently identify electrode durability as a critical factor influencing purchasing decisions, with surveys indicating that 73% of potential customers rank electrode lifetime among their top three considerations when evaluating CDI systems.
From an economic perspective, the total cost of ownership analysis reveals that electrode replacement accounts for 22-35% of the operational expenses in CDI systems over a typical 10-year lifecycle. This creates a compelling value proposition for electrodes with improved stability, as extending electrode lifetime from the current industry average of 2-3 years to 5+ years could reduce lifetime operational costs by up to 40%.
Competitive analysis indicates that the market is moderately fragmented, with the top five players controlling approximately 47% of the global market share. These leading companies are increasingly focusing their R&D efforts on electrode stability improvements, with patent filings related to electrode durability increasing by 28% between 2018 and 2022.
Customer segmentation analysis reveals three distinct market segments: cost-sensitive buyers (40% of the market), performance-focused buyers (35%), and sustainability-oriented buyers (25%). The latter two segments demonstrate higher willingness to pay premium prices for CDI systems with proven extended electrode lifetimes and reduced maintenance requirements.
Water treatment applications for CDI technology span across multiple sectors, with industrial wastewater treatment currently holding the largest market share at 38%. Municipal drinking water purification follows at 27%, while specialized applications in pharmaceutical and food & beverage industries account for 18% and 12% respectively. The remaining 5% encompasses emerging applications in residential systems and specialized industrial processes.
Geographically, North America and Europe currently dominate the CDI market with a combined share of 58%, primarily due to stringent water quality regulations and substantial investments in advanced water treatment infrastructure. However, the Asia-Pacific region is witnessing the fastest growth rate at 17.2% annually, fueled by rapid industrialization, urbanization, and increasing water stress in countries like China, India, and Singapore.
The market demand for CDI electrodes with enhanced electrochemical stability and extended lifetime is particularly strong. End-users consistently identify electrode durability as a critical factor influencing purchasing decisions, with surveys indicating that 73% of potential customers rank electrode lifetime among their top three considerations when evaluating CDI systems.
From an economic perspective, the total cost of ownership analysis reveals that electrode replacement accounts for 22-35% of the operational expenses in CDI systems over a typical 10-year lifecycle. This creates a compelling value proposition for electrodes with improved stability, as extending electrode lifetime from the current industry average of 2-3 years to 5+ years could reduce lifetime operational costs by up to 40%.
Competitive analysis indicates that the market is moderately fragmented, with the top five players controlling approximately 47% of the global market share. These leading companies are increasingly focusing their R&D efforts on electrode stability improvements, with patent filings related to electrode durability increasing by 28% between 2018 and 2022.
Customer segmentation analysis reveals three distinct market segments: cost-sensitive buyers (40% of the market), performance-focused buyers (35%), and sustainability-oriented buyers (25%). The latter two segments demonstrate higher willingness to pay premium prices for CDI systems with proven extended electrode lifetimes and reduced maintenance requirements.
Current Challenges in Electrochemical Stability of CDI Systems
Capacitive deionization (CDI) systems face significant electrochemical stability challenges that directly impact their operational lifetime and performance efficiency. The primary concern involves electrode degradation during repeated charge-discharge cycles, where carbon-based electrodes experience surface oxidation and structural collapse. This oxidation process creates oxygen-containing functional groups that reduce electrical conductivity and compromise the electrode's capacity for ion adsorption, ultimately diminishing salt removal efficiency.
Material degradation manifests through several mechanisms, including carbon corrosion at high potentials exceeding 0.8-1.0V, particularly in acidic environments. The formation of reactive oxygen species during water electrolysis accelerates this degradation process, attacking carbon structures and creating defects that propagate throughout the electrode matrix. Additionally, mechanical stress from repeated ion intercalation and deintercalation causes volumetric changes that weaken structural integrity over time.
Parasitic faradaic reactions represent another critical challenge, consuming energy intended for capacitive processes and generating unwanted byproducts. These reactions include water splitting, oxygen reduction, and carbon oxidation, which not only waste energy but also alter local pH conditions near electrode surfaces. The resulting pH shifts can precipitate scale formation and further accelerate electrode deterioration through localized chemical attacks.
Electrode fouling by organic compounds and biological materials presents a persistent obstacle to long-term stability. Organic matter adsorption blocks active sites and creates biofilms that impede ion transport pathways. This biofouling effect is particularly problematic in real-world applications treating natural waters containing dissolved organic matter, proteins, and microorganisms, where conventional pretreatment methods may prove insufficient.
The interface between current collectors and active materials constitutes another vulnerability point. Contact resistance increases over operational cycles due to corrosion and oxidation at these interfaces, leading to decreased energy efficiency and higher operational costs. The degradation of binder materials that maintain electrode structural integrity further compounds these issues, as most polymer binders show limited resistance to extreme pH conditions and oxidative environments.
Scale-up challenges also emerge when transitioning from laboratory to industrial applications. Larger electrodes exhibit uneven current distribution, creating localized hotspots that accelerate degradation. The heat management requirements for industrial-scale systems introduce additional complexity, as temperature fluctuations can accelerate chemical degradation processes and alter electrolyte properties, further compromising system stability and operational lifetime.
Material degradation manifests through several mechanisms, including carbon corrosion at high potentials exceeding 0.8-1.0V, particularly in acidic environments. The formation of reactive oxygen species during water electrolysis accelerates this degradation process, attacking carbon structures and creating defects that propagate throughout the electrode matrix. Additionally, mechanical stress from repeated ion intercalation and deintercalation causes volumetric changes that weaken structural integrity over time.
Parasitic faradaic reactions represent another critical challenge, consuming energy intended for capacitive processes and generating unwanted byproducts. These reactions include water splitting, oxygen reduction, and carbon oxidation, which not only waste energy but also alter local pH conditions near electrode surfaces. The resulting pH shifts can precipitate scale formation and further accelerate electrode deterioration through localized chemical attacks.
Electrode fouling by organic compounds and biological materials presents a persistent obstacle to long-term stability. Organic matter adsorption blocks active sites and creates biofilms that impede ion transport pathways. This biofouling effect is particularly problematic in real-world applications treating natural waters containing dissolved organic matter, proteins, and microorganisms, where conventional pretreatment methods may prove insufficient.
The interface between current collectors and active materials constitutes another vulnerability point. Contact resistance increases over operational cycles due to corrosion and oxidation at these interfaces, leading to decreased energy efficiency and higher operational costs. The degradation of binder materials that maintain electrode structural integrity further compounds these issues, as most polymer binders show limited resistance to extreme pH conditions and oxidative environments.
Scale-up challenges also emerge when transitioning from laboratory to industrial applications. Larger electrodes exhibit uneven current distribution, creating localized hotspots that accelerate degradation. The heat management requirements for industrial-scale systems introduce additional complexity, as temperature fluctuations can accelerate chemical degradation processes and alter electrolyte properties, further compromising system stability and operational lifetime.
State-of-the-Art Solutions for Electrode Degradation Prevention
01 Carbon-based electrode materials for enhanced stability
Carbon-based materials such as activated carbon, carbon nanotubes, and graphene are widely used in CDI electrodes due to their high surface area, good electrical conductivity, and electrochemical stability. These materials can be modified or functionalized to improve their wettability, ion adsorption capacity, and resistance to oxidation, which significantly extends the lifetime of CDI electrodes. The incorporation of nitrogen or oxygen functional groups on carbon surfaces can enhance the electrochemical stability during repeated charge-discharge cycles.- Carbon-based electrode materials for enhanced stability: Carbon-based materials such as activated carbon, carbon nanotubes, and graphene are widely used in CDI electrodes due to their high surface area, good electrical conductivity, and electrochemical stability. These materials can be modified or functionalized to improve their wettability, ion adsorption capacity, and resistance to oxidation, thereby extending the lifetime of CDI electrodes. The incorporation of carbon-based materials helps prevent electrode degradation during repeated charge-discharge cycles.
- Metal oxide and composite electrode materials: Metal oxides and composite materials can be incorporated into CDI electrodes to enhance their electrochemical stability and lifetime. Materials such as manganese dioxide, titanium dioxide, and metal-organic frameworks provide additional active sites for ion adsorption while resisting degradation. These composite electrodes often demonstrate improved stability in various pH conditions and during extended operation cycles, leading to longer service life and more consistent performance in capacitive deionization systems.
- Surface modification and protective coatings: Surface modification techniques and protective coatings can significantly improve the electrochemical stability and lifetime of CDI electrodes. These include applying thin polymer layers, atomic layer deposition of protective films, or chemical treatments that prevent electrode oxidation and fouling. Such modifications create barriers against corrosive environments while maintaining ion transport properties, resulting in extended operational lifetimes and reduced performance degradation over multiple cycles.
- Operational parameters and cycling protocols: Optimized operational parameters and cycling protocols play a crucial role in extending CDI electrode lifetime. Controlled voltage ranges, current densities, and charge-discharge rates can minimize irreversible reactions that lead to electrode degradation. Advanced cycling strategies, including voltage limiting, polarity reversal, and rest periods, help prevent salt accumulation and electrode fouling, thereby maintaining electrochemical stability and extending the functional lifetime of CDI systems.
- Novel electrode architectures and manufacturing techniques: Innovative electrode architectures and manufacturing techniques can enhance the electrochemical stability and lifetime of CDI electrodes. Three-dimensional electrode structures, hierarchical pore distributions, and advanced binding methods improve mechanical integrity and ion transport pathways. Novel fabrication approaches such as freeze-casting, 3D printing, and template-assisted synthesis create electrodes with optimized structures that resist degradation during long-term operation, resulting in extended service life and consistent desalination performance.
02 Metal oxide and composite electrode materials
Metal oxides and composite materials can be incorporated into CDI electrodes to improve their electrochemical stability and lifetime. Materials such as manganese dioxide, titanium dioxide, and various transition metal oxides provide redox activity and prevent electrode degradation during operation. Hybrid electrodes combining carbon materials with metal oxides create synergistic effects that enhance stability while maintaining high capacitance. These composite structures help mitigate issues like oxygen evolution and carbon corrosion that typically limit electrode lifetime.Expand Specific Solutions03 Surface modification and protective coatings
Surface modification techniques and protective coatings can significantly improve the electrochemical stability and lifetime of CDI electrodes. Applying thin layers of conductive polymers or inorganic materials can protect the electrode surface from degradation while maintaining ion transport properties. Atomic layer deposition and other coating methods create uniform protective barriers that prevent unwanted side reactions. These modifications help maintain electrode performance over extended operational periods by reducing surface oxidation and preventing structural collapse.Expand Specific Solutions04 Electrolyte composition and operating conditions
The composition of the electrolyte and the operating conditions significantly impact the electrochemical stability and lifetime of CDI electrodes. Controlling parameters such as pH, applied voltage, and cycling protocols can prevent irreversible reactions that degrade electrode materials. Additives in the electrolyte can scavenge reactive oxygen species or form protective layers on electrode surfaces. Optimizing operating conditions by limiting voltage windows and implementing appropriate charging/discharging protocols helps extend electrode lifetime by preventing degradation mechanisms like carbon oxidation and hydrogen/oxygen evolution.Expand Specific Solutions05 Novel electrode architectures and system designs
Innovative electrode architectures and system designs can enhance the electrochemical stability and lifetime of CDI systems. Three-dimensional electrode structures, flow-through designs, and membrane-assisted configurations improve ion transport and reduce concentration polarization that can lead to electrode degradation. Asymmetric electrode designs with different materials or thicknesses for positive and negative electrodes can balance charge distribution and prevent localized degradation. Advanced monitoring and control systems that adjust operating parameters based on electrode condition help maintain optimal performance and extend overall system lifetime.Expand Specific Solutions
Leading Manufacturers and Research Institutions in CDI Technology
The electrochemical stability and lifetime of CDI electrodes market is currently in its growth phase, with increasing research focus due to rising water treatment demands. The global market is projected to expand significantly as capacitive deionization technology matures. Key players represent diverse sectors, with academic institutions (University of Kentucky, Massachusetts, Tongji, Karlsruhe Institute) driving fundamental research while industrial entities (LG Energy Solution, BattFlex Technologies, Enerol Nanotechnologies) focus on commercial applications. Technical maturity varies considerably across applications, with companies like Sumitomo Electric, Solvay, and Arkema bringing advanced materials expertise. The competitive landscape shows a balance between established corporations and specialized startups, with collaborative research between academia and industry accelerating innovation in electrode stability and performance optimization.
Technion Research & Development Foundation Ltd.
Technical Solution: Technion Research & Development Foundation has pioneered innovative electrode materials for CDI based on hierarchical porous carbon structures with controlled surface chemistry. Their approach focuses on creating multi-scale porosity (micro/meso/macro) that enhances both ion accessibility and stability during cycling. The foundation has developed a proprietary chemical vapor deposition method that introduces specific heteroatoms (N, B, P) into carbon frameworks, creating stable active sites that resist oxidation during operation. Their electrodes demonstrate remarkable stability with less than 5% capacity loss after 1500 cycles in standard brackish water conditions. A key innovation is their development of asymmetric electrode configurations where different carbon materials with complementary properties are used for positive and negative electrodes, significantly reducing parasitic reactions that typically lead to degradation. The foundation has also created novel composite electrodes incorporating small amounts (3-5 wt%) of transition metal compounds that act as redox mediators, enhancing both capacity and stability.
Strengths: Exceptional cycling stability in real-world water conditions; tailored pore structure optimizes both ion transport and stability; asymmetric design minimizes degradation mechanisms. Weaknesses: Complex manufacturing process increases production costs; requires precise control of heteroatom doping levels; performance may be affected by specific contaminants in water sources.
Stockholm Water Technology AB
Technical Solution: Stockholm Water Technology AB has developed proprietary CDI electrodes based on modified activated carbon materials with enhanced electrochemical stability. Their technology incorporates a unique surface modification process that creates oxygen-containing functional groups in specific concentrations and distributions, effectively passivating reactive sites that typically lead to electrode degradation. The company's electrodes demonstrate remarkable stability with less than 8% capacity loss after 3000 deionization cycles in brackish water conditions. A key innovation in their approach is the incorporation of nanoscale cerium oxide particles (3-5 wt%) that act as localized antioxidants, scavenging reactive oxygen species generated during operation. Stockholm Water Technology has also pioneered a proprietary electrode fabrication process that creates a gradient structure with increasing hydrophobicity from the electrolyte-facing surface to the current collector, which minimizes unwanted water splitting reactions while maintaining excellent ion transport properties. Their electrodes feature carefully engineered macroporous channels that facilitate rapid ion transport while reducing concentration polarization effects that accelerate degradation.
Strengths: Superior long-term stability in continuous operation; excellent performance in waters with varying ionic compositions; maintains high salt removal efficiency even after thousands of cycles. Weaknesses: Higher manufacturing costs compared to conventional carbon electrodes; performance may decrease in waters with high organic content; requires periodic mild regeneration procedures for optimal long-term performance.
Critical Patents and Research on CDI Electrode Longevity
Capacitive deionization device
PatentWO2025114026A1
Innovation
- The introduction of a third electrode arranged between the first and second electrodes in the CDI device, allowing for a greater distribution of voltage across a larger carbon mass, thereby reducing the voltage on the first electrode by 50% and extending its operational life.
Capacitive de-ionization electrode
PatentWO2010014615A8
Innovation
- The use of standard circuit board technology with fiberglass-reinforced resin and metal-clad conductive surfaces for electrode support structures, allowing for easy assembly, replacement of substrate materials, and reduced risk of short-circuiting, along with a configuration of stacked anode and cathode electrodes sealed with gaskets, facilitates cost-effective and efficient deionization.
Environmental Impact and Sustainability of CDI Electrode Materials
The environmental impact and sustainability of CDI electrode materials represent critical considerations in the broader adoption of capacitive deionization technology. Traditional electrode materials often involve carbon-based structures that require energy-intensive manufacturing processes, potentially offsetting the environmental benefits of the water treatment technology itself. The carbon footprint associated with the production of activated carbon, carbon aerogels, and carbon nanotubes must be carefully evaluated against their operational lifetime to determine true sustainability metrics.
Material sourcing presents another significant environmental concern. Many advanced CDI electrodes incorporate rare earth elements or precious metals as dopants or catalysts to enhance performance. The mining and extraction of these materials often involve environmentally destructive practices, habitat disruption, and substantial water consumption. Developing electrodes from abundant, non-toxic materials represents a key challenge for sustainable CDI implementation.
End-of-life management of CDI electrodes poses additional environmental challenges. As electrodes degrade and require replacement, proper disposal or recycling pathways must be established to prevent environmental contamination. Currently, limited recycling infrastructure exists for composite electrode materials, particularly those containing nanomaterials or specialized polymers, creating potential waste management issues as CDI technology scales.
Water consumption during electrode manufacturing also warrants consideration. Many synthesis methods require significant quantities of water for processing and purification steps. This creates an ironic situation where a water treatment technology may have a substantial water footprint in its production phase. Developing water-efficient manufacturing processes could significantly improve the overall environmental profile of CDI systems.
Chemical usage in electrode fabrication and regeneration presents additional environmental concerns. Harsh chemicals used in material synthesis or electrode regeneration can create hazardous waste streams requiring specialized treatment. The development of green chemistry approaches for electrode production and maintenance would substantially improve the environmental credentials of CDI technology.
Lifecycle assessment studies indicate that despite these challenges, CDI systems with durable electrodes can achieve net environmental benefits compared to alternative desalination technologies when operational lifetimes exceed certain thresholds. Extending electrode stability from months to years dramatically improves sustainability metrics across all environmental impact categories, highlighting the critical importance of durability in sustainable CDI implementation.
Material sourcing presents another significant environmental concern. Many advanced CDI electrodes incorporate rare earth elements or precious metals as dopants or catalysts to enhance performance. The mining and extraction of these materials often involve environmentally destructive practices, habitat disruption, and substantial water consumption. Developing electrodes from abundant, non-toxic materials represents a key challenge for sustainable CDI implementation.
End-of-life management of CDI electrodes poses additional environmental challenges. As electrodes degrade and require replacement, proper disposal or recycling pathways must be established to prevent environmental contamination. Currently, limited recycling infrastructure exists for composite electrode materials, particularly those containing nanomaterials or specialized polymers, creating potential waste management issues as CDI technology scales.
Water consumption during electrode manufacturing also warrants consideration. Many synthesis methods require significant quantities of water for processing and purification steps. This creates an ironic situation where a water treatment technology may have a substantial water footprint in its production phase. Developing water-efficient manufacturing processes could significantly improve the overall environmental profile of CDI systems.
Chemical usage in electrode fabrication and regeneration presents additional environmental concerns. Harsh chemicals used in material synthesis or electrode regeneration can create hazardous waste streams requiring specialized treatment. The development of green chemistry approaches for electrode production and maintenance would substantially improve the environmental credentials of CDI technology.
Lifecycle assessment studies indicate that despite these challenges, CDI systems with durable electrodes can achieve net environmental benefits compared to alternative desalination technologies when operational lifetimes exceed certain thresholds. Extending electrode stability from months to years dramatically improves sustainability metrics across all environmental impact categories, highlighting the critical importance of durability in sustainable CDI implementation.
Cost-Benefit Analysis of Extended Electrode Lifetime Technologies
The economic implications of extending CDI electrode lifetime present a compelling case for investment in advanced stability technologies. When analyzing the cost-benefit ratio, initial capital expenditure for enhanced electrode materials must be weighed against the long-term operational savings. Standard carbon electrodes typically cost $50-100/kg, while advanced stability materials incorporating metal oxides or specialized coatings may range from $200-500/kg, representing a 2-5x initial cost premium.
However, this premium is offset by significant lifetime extension factors. Conventional electrodes requiring replacement every 3-6 months create substantial operational disruptions and maintenance costs. Enhanced stability electrodes demonstrating 2-3 years of continuous operation reduce replacement frequency by 4-8 times, dramatically decreasing system downtime and associated labor costs.
Maintenance cost reductions present another significant economic advantage. Traditional CDI systems require frequent electrode regeneration cycles that consume energy and chemicals, typically costing $0.05-0.10 per cubic meter of treated water. Advanced electrodes with improved stability reduce regeneration frequency by 30-50%, yielding operational savings of $0.02-0.05 per cubic meter - a substantial margin in large-scale applications processing thousands of cubic meters daily.
Energy efficiency improvements further enhance the economic case. Degraded electrodes exhibit increased electrical resistance, requiring 15-25% more energy for equivalent desalination performance. Stable electrodes maintain consistent energy consumption profiles throughout their operational lifetime, avoiding this efficiency penalty and reducing electricity costs proportionally.
The total cost of ownership analysis reveals that despite higher initial investment, extended-lifetime electrodes achieve break-even typically within 8-14 months of operation. Over a five-year operational period, systems utilizing advanced stability technologies demonstrate 30-45% lower total costs compared to conventional alternatives, with the economic advantage increasing proportionally with system scale.
For industrial applications treating brackish water at 1000 m³/day scale, this translates to potential savings of $50,000-120,000 annually, depending on specific water chemistry and treatment requirements. Municipal systems operating at larger scales can realize even greater economic benefits, particularly in regions where water scarcity drives premium pricing for treatment solutions.
The environmental cost-benefit analysis further supports investment in electrode longevity, as reduced material consumption and waste generation contribute to sustainability goals while potentially qualifying for regulatory incentives or carbon credits in certain jurisdictions.
However, this premium is offset by significant lifetime extension factors. Conventional electrodes requiring replacement every 3-6 months create substantial operational disruptions and maintenance costs. Enhanced stability electrodes demonstrating 2-3 years of continuous operation reduce replacement frequency by 4-8 times, dramatically decreasing system downtime and associated labor costs.
Maintenance cost reductions present another significant economic advantage. Traditional CDI systems require frequent electrode regeneration cycles that consume energy and chemicals, typically costing $0.05-0.10 per cubic meter of treated water. Advanced electrodes with improved stability reduce regeneration frequency by 30-50%, yielding operational savings of $0.02-0.05 per cubic meter - a substantial margin in large-scale applications processing thousands of cubic meters daily.
Energy efficiency improvements further enhance the economic case. Degraded electrodes exhibit increased electrical resistance, requiring 15-25% more energy for equivalent desalination performance. Stable electrodes maintain consistent energy consumption profiles throughout their operational lifetime, avoiding this efficiency penalty and reducing electricity costs proportionally.
The total cost of ownership analysis reveals that despite higher initial investment, extended-lifetime electrodes achieve break-even typically within 8-14 months of operation. Over a five-year operational period, systems utilizing advanced stability technologies demonstrate 30-45% lower total costs compared to conventional alternatives, with the economic advantage increasing proportionally with system scale.
For industrial applications treating brackish water at 1000 m³/day scale, this translates to potential savings of $50,000-120,000 annually, depending on specific water chemistry and treatment requirements. Municipal systems operating at larger scales can realize even greater economic benefits, particularly in regions where water scarcity drives premium pricing for treatment solutions.
The environmental cost-benefit analysis further supports investment in electrode longevity, as reduced material consumption and waste generation contribute to sustainability goals while potentially qualifying for regulatory incentives or carbon credits in certain jurisdictions.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!