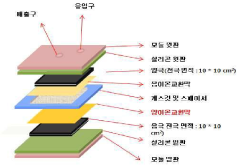
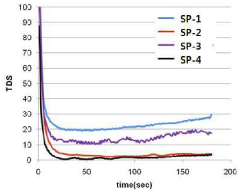
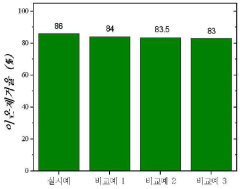
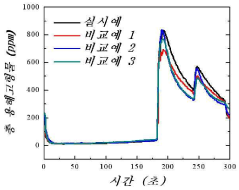
Electrode Materials For High-Efficiency CDI Systems
AUG 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CDI Electrode Materials Background and Objectives
Capacitive deionization (CDI) technology has emerged as a promising approach for water desalination and purification over the past several decades. The evolution of this technology can be traced back to the 1960s when the concept of removing ions from water using electrical double layers was first introduced. Since then, CDI has undergone significant advancements, particularly in electrode material development, which has been the primary driver of efficiency improvements.
The trajectory of CDI electrode materials has evolved from traditional carbon-based materials to more sophisticated nanostructured and composite materials. Initially, activated carbon dominated the field due to its high surface area and relatively low cost. However, limitations in ion adsorption capacity and electrical conductivity prompted research into alternative materials. The introduction of carbon nanotubes, graphene, and mesoporous carbon in the early 2000s marked a significant turning point, offering enhanced surface area and improved electrical properties.
Recent years have witnessed the emergence of hybrid and composite electrode materials that combine the advantages of different components. Metal oxides, conducting polymers, and MXenes have been incorporated into carbon matrices to enhance specific capacitance and ion selectivity. This trend reflects the growing recognition that optimizing multiple material properties simultaneously is essential for achieving high-efficiency CDI systems.
The current technical objectives in CDI electrode material development focus on several key parameters. First, maximizing the specific surface area remains crucial for providing abundant adsorption sites for ions. Second, optimizing pore size distribution is essential for facilitating ion transport and enhancing adsorption kinetics. Third, improving electrical conductivity is necessary for reducing energy consumption during the charging/discharging process. Fourth, enhancing wettability ensures effective contact between the electrode surface and the aqueous solution.
Beyond these fundamental properties, emerging objectives include developing materials with selective ion removal capabilities, particularly for applications requiring the extraction of specific contaminants such as heavy metals or nitrates. Additionally, there is growing interest in materials that demonstrate stability over extended operational cycles, as commercial viability depends on long-term performance consistency.
The ultimate goal of electrode material research for CDI systems is to achieve a balance between desalination capacity, energy efficiency, operational stability, and cost-effectiveness. This requires a multidisciplinary approach that integrates materials science, electrochemistry, and engineering principles to design and fabricate electrode materials tailored for specific water treatment applications.
The trajectory of CDI electrode materials has evolved from traditional carbon-based materials to more sophisticated nanostructured and composite materials. Initially, activated carbon dominated the field due to its high surface area and relatively low cost. However, limitations in ion adsorption capacity and electrical conductivity prompted research into alternative materials. The introduction of carbon nanotubes, graphene, and mesoporous carbon in the early 2000s marked a significant turning point, offering enhanced surface area and improved electrical properties.
Recent years have witnessed the emergence of hybrid and composite electrode materials that combine the advantages of different components. Metal oxides, conducting polymers, and MXenes have been incorporated into carbon matrices to enhance specific capacitance and ion selectivity. This trend reflects the growing recognition that optimizing multiple material properties simultaneously is essential for achieving high-efficiency CDI systems.
The current technical objectives in CDI electrode material development focus on several key parameters. First, maximizing the specific surface area remains crucial for providing abundant adsorption sites for ions. Second, optimizing pore size distribution is essential for facilitating ion transport and enhancing adsorption kinetics. Third, improving electrical conductivity is necessary for reducing energy consumption during the charging/discharging process. Fourth, enhancing wettability ensures effective contact between the electrode surface and the aqueous solution.
Beyond these fundamental properties, emerging objectives include developing materials with selective ion removal capabilities, particularly for applications requiring the extraction of specific contaminants such as heavy metals or nitrates. Additionally, there is growing interest in materials that demonstrate stability over extended operational cycles, as commercial viability depends on long-term performance consistency.
The ultimate goal of electrode material research for CDI systems is to achieve a balance between desalination capacity, energy efficiency, operational stability, and cost-effectiveness. This requires a multidisciplinary approach that integrates materials science, electrochemistry, and engineering principles to design and fabricate electrode materials tailored for specific water treatment applications.
Market Analysis for CDI Water Treatment Technologies
The global Capacitive Deionization (CDI) water treatment market is experiencing significant growth, driven by increasing water scarcity concerns and the need for energy-efficient desalination technologies. Current market valuations place the CDI sector at approximately $320 million in 2023, with projections indicating a compound annual growth rate of 9.8% through 2030, potentially reaching $580 million by the end of the decade.
The industrial sector represents the largest market segment for CDI technologies, accounting for roughly 45% of total market share. This dominance stems from stringent water quality requirements in manufacturing processes, particularly in electronics, pharmaceuticals, and food and beverage industries where ultrapure water is essential. Municipal water treatment follows as the second-largest segment at 30%, with growing adoption in regions facing freshwater shortages.
Geographically, North America currently leads the CDI market with 35% share, followed closely by Asia-Pacific at 32%. The Asia-Pacific region, however, demonstrates the highest growth potential with a projected CAGR of 12.3%, driven primarily by rapid industrialization in China and India, coupled with increasing government investments in water infrastructure development.
Market penetration analysis reveals that CDI technologies have achieved only 15% of their potential market reach, indicating substantial room for growth. The primary limiting factors include high initial capital costs compared to conventional technologies and limited awareness among potential end-users about CDI's long-term operational benefits.
Customer segmentation shows three distinct buyer profiles: large industrial corporations seeking advanced water treatment solutions (40% of current customers), municipal water authorities in water-stressed regions (35%), and specialized applications such as laboratory water purification systems (25%). Each segment demonstrates different purchasing behaviors and value propositions, with industrial users prioritizing performance and reliability, while municipal customers focus more on long-term cost efficiency.
Competitive analysis indicates a fragmented market with over 50 companies offering CDI solutions globally. However, five major players control approximately 60% of the market share, with technological differentiation primarily focused on electrode material innovations. Recent market trends show increasing strategic partnerships between electrode material manufacturers and system integrators to develop comprehensive CDI solutions.
The pricing structure for CDI systems varies significantly based on treatment capacity and application, ranging from $5,000 for small-scale laboratory units to over $2 million for industrial-scale installations. The average return on investment period currently stands at 3.5 years, though this is expected to decrease as electrode materials advance and manufacturing scales improve.
The industrial sector represents the largest market segment for CDI technologies, accounting for roughly 45% of total market share. This dominance stems from stringent water quality requirements in manufacturing processes, particularly in electronics, pharmaceuticals, and food and beverage industries where ultrapure water is essential. Municipal water treatment follows as the second-largest segment at 30%, with growing adoption in regions facing freshwater shortages.
Geographically, North America currently leads the CDI market with 35% share, followed closely by Asia-Pacific at 32%. The Asia-Pacific region, however, demonstrates the highest growth potential with a projected CAGR of 12.3%, driven primarily by rapid industrialization in China and India, coupled with increasing government investments in water infrastructure development.
Market penetration analysis reveals that CDI technologies have achieved only 15% of their potential market reach, indicating substantial room for growth. The primary limiting factors include high initial capital costs compared to conventional technologies and limited awareness among potential end-users about CDI's long-term operational benefits.
Customer segmentation shows three distinct buyer profiles: large industrial corporations seeking advanced water treatment solutions (40% of current customers), municipal water authorities in water-stressed regions (35%), and specialized applications such as laboratory water purification systems (25%). Each segment demonstrates different purchasing behaviors and value propositions, with industrial users prioritizing performance and reliability, while municipal customers focus more on long-term cost efficiency.
Competitive analysis indicates a fragmented market with over 50 companies offering CDI solutions globally. However, five major players control approximately 60% of the market share, with technological differentiation primarily focused on electrode material innovations. Recent market trends show increasing strategic partnerships between electrode material manufacturers and system integrators to develop comprehensive CDI solutions.
The pricing structure for CDI systems varies significantly based on treatment capacity and application, ranging from $5,000 for small-scale laboratory units to over $2 million for industrial-scale installations. The average return on investment period currently stands at 3.5 years, though this is expected to decrease as electrode materials advance and manufacturing scales improve.
Current Challenges in CDI Electrode Development
Despite significant advancements in capacitive deionization (CDI) technology, electrode material development remains a critical bottleneck limiting widespread commercial adoption. Current carbon-based electrodes face persistent challenges in achieving optimal performance metrics necessary for industrial-scale implementation. The primary limitation is insufficient salt adsorption capacity (SAC), with most conventional carbon electrodes achieving only 10-15 mg/g, far below theoretical predictions and practical requirements for competitive desalination.
Electrode stability presents another significant hurdle, as performance degradation occurs after repeated charge-discharge cycles. This degradation manifests through decreased salt removal efficiency and increased energy consumption, with most current materials showing 15-30% capacity loss after 100-200 cycles. Such instability substantially increases operational costs and maintenance requirements in real-world applications.
The trade-off between specific surface area and ion transport kinetics remains unresolved. While high surface area carbons (>2000 m²/g) offer greater theoretical adsorption sites, their intricate pore structures often create diffusion limitations that reduce actual performance under practical operating conditions. This paradox has complicated material optimization efforts and hindered the development of universally effective electrode designs.
Energy efficiency challenges persist across electrode types. Current materials exhibit high electrical resistance and suboptimal charge efficiency, resulting in energy consumption rates of 1-2 kWh/m³ for brackish water treatment—significantly higher than competing technologies like reverse osmosis. This energy penalty undermines CDI's value proposition, particularly in energy-constrained environments.
Manufacturing scalability presents additional complications. Laboratory-scale materials with promising performance characteristics often face prohibitive production costs or complex synthesis procedures that impede industrial-scale manufacturing. The absence of standardized production methods further complicates quality control and performance reproducibility across different manufacturing batches.
Selectivity limitations represent an emerging concern as CDI applications diversify. Current electrode materials demonstrate poor ion selectivity, limiting their effectiveness in mixed-ion environments common in real-world water sources. This deficiency restricts CDI application in scenarios requiring preferential removal of specific contaminants like heavy metals or nitrates.
Environmental sustainability concerns have also emerged regarding electrode materials. Many advanced carbon composites incorporate potentially harmful components like heavy metals or synthetic polymers that may leach during operation or create disposal challenges at end-of-life. This contradicts the inherently environmentally friendly premise of CDI technology and creates regulatory hurdles for implementation.
Electrode stability presents another significant hurdle, as performance degradation occurs after repeated charge-discharge cycles. This degradation manifests through decreased salt removal efficiency and increased energy consumption, with most current materials showing 15-30% capacity loss after 100-200 cycles. Such instability substantially increases operational costs and maintenance requirements in real-world applications.
The trade-off between specific surface area and ion transport kinetics remains unresolved. While high surface area carbons (>2000 m²/g) offer greater theoretical adsorption sites, their intricate pore structures often create diffusion limitations that reduce actual performance under practical operating conditions. This paradox has complicated material optimization efforts and hindered the development of universally effective electrode designs.
Energy efficiency challenges persist across electrode types. Current materials exhibit high electrical resistance and suboptimal charge efficiency, resulting in energy consumption rates of 1-2 kWh/m³ for brackish water treatment—significantly higher than competing technologies like reverse osmosis. This energy penalty undermines CDI's value proposition, particularly in energy-constrained environments.
Manufacturing scalability presents additional complications. Laboratory-scale materials with promising performance characteristics often face prohibitive production costs or complex synthesis procedures that impede industrial-scale manufacturing. The absence of standardized production methods further complicates quality control and performance reproducibility across different manufacturing batches.
Selectivity limitations represent an emerging concern as CDI applications diversify. Current electrode materials demonstrate poor ion selectivity, limiting their effectiveness in mixed-ion environments common in real-world water sources. This deficiency restricts CDI application in scenarios requiring preferential removal of specific contaminants like heavy metals or nitrates.
Environmental sustainability concerns have also emerged regarding electrode materials. Many advanced carbon composites incorporate potentially harmful components like heavy metals or synthetic polymers that may leach during operation or create disposal challenges at end-of-life. This contradicts the inherently environmentally friendly premise of CDI technology and creates regulatory hurdles for implementation.
State-of-the-Art Electrode Materials for CDI Systems
01 Carbon-based electrode materials for CDI systems
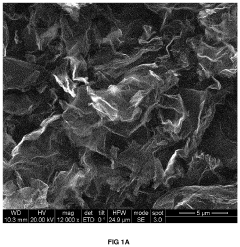
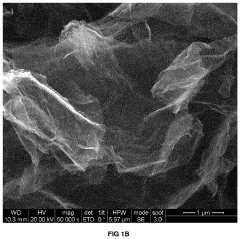
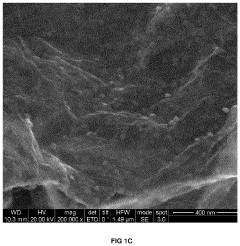
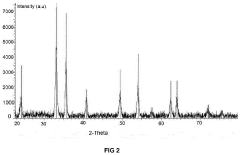
Carbon-based materials are widely used as electrodes in capacitive deionization (CDI) systems due to their high surface area, good electrical conductivity, and chemical stability. These materials include activated carbon, carbon nanotubes, graphene, and carbon aerogels. The high surface area of these materials allows for increased ion adsorption capacity, while their electrical conductivity facilitates efficient charge transfer, resulting in improved CDI system efficiency.- Carbon-based electrode materials for CDI systems: Carbon-based materials are widely used as electrodes in capacitive deionization (CDI) systems due to their high surface area, good electrical conductivity, and chemical stability. These materials include activated carbon, carbon nanotubes, graphene, and carbon aerogels. The high surface area of these materials allows for increased ion adsorption capacity, while their electrical conductivity facilitates efficient charge transfer, resulting in improved CDI system efficiency. Various modifications and treatments can be applied to carbon-based materials to enhance their performance in CDI applications.
- Metal oxide and composite electrode materials: Metal oxide and composite materials offer enhanced electrochemical properties for CDI electrodes. These materials, including titanium dioxide, manganese dioxide, and various metal oxide composites, provide improved ion adsorption capacity and selectivity. Composite electrodes combining carbon materials with metal oxides can synergistically enhance CDI performance by increasing the specific capacitance and improving the stability of the electrodes. The incorporation of metal oxides can also modify the surface charge properties of the electrodes, allowing for better ion selectivity in the deionization process.
- Novel electrode structures and architectures: Innovative electrode structures and architectures are being developed to enhance CDI efficiency. These include 3D porous structures, hierarchical pore distributions, and flow-through electrode designs that maximize the contact area between the electrode and the solution while minimizing ion diffusion distances. Such structures facilitate faster ion transport and adsorption kinetics, leading to improved deionization rates and energy efficiency. Advanced manufacturing techniques like 3D printing and template-assisted synthesis are employed to create these optimized electrode architectures.
- Surface-modified electrodes for enhanced performance: Surface modification techniques are applied to electrode materials to enhance their performance in CDI systems. These modifications include functionalization with specific chemical groups, doping with heteroatoms, and coating with ion-selective membranes. Such treatments can improve the wettability, ion selectivity, and charge efficiency of the electrodes. Surface-modified electrodes exhibit reduced co-ion effects, decreased energy consumption, and improved salt adsorption capacity, contributing to the overall efficiency of CDI systems.
- Sustainable and low-cost electrode materials: Research is focused on developing sustainable and cost-effective electrode materials for CDI systems to enable widespread application. These materials include biomass-derived carbons, waste-derived materials, and naturally abundant compounds. The use of environmentally friendly synthesis methods and renewable precursors reduces the environmental impact and cost of electrode production. These sustainable materials can achieve comparable or even superior performance to conventional electrodes while offering economic and ecological advantages for large-scale CDI implementation.
02 Metal oxide-based electrode materials
Metal oxide-based materials, such as titanium dioxide, manganese dioxide, and iron oxide, can be used as electrodes in CDI systems to enhance performance. These materials offer high specific capacitance, good stability, and selective ion adsorption properties. By incorporating metal oxides into electrode materials, the efficiency of CDI systems can be significantly improved, particularly in terms of ion removal capacity and cycling stability.Expand Specific Solutions03 Composite electrode materials for enhanced performance
Composite electrode materials, combining carbon-based materials with metal oxides or polymers, can offer synergistic effects that enhance CDI system efficiency. These composites typically exhibit improved electrical conductivity, increased surface area, and enhanced ion adsorption capacity compared to single-component materials. The integration of different materials allows for tailored properties that address specific challenges in CDI applications, such as selectivity, stability, and energy efficiency.Expand Specific Solutions04 Surface-modified electrode materials
Surface modification of electrode materials through functionalization or doping can significantly improve their performance in CDI systems. Techniques such as nitrogen doping, oxygen functionalization, or introduction of specific functional groups can enhance the wettability, ion selectivity, and adsorption capacity of the electrodes. These modifications can be tailored to target specific ions or to improve the overall efficiency of the CDI process.Expand Specific Solutions05 Novel electrode architectures and structures
Innovative electrode architectures, such as hierarchical porous structures, 3D frameworks, and flow-through electrodes, can significantly enhance CDI system efficiency. These structures are designed to optimize ion transport pathways, minimize diffusion limitations, and maximize the utilization of electrode surface area. By engineering the physical structure of electrodes at multiple scales, from nano to macro, the performance of CDI systems can be substantially improved in terms of desalination rate, energy consumption, and operational stability.Expand Specific Solutions
Leading Companies and Research Institutions in CDI Field
The Capacitive Deionization (CDI) electrode materials market is currently in a growth phase, with increasing adoption driven by water treatment applications. The market size is expanding rapidly, projected to reach significant value as water scarcity concerns intensify globally. Technologically, the field shows moderate maturity with ongoing innovation. Leading players include Samsung Electronics and Corning, who leverage their materials expertise; academic institutions like Zhejiang University of Technology and Tianjin University contributing fundamental research; and specialized companies such as Pureechem Co. focusing exclusively on CDI technology. South Korean entities (KIST, Doosan Enerbility) demonstrate particular strength in this domain, while collaborative efforts between industry and research institutions are accelerating development of high-efficiency electrode materials with improved performance metrics.
Samsung Electronics Co., Ltd.
Technical Solution: Samsung Electronics has developed advanced carbon-based electrode materials for high-efficiency CDI systems, focusing on graphene-derived structures with optimized surface properties. Their proprietary technology utilizes reduced graphene oxide (rGO) sheets with controlled oxygen functional groups that enhance hydrophilicity while maintaining excellent electrical conductivity (>200 S/cm)[1]. Samsung's manufacturing process incorporates a unique freeze-drying technique followed by thermal annealing to create three-dimensional porous architectures with high specific surface area (>1000 m²/g) and interconnected channels for efficient ion transport. Their electrodes feature a hierarchical pore structure with optimized micro/mesopore distributions that maximize ion adsorption capacity while minimizing diffusion limitations. Recent innovations include graphene-carbon nanotube composite electrodes that demonstrate enhanced mechanical stability and improved salt removal efficiency (up to 20 mg/g) at low applied voltages (<1.2V)[2]. The company has also developed specialized coating techniques that reduce electrode fouling in real-world applications, extending operational lifetimes and reducing maintenance requirements for CDI systems deployed in industrial water treatment facilities.
Strengths: Excellent electrical conductivity reducing energy consumption; superior mechanical stability for extended operational lifetime; advanced manufacturing capabilities for consistent quality. Weaknesses: Higher production costs compared to conventional carbon materials; complex fabrication process requiring specialized equipment; intellectual property restrictions may limit broader adoption.
Central South University
Technical Solution: Central South University has pioneered the development of novel electrode materials for CDI systems based on hierarchically porous carbon derived from sustainable biomass sources. Their technology utilizes agricultural waste materials (rice husks, corn stalks) as carbon precursors, which undergo a controlled pyrolysis and activation process to create electrodes with high surface area (1200-1800 m²/g) and optimized pore size distributions[1]. The university's research team has developed a proprietary chemical activation method that creates interconnected micro/mesoporous structures with enhanced ion accessibility and reduced diffusion resistance. Their electrodes incorporate nitrogen and oxygen functional groups that improve hydrophilicity and create additional ion adsorption sites, resulting in salt removal capacities of 15-18 mg/g at low energy consumption (0.3-0.5 kWh/m³)[2]. Recent innovations include carbon-MnO2 composite electrodes that demonstrate pseudocapacitive behavior, enhancing ion removal through both physical adsorption and faradaic reactions. The electrodes maintain stable performance over extended operation (>500 cycles) with minimal capacity degradation, addressing durability concerns in practical applications.
Strengths: Cost-effective production using sustainable biomass precursors; excellent combination of physical adsorption and pseudocapacitive mechanisms; good cycling stability. Weaknesses: Potential variability in performance due to biomass source differences; lower electrical conductivity compared to graphene-based materials; may require additional processing to ensure consistent quality.
Key Patents and Research Breakthroughs in CDI Technology
Capacitive deionization electrode
PatentActiveUS20210363015A1
Innovation
- A 3D reduced graphene oxide/Fe2O3 material is developed through a process involving a graphene oxide water dispersion treated with iron (II) sulfate, followed by hydrothermal treatment, freezing, and lyophilization, resulting in a material with enhanced porosity and specific capacitance for improved ion removal in CDI devices.
Manufacturing Method of Capacitive Deionization Electrode
PatentInactiveKR1020120057750A
Innovation
- A method for manufacturing an electrosorption-type ion removal electrode by directly coating a polymer solution with specific ion exchange groups onto a carbon material electrode, minimizing interface resistance and simplifying the process by omitting separate ion exchange membrane formation.
Environmental Impact and Sustainability Assessment
The environmental impact of electrode materials in Capacitive Deionization (CDI) systems represents a critical consideration for sustainable water treatment technologies. Traditional electrode materials often involve carbon-based substances that require energy-intensive manufacturing processes, potentially offsetting the environmental benefits of CDI technology. The carbon footprint associated with producing activated carbon, carbon aerogels, and carbon nanotubes must be carefully evaluated against their operational efficiency and lifespan in CDI applications.
Life cycle assessment (LCA) studies indicate that while CDI systems generally consume less energy during operation compared to reverse osmosis, the environmental burden of electrode material production can be substantial. Manufacturing processes for advanced carbon materials like graphene and carbon nanotubes typically involve high-temperature treatments and chemical processing that generate significant greenhouse gas emissions and consume considerable energy resources.
Material sourcing presents another environmental consideration, as rare earth elements and precious metals used in some high-performance electrodes face supply constraints and often involve environmentally damaging extraction practices. The mining operations associated with these materials can lead to habitat destruction, water pollution, and soil contamination in source regions, raising ethical concerns about the true sustainability of these technological solutions.
End-of-life management of electrode materials presents both challenges and opportunities. Many current electrode materials lack established recycling pathways, potentially contributing to electronic waste streams. However, emerging research demonstrates promising approaches for electrode material recovery and reuse, which could significantly improve the sustainability profile of CDI systems through circular economy principles.
Water treatment residuals from CDI operations, including concentrated brine streams and degraded electrode materials, require proper management to prevent secondary environmental contamination. The fate of adsorbed contaminants, particularly heavy metals and persistent organic pollutants, must be addressed through appropriate disposal or treatment protocols to ensure complete environmental protection.
Recent innovations in bio-based and renewable electrode materials offer promising alternatives to conventional options. Materials derived from biomass waste, such as activated biochar from agricultural residues, demonstrate comparable performance while significantly reducing environmental impacts. These sustainable alternatives align with green chemistry principles and contribute to waste valorization efforts across industrial sectors.
The energy efficiency of CDI systems directly correlates with electrode material properties, creating an important sustainability feedback loop. Materials that enable lower energy consumption during operation can compensate for higher production impacts, emphasizing the importance of holistic environmental assessment approaches that consider the entire technology lifecycle rather than isolated environmental metrics.
Life cycle assessment (LCA) studies indicate that while CDI systems generally consume less energy during operation compared to reverse osmosis, the environmental burden of electrode material production can be substantial. Manufacturing processes for advanced carbon materials like graphene and carbon nanotubes typically involve high-temperature treatments and chemical processing that generate significant greenhouse gas emissions and consume considerable energy resources.
Material sourcing presents another environmental consideration, as rare earth elements and precious metals used in some high-performance electrodes face supply constraints and often involve environmentally damaging extraction practices. The mining operations associated with these materials can lead to habitat destruction, water pollution, and soil contamination in source regions, raising ethical concerns about the true sustainability of these technological solutions.
End-of-life management of electrode materials presents both challenges and opportunities. Many current electrode materials lack established recycling pathways, potentially contributing to electronic waste streams. However, emerging research demonstrates promising approaches for electrode material recovery and reuse, which could significantly improve the sustainability profile of CDI systems through circular economy principles.
Water treatment residuals from CDI operations, including concentrated brine streams and degraded electrode materials, require proper management to prevent secondary environmental contamination. The fate of adsorbed contaminants, particularly heavy metals and persistent organic pollutants, must be addressed through appropriate disposal or treatment protocols to ensure complete environmental protection.
Recent innovations in bio-based and renewable electrode materials offer promising alternatives to conventional options. Materials derived from biomass waste, such as activated biochar from agricultural residues, demonstrate comparable performance while significantly reducing environmental impacts. These sustainable alternatives align with green chemistry principles and contribute to waste valorization efforts across industrial sectors.
The energy efficiency of CDI systems directly correlates with electrode material properties, creating an important sustainability feedback loop. Materials that enable lower energy consumption during operation can compensate for higher production impacts, emphasizing the importance of holistic environmental assessment approaches that consider the entire technology lifecycle rather than isolated environmental metrics.
Cost-Performance Analysis of Advanced CDI Materials
The economic viability of Capacitive Deionization (CDI) systems is heavily dependent on the cost-performance ratio of electrode materials. Carbon-based materials, particularly activated carbon, offer the most cost-effective solution at approximately $10-30 per kilogram while delivering moderate performance with specific capacitance ranging from 100-200 F/g. These materials benefit from established manufacturing processes and widespread availability, making them the current market standard despite their performance limitations.
Advanced carbon nanomaterials such as graphene and carbon nanotubes demonstrate superior electrochemical properties with specific capacitance reaching 200-300 F/g, but at significantly higher costs ($200-1000/kg). This substantial price premium creates a barrier to widespread commercial adoption despite their technical advantages. The cost-performance gap remains a critical challenge for industry implementation.
Metal oxide-based electrodes, including manganese dioxide and titanium dioxide, present a middle-ground option with moderate costs ($50-150/kg) and improved performance characteristics compared to traditional carbon materials. However, their manufacturing complexity and scalability issues continue to limit their market penetration in commercial CDI systems.
Lifecycle cost analysis reveals that while advanced materials require higher initial investment, their extended operational lifespan and reduced maintenance requirements can potentially offset these costs over time. For instance, graphene-based electrodes may last 2-3 times longer than conventional carbon electrodes, reducing replacement frequency and associated operational disruptions.
Energy efficiency calculations demonstrate that high-performance electrode materials can reduce energy consumption by 20-40% compared to conventional materials, translating to significant operational savings in large-scale desalination operations. This energy reduction becomes particularly valuable in regions with high electricity costs or renewable energy integration challenges.
Market analysis indicates a clear correlation between material cost reduction and CDI system adoption rates. Historical data shows that a 30% reduction in electrode material costs typically results in a 15-25% increase in market adoption, highlighting the price sensitivity of this technology sector. Industry projections suggest that achieving a cost threshold below $50/kg for high-performance materials would trigger widespread commercial implementation.
The development of hybrid materials and composite electrodes represents a promising approach to optimizing the cost-performance ratio. These engineered materials strategically combine low-cost carbon substrates with selective high-performance components to achieve enhanced functionality without proportional cost increases. Recent research demonstrates that such hybrid approaches can deliver 70-80% of the performance benefits of premium materials at only 30-40% of their cost.
Advanced carbon nanomaterials such as graphene and carbon nanotubes demonstrate superior electrochemical properties with specific capacitance reaching 200-300 F/g, but at significantly higher costs ($200-1000/kg). This substantial price premium creates a barrier to widespread commercial adoption despite their technical advantages. The cost-performance gap remains a critical challenge for industry implementation.
Metal oxide-based electrodes, including manganese dioxide and titanium dioxide, present a middle-ground option with moderate costs ($50-150/kg) and improved performance characteristics compared to traditional carbon materials. However, their manufacturing complexity and scalability issues continue to limit their market penetration in commercial CDI systems.
Lifecycle cost analysis reveals that while advanced materials require higher initial investment, their extended operational lifespan and reduced maintenance requirements can potentially offset these costs over time. For instance, graphene-based electrodes may last 2-3 times longer than conventional carbon electrodes, reducing replacement frequency and associated operational disruptions.
Energy efficiency calculations demonstrate that high-performance electrode materials can reduce energy consumption by 20-40% compared to conventional materials, translating to significant operational savings in large-scale desalination operations. This energy reduction becomes particularly valuable in regions with high electricity costs or renewable energy integration challenges.
Market analysis indicates a clear correlation between material cost reduction and CDI system adoption rates. Historical data shows that a 30% reduction in electrode material costs typically results in a 15-25% increase in market adoption, highlighting the price sensitivity of this technology sector. Industry projections suggest that achieving a cost threshold below $50/kg for high-performance materials would trigger widespread commercial implementation.
The development of hybrid materials and composite electrodes represents a promising approach to optimizing the cost-performance ratio. These engineered materials strategically combine low-cost carbon substrates with selective high-performance components to achieve enhanced functionality without proportional cost increases. Recent research demonstrates that such hybrid approaches can deliver 70-80% of the performance benefits of premium materials at only 30-40% of their cost.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!