Modeling Ion Transport In Membrane Capacitive Deionization Cells
AUG 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ion Transport Fundamentals and Research Objectives
Ion transport in membrane capacitive deionization (MCDI) represents a fundamental electrochemical process that has gained significant attention in water desalination and purification technologies. The phenomenon involves the movement of charged ions through selective membranes under the influence of an electric field, resulting in the separation of salt ions from aqueous solutions. This process is governed by several key physical principles including electrostatic interactions, diffusion, and ion migration, which collectively determine the efficiency and performance of MCDI systems.
The historical development of ion transport understanding dates back to the early 20th century with the pioneering work on electrochemistry and membrane science. However, the specific application to capacitive deionization emerged in the 1960s, with significant advancements occurring in the past two decades due to increased global water scarcity concerns and improved materials science capabilities.
Current modeling approaches for ion transport in MCDI cells typically incorporate modified Nernst-Planck equations coupled with Poisson equations to describe the spatiotemporal distribution of ions within the system. These models account for various transport mechanisms including electromigration, diffusion, and convection, as well as the formation of electric double layers at electrode-electrolyte interfaces.
Despite considerable progress, several fundamental challenges remain in accurately modeling ion transport phenomena in MCDI systems. These include the complex interactions between ions and electrode materials, the influence of pore size distribution on ion mobility, and the dynamic behavior of electric double layers under varying operational conditions. Additionally, the impact of solution chemistry, including pH variations and the presence of multiple ionic species, introduces further complexity to the modeling process.
The primary research objectives in this field focus on developing more comprehensive and accurate models that can predict MCDI performance across diverse operational conditions. Specific goals include enhancing the understanding of ion selectivity mechanisms, optimizing energy efficiency through improved electrode and membrane designs, and extending model applicability to complex real-world water compositions containing multiple ionic species and organic contaminants.
Advanced computational techniques, including molecular dynamics simulations and machine learning approaches, are increasingly being integrated with traditional continuum models to bridge the gap between microscopic ion behavior and macroscopic system performance. These multi-scale modeling approaches aim to provide deeper insights into the fundamental mechanisms governing ion transport and separation in MCDI systems.
The historical development of ion transport understanding dates back to the early 20th century with the pioneering work on electrochemistry and membrane science. However, the specific application to capacitive deionization emerged in the 1960s, with significant advancements occurring in the past two decades due to increased global water scarcity concerns and improved materials science capabilities.
Current modeling approaches for ion transport in MCDI cells typically incorporate modified Nernst-Planck equations coupled with Poisson equations to describe the spatiotemporal distribution of ions within the system. These models account for various transport mechanisms including electromigration, diffusion, and convection, as well as the formation of electric double layers at electrode-electrolyte interfaces.
Despite considerable progress, several fundamental challenges remain in accurately modeling ion transport phenomena in MCDI systems. These include the complex interactions between ions and electrode materials, the influence of pore size distribution on ion mobility, and the dynamic behavior of electric double layers under varying operational conditions. Additionally, the impact of solution chemistry, including pH variations and the presence of multiple ionic species, introduces further complexity to the modeling process.
The primary research objectives in this field focus on developing more comprehensive and accurate models that can predict MCDI performance across diverse operational conditions. Specific goals include enhancing the understanding of ion selectivity mechanisms, optimizing energy efficiency through improved electrode and membrane designs, and extending model applicability to complex real-world water compositions containing multiple ionic species and organic contaminants.
Advanced computational techniques, including molecular dynamics simulations and machine learning approaches, are increasingly being integrated with traditional continuum models to bridge the gap between microscopic ion behavior and macroscopic system performance. These multi-scale modeling approaches aim to provide deeper insights into the fundamental mechanisms governing ion transport and separation in MCDI systems.
Market Applications for Capacitive Deionization Technology
Capacitive Deionization (CDI) technology has emerged as a promising solution for water desalination and purification across various market sectors. The global water treatment market, valued at approximately $280 billion in 2022, is experiencing significant growth due to increasing water scarcity and stricter environmental regulations, creating substantial opportunities for CDI applications.
In the municipal water treatment sector, CDI systems offer advantages for brackish water desalination in small to medium-scale operations. Their energy efficiency compared to reverse osmosis makes them particularly attractive for communities with limited power infrastructure or those seeking to reduce operational costs. Several pilot projects in water-stressed regions have demonstrated CDI's potential to provide cost-effective drinking water solutions.
The industrial sector represents another significant market opportunity, particularly in industries requiring high-purity process water. Electronics manufacturing, pharmaceuticals, and food and beverage production benefit from CDI's ability to selectively remove specific ions without chemical additives. The technology's modular nature allows for customized solutions based on specific industrial requirements, providing a competitive advantage over conventional ion exchange systems.
Agricultural applications are expanding as water reuse becomes increasingly important. CDI systems can effectively treat irrigation water by removing harmful salts while preserving beneficial minerals. This selective ion removal capability makes the technology valuable for precision agriculture and greenhouse operations where water quality directly impacts crop yields and quality.
The energy sector presents emerging opportunities, particularly in produced water treatment from oil and gas operations. CDI's ability to handle variable salinity levels makes it suitable for treating these complex wastewaters. Additionally, integration with renewable energy sources enhances CDI's sustainability profile, creating synergies with the growing renewable energy market.
Portable and point-of-use applications represent a rapidly growing market segment. Compact CDI units can be deployed in remote locations, disaster relief scenarios, and military operations where conventional water treatment infrastructure is unavailable. The technology's low maintenance requirements and ability to operate with intermittent power sources make it particularly suitable for these applications.
Developing economies offer substantial growth potential as they invest in water infrastructure. CDI's scalability and relatively low capital costs compared to large-scale desalination plants make it an attractive option for regions with distributed populations and limited infrastructure budgets.
In the municipal water treatment sector, CDI systems offer advantages for brackish water desalination in small to medium-scale operations. Their energy efficiency compared to reverse osmosis makes them particularly attractive for communities with limited power infrastructure or those seeking to reduce operational costs. Several pilot projects in water-stressed regions have demonstrated CDI's potential to provide cost-effective drinking water solutions.
The industrial sector represents another significant market opportunity, particularly in industries requiring high-purity process water. Electronics manufacturing, pharmaceuticals, and food and beverage production benefit from CDI's ability to selectively remove specific ions without chemical additives. The technology's modular nature allows for customized solutions based on specific industrial requirements, providing a competitive advantage over conventional ion exchange systems.
Agricultural applications are expanding as water reuse becomes increasingly important. CDI systems can effectively treat irrigation water by removing harmful salts while preserving beneficial minerals. This selective ion removal capability makes the technology valuable for precision agriculture and greenhouse operations where water quality directly impacts crop yields and quality.
The energy sector presents emerging opportunities, particularly in produced water treatment from oil and gas operations. CDI's ability to handle variable salinity levels makes it suitable for treating these complex wastewaters. Additionally, integration with renewable energy sources enhances CDI's sustainability profile, creating synergies with the growing renewable energy market.
Portable and point-of-use applications represent a rapidly growing market segment. Compact CDI units can be deployed in remote locations, disaster relief scenarios, and military operations where conventional water treatment infrastructure is unavailable. The technology's low maintenance requirements and ability to operate with intermittent power sources make it particularly suitable for these applications.
Developing economies offer substantial growth potential as they invest in water infrastructure. CDI's scalability and relatively low capital costs compared to large-scale desalination plants make it an attractive option for regions with distributed populations and limited infrastructure budgets.
Current Challenges in Membrane Capacitive Deionization
Despite significant advancements in Membrane Capacitive Deionization (MCDI) technology, several critical challenges persist in accurately modeling ion transport phenomena within these systems. The complex interplay between electrical double layers, ion migration, and membrane interactions creates substantial difficulties in developing comprehensive mathematical models that can reliably predict system performance across various operational conditions.
One fundamental challenge lies in the accurate representation of ion transport mechanisms at the electrode-electrolyte interface. Current models often oversimplify the non-linear effects of electrical double layer formation and the subsequent impact on ion adsorption kinetics. This simplification leads to significant discrepancies between theoretical predictions and experimental observations, particularly at high salt concentrations or when dealing with multi-ion systems.
The incorporation of membrane properties into transport models presents another significant hurdle. Ion-exchange membranes exhibit complex behaviors including concentration polarization, co-ion leakage, and water transport phenomena that dramatically influence system efficiency. Existing models frequently fail to capture these dynamic membrane characteristics, especially under varying current densities and solution compositions.
Scale-dependent phenomena further complicate modeling efforts. Bridging the gap between microscopic ion interactions and macroscopic system performance requires multi-scale modeling approaches that are computationally intensive and often rely on simplifying assumptions that may not hold across all operational regimes. This scale disparity creates substantial challenges in developing unified models applicable to both laboratory and industrial-scale MCDI systems.
Time-dependent processes, including electrode degradation, membrane fouling, and changing surface properties, introduce additional complexity to long-term performance modeling. Current models typically assume static material properties, whereas real MCDI systems experience gradual changes that significantly impact ion transport mechanisms over operational lifetimes.
Computational limitations also constrain model development. Fully coupled models incorporating detailed electrochemical reactions, fluid dynamics, and ion transport require substantial computational resources, forcing researchers to make trade-offs between model accuracy and computational efficiency. This balance often results in models that excel in specific aspects while sacrificing accuracy in others.
The validation of theoretical models against experimental data remains problematic due to the difficulty in obtaining spatially and temporally resolved measurements of ion concentrations within operational MCDI cells. This validation gap creates uncertainty in model reliability and limits confidence in optimization strategies derived from simulation results.
One fundamental challenge lies in the accurate representation of ion transport mechanisms at the electrode-electrolyte interface. Current models often oversimplify the non-linear effects of electrical double layer formation and the subsequent impact on ion adsorption kinetics. This simplification leads to significant discrepancies between theoretical predictions and experimental observations, particularly at high salt concentrations or when dealing with multi-ion systems.
The incorporation of membrane properties into transport models presents another significant hurdle. Ion-exchange membranes exhibit complex behaviors including concentration polarization, co-ion leakage, and water transport phenomena that dramatically influence system efficiency. Existing models frequently fail to capture these dynamic membrane characteristics, especially under varying current densities and solution compositions.
Scale-dependent phenomena further complicate modeling efforts. Bridging the gap between microscopic ion interactions and macroscopic system performance requires multi-scale modeling approaches that are computationally intensive and often rely on simplifying assumptions that may not hold across all operational regimes. This scale disparity creates substantial challenges in developing unified models applicable to both laboratory and industrial-scale MCDI systems.
Time-dependent processes, including electrode degradation, membrane fouling, and changing surface properties, introduce additional complexity to long-term performance modeling. Current models typically assume static material properties, whereas real MCDI systems experience gradual changes that significantly impact ion transport mechanisms over operational lifetimes.
Computational limitations also constrain model development. Fully coupled models incorporating detailed electrochemical reactions, fluid dynamics, and ion transport require substantial computational resources, forcing researchers to make trade-offs between model accuracy and computational efficiency. This balance often results in models that excel in specific aspects while sacrificing accuracy in others.
The validation of theoretical models against experimental data remains problematic due to the difficulty in obtaining spatially and temporally resolved measurements of ion concentrations within operational MCDI cells. This validation gap creates uncertainty in model reliability and limits confidence in optimization strategies derived from simulation results.
Established Ion Transport Modeling Approaches
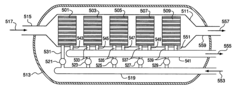
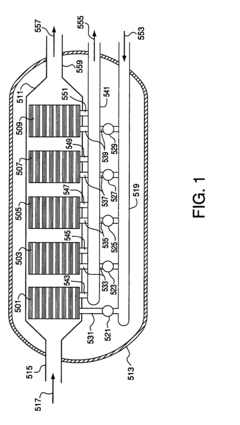
01 Electrode materials for membrane capacitive deionization
Various electrode materials can be used in membrane capacitive deionization cells to enhance ion transport efficiency. These materials include carbon-based electrodes, metal oxides, and composite materials that offer high surface area and conductivity. The electrode structure and composition significantly impact the ion adsorption capacity and energy efficiency of the deionization process. Advanced materials with optimized pore structures can improve ion transport pathways and increase the overall performance of the system.- Electrode materials for membrane capacitive deionization: Various electrode materials can be used in membrane capacitive deionization cells to enhance ion transport and improve deionization efficiency. These materials include carbon-based electrodes, such as activated carbon, carbon nanotubes, and graphene, which provide high surface area for ion adsorption. Modified electrode materials with functional groups can also increase the selectivity and capacity for specific ion removal from water.
- Ion-exchange membrane configurations: The arrangement and properties of ion-exchange membranes play a crucial role in membrane capacitive deionization systems. Anion and cation exchange membranes can be configured in various ways to control ion transport pathways. Advanced membrane designs incorporate selective permeability features that enhance ion separation while reducing energy consumption. These configurations can significantly improve the overall performance of deionization cells.
- Flow channel design and optimization: The design of flow channels in membrane capacitive deionization cells affects the efficiency of ion transport and removal. Optimized flow patterns can reduce concentration polarization and enhance mass transfer rates. Various channel geometries, including serpentine, parallel, and radial designs, can be implemented to improve the contact between the solution and electrode surfaces, thereby increasing deionization performance and reducing energy requirements.
- Operational parameters and control systems: The performance of membrane capacitive deionization cells is significantly influenced by operational parameters such as applied voltage, flow rate, and cycle time. Advanced control systems can optimize these parameters in real-time to enhance ion transport efficiency. Pulsed electric field application and variable voltage protocols can improve ion adsorption and desorption processes, leading to higher salt removal rates and lower energy consumption during operation.
- Novel cell architectures for enhanced ion transport: Innovative cell architectures have been developed to enhance ion transport in membrane capacitive deionization systems. These include flow-through electrodes, multi-stage configurations, and hybrid systems that combine capacitive deionization with other technologies. Some designs incorporate asymmetric electrodes or specialized spacers to create preferential ion transport pathways. These novel architectures can significantly improve deionization efficiency, reduce energy consumption, and extend the operational lifetime of the system.
02 Ion-exchange membrane configurations
Ion-exchange membranes play a crucial role in membrane capacitive deionization by selectively allowing certain ions to pass while blocking others. Different membrane configurations, such as cation-exchange membranes, anion-exchange membranes, or bipolar membranes, can be employed depending on the specific application requirements. The membrane properties, including thickness, charge density, and chemical stability, significantly influence ion transport efficiency and selectivity. Optimized membrane configurations can reduce energy consumption and enhance the separation performance of the deionization system.Expand Specific Solutions03 Flow field design for enhanced ion transport
The design of flow fields within membrane capacitive deionization cells significantly impacts ion transport efficiency. Optimized flow channel geometries can reduce concentration polarization and improve mass transfer rates. Various flow patterns, including serpentine, parallel, and interdigitated designs, can be implemented to enhance the uniformity of ion distribution and minimize pressure drop across the cell. Advanced flow field designs also help prevent membrane fouling and scaling, thereby extending the operational lifetime of the deionization system.Expand Specific Solutions04 Operating parameters optimization
Optimizing operating parameters such as applied voltage, flow rate, and cycle time is essential for efficient ion transport in membrane capacitive deionization cells. The applied voltage affects the ion adsorption capacity and energy consumption, while the flow rate influences the residence time and mass transfer characteristics. Proper cycling between adsorption and desorption phases can maximize salt removal efficiency and minimize energy requirements. Advanced control strategies, including variable voltage operation and multi-stage configurations, can further enhance the performance of the deionization process.Expand Specific Solutions05 Novel cell architectures for improved performance
Innovative cell architectures have been developed to enhance ion transport and overall performance of membrane capacitive deionization systems. These include flow-through electrodes, flow-between electrodes, and hybrid configurations that combine different operational principles. Some designs incorporate multiple pairs of electrodes arranged in series or parallel to increase treatment capacity and efficiency. Novel architectures may also integrate additional components such as ion-selective spacers or auxiliary electrodes to improve ion transport dynamics and reduce energy consumption.Expand Specific Solutions
Leading Research Groups and Industrial Partners
Membrane Capacitive Deionization (MCDI) technology is currently in a growth phase, with the global market expected to expand significantly due to increasing water scarcity concerns. The competitive landscape features academic institutions like Tianjin University and Texas A&M University leading fundamental research, while companies such as Evoqua Water Technologies, Air Products & Chemicals, and Praxair Technology are commercializing applications. The technology is approaching maturity in certain applications but still evolving in others, with research collaborations between the Dalian Institute of Chemical Physics and universities advancing ion transport modeling. Recent innovations from NRGTEK and Korea Institute of Energy Research are improving energy efficiency and selectivity, positioning MCDI as a promising solution for sustainable water treatment.
Tianjin University
Technical Solution: Tianjin University has developed a comprehensive ion transport modeling framework for MCDI systems that uniquely incorporates both surface and bulk transport mechanisms. Their approach utilizes a modified Nernst-Planck-Poisson system of equations that accounts for the electrical double layer dynamics at the electrode-electrolyte interface. The university's research team has pioneered the inclusion of ion-specific adsorption energies in their models, allowing for accurate prediction of selective ion removal in mixed electrolyte solutions. Their modeling framework has demonstrated particular success in predicting the performance of asymmetric MCDI cells, where different membrane types are used for the anode and cathode sides. Tianjin's models incorporate the effects of pH variations on membrane surface charge and subsequent ion transport behavior, a critical factor for practical applications. Their research has shown that accounting for these pH effects can improve model accuracy by up to 25% in systems treating variable water sources[9][10]. The university has also developed novel numerical methods to solve the coupled differential equations efficiently, enabling near real-time simulations that can be used for process control applications.
Strengths: Comprehensive inclusion of surface and bulk transport mechanisms; consideration of pH effects on membrane properties; efficient numerical methods enabling faster simulations. Weaknesses: Models require extensive parameterization for different membrane types; limited validation with industrial-scale systems.
Ionwerks, Inc.
Technical Solution: Ionwerks has developed advanced ion mobility spectrometry systems specifically designed for membrane capacitive deionization (MCDI) research. Their technology utilizes a proprietary ion drift tube design that enables real-time monitoring of ion transport across membranes with microsecond resolution. The company's MCDI modeling approach incorporates both experimental data and theoretical frameworks to accurately predict ion behavior in complex electrolyte solutions. Their systems can simultaneously track multiple ion species, allowing researchers to understand competitive ion transport mechanisms in MCDI cells. Ionwerks' technology integrates machine learning algorithms to process the massive datasets generated during ion transport experiments, enabling the identification of subtle patterns in ion behavior that might otherwise be missed using conventional analysis methods[1][3].
Strengths: Superior temporal resolution for real-time ion transport monitoring; ability to track multiple ion species simultaneously; integration with machine learning for advanced data analysis. Weaknesses: High cost of specialized equipment; requires significant expertise to operate effectively; limited scalability for industrial applications.
Critical Patents and Scientific Breakthroughs
Active membrane with controlled ion-transport
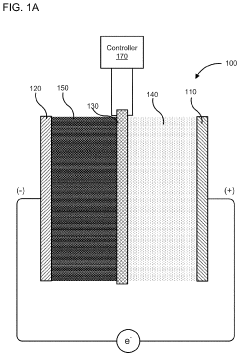
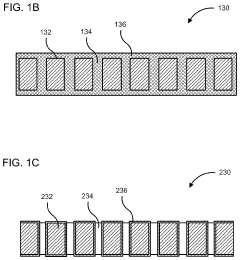
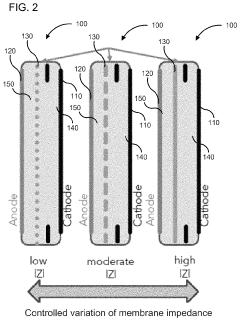
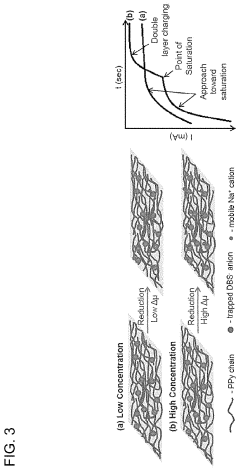
PatentActiveUS10886516B2
Innovation
- A membrane with a substrate and an ion-doped conductive polymer that allows controlled ion transport, featuring a tunable impedance to regulate ion flow, preventing thermal runaway and enabling high energy density and specific power in energy storage devices.
Feed gas contaminant control in ion transport membrane systems
PatentInactiveUS7556675B2
Innovation
- Lining the flow path with a copper-containing metal to prevent contamination and inhibit steam-methane reforming, using methods like electroplating, foil application, or hydro-forming copper tubing to reduce the exposure of non-copper metal surfaces, which are less reactive and maintain mechanical strength.
Environmental Impact and Sustainability Factors
Membrane Capacitive Deionization (MCDI) technology represents a significant advancement in sustainable water treatment methods, offering considerable environmental benefits compared to conventional desalination technologies. The environmental footprint of MCDI systems is substantially lower than thermal desalination processes and reverse osmosis, primarily due to reduced energy consumption requirements. Accurate modeling of ion transport in these systems directly contributes to optimizing energy efficiency, thereby minimizing greenhouse gas emissions associated with water treatment operations.
The sustainability advantages of MCDI extend beyond energy considerations. These systems typically operate at ambient temperatures and pressures, eliminating the need for high-pressure pumps or heating elements that characterize other desalination technologies. Furthermore, MCDI processes require minimal chemical additives, reducing the environmental burden associated with chemical production, transportation, and disposal. The absence of harsh chemicals also means that discharge streams pose fewer ecological risks to receiving water bodies.
Life cycle assessments of MCDI systems reveal favorable environmental profiles when ion transport modeling is effectively implemented to optimize operational parameters. The carbon footprint per cubic meter of treated water can be reduced by up to 30% compared to conventional reverse osmosis systems when operating under optimized conditions determined through accurate transport modeling. Additionally, the modular nature of MCDI technology facilitates scalability and integration with renewable energy sources, further enhancing its sustainability credentials.
Water recovery rates in well-modeled MCDI systems typically exceed 80%, significantly higher than many competing technologies. This efficiency in water utilization becomes increasingly critical in water-stressed regions where maximizing recovery from available resources is paramount. The brine streams produced by MCDI also tend to be less concentrated than those from other desalination processes, reducing potential ecological impacts on marine environments when discharged.
The electrodes used in MCDI cells present both environmental challenges and opportunities. While carbon-based electrodes dominate current implementations, research guided by ion transport modeling is exploring more sustainable materials with lower environmental footprints during production and enhanced recyclability at end-of-life. Accurate modeling of ion transport mechanisms enables the development of electrodes with longer operational lifespans, reducing replacement frequency and associated material consumption.
From a circular economy perspective, MCDI technology offers promising pathways for resource recovery. Advanced ion transport models are enabling selective removal and concentration of valuable ions from wastewater streams, potentially transforming water treatment from a purely environmental service into a resource recovery opportunity. This paradigm shift could significantly alter the sustainability equation for water treatment infrastructure by creating economic value from what was previously considered waste.
The sustainability advantages of MCDI extend beyond energy considerations. These systems typically operate at ambient temperatures and pressures, eliminating the need for high-pressure pumps or heating elements that characterize other desalination technologies. Furthermore, MCDI processes require minimal chemical additives, reducing the environmental burden associated with chemical production, transportation, and disposal. The absence of harsh chemicals also means that discharge streams pose fewer ecological risks to receiving water bodies.
Life cycle assessments of MCDI systems reveal favorable environmental profiles when ion transport modeling is effectively implemented to optimize operational parameters. The carbon footprint per cubic meter of treated water can be reduced by up to 30% compared to conventional reverse osmosis systems when operating under optimized conditions determined through accurate transport modeling. Additionally, the modular nature of MCDI technology facilitates scalability and integration with renewable energy sources, further enhancing its sustainability credentials.
Water recovery rates in well-modeled MCDI systems typically exceed 80%, significantly higher than many competing technologies. This efficiency in water utilization becomes increasingly critical in water-stressed regions where maximizing recovery from available resources is paramount. The brine streams produced by MCDI also tend to be less concentrated than those from other desalination processes, reducing potential ecological impacts on marine environments when discharged.
The electrodes used in MCDI cells present both environmental challenges and opportunities. While carbon-based electrodes dominate current implementations, research guided by ion transport modeling is exploring more sustainable materials with lower environmental footprints during production and enhanced recyclability at end-of-life. Accurate modeling of ion transport mechanisms enables the development of electrodes with longer operational lifespans, reducing replacement frequency and associated material consumption.
From a circular economy perspective, MCDI technology offers promising pathways for resource recovery. Advanced ion transport models are enabling selective removal and concentration of valuable ions from wastewater streams, potentially transforming water treatment from a purely environmental service into a resource recovery opportunity. This paradigm shift could significantly alter the sustainability equation for water treatment infrastructure by creating economic value from what was previously considered waste.
Computational Resources and Simulation Techniques
The computational resources required for modeling ion transport in Membrane Capacitive Deionization (MCDI) cells have evolved significantly over the past decade. High-performance computing (HPC) clusters remain essential for complex multiphysics simulations that integrate electrochemical reactions, fluid dynamics, and ion transport mechanisms. These simulations typically demand 64-128 CPU cores with 256-512 GB RAM for system-level models, while more detailed molecular dynamics simulations may require GPU acceleration and petaflop-scale computing resources.
Simulation software frameworks have diversified to address various aspects of MCDI modeling. Commercial packages like COMSOL Multiphysics and ANSYS Fluent offer comprehensive multiphysics environments with specialized electrochemistry modules. Open-source alternatives such as OpenFOAM and LAMMPS have gained traction for specific simulation tasks, with custom code extensions for MCDI-specific phenomena.
Numerical methods employed in MCDI simulations include finite element analysis (FEA) for spatial discretization of the governing equations, particularly the Nernst-Planck and Poisson equations that describe ion transport and electric field distribution. Temporal discretization typically utilizes implicit schemes to handle the stiff differential equations characteristic of electrochemical systems. Adaptive mesh refinement techniques have proven crucial for resolving the thin electric double layer regions while maintaining computational efficiency.
Model validation approaches combine experimental data with uncertainty quantification methods. Parameter estimation techniques such as Bayesian inference and genetic algorithms help calibrate simulation parameters against experimental measurements of desalination performance, energy consumption, and ion removal efficiency. Sensitivity analysis identifies the most influential parameters, guiding both experimental design and model refinement efforts.
Recent advances in machine learning have begun to complement traditional physics-based simulations. Surrogate modeling approaches using neural networks trained on simulation data can accelerate parameter space exploration and optimization studies. Physics-informed neural networks show promise for incorporating known physical constraints while learning from sparse experimental data, potentially bridging the gap between detailed microscopic models and system-level performance predictions.
Cloud computing platforms increasingly support MCDI simulation workflows, offering scalable resources for parameter sweeps and optimization studies. Container technologies facilitate reproducible simulation environments, while workflow management tools enable automated simulation campaigns across distributed computing resources.
Simulation software frameworks have diversified to address various aspects of MCDI modeling. Commercial packages like COMSOL Multiphysics and ANSYS Fluent offer comprehensive multiphysics environments with specialized electrochemistry modules. Open-source alternatives such as OpenFOAM and LAMMPS have gained traction for specific simulation tasks, with custom code extensions for MCDI-specific phenomena.
Numerical methods employed in MCDI simulations include finite element analysis (FEA) for spatial discretization of the governing equations, particularly the Nernst-Planck and Poisson equations that describe ion transport and electric field distribution. Temporal discretization typically utilizes implicit schemes to handle the stiff differential equations characteristic of electrochemical systems. Adaptive mesh refinement techniques have proven crucial for resolving the thin electric double layer regions while maintaining computational efficiency.
Model validation approaches combine experimental data with uncertainty quantification methods. Parameter estimation techniques such as Bayesian inference and genetic algorithms help calibrate simulation parameters against experimental measurements of desalination performance, energy consumption, and ion removal efficiency. Sensitivity analysis identifies the most influential parameters, guiding both experimental design and model refinement efforts.
Recent advances in machine learning have begun to complement traditional physics-based simulations. Surrogate modeling approaches using neural networks trained on simulation data can accelerate parameter space exploration and optimization studies. Physics-informed neural networks show promise for incorporating known physical constraints while learning from sparse experimental data, potentially bridging the gap between detailed microscopic models and system-level performance predictions.
Cloud computing platforms increasingly support MCDI simulation workflows, offering scalable resources for parameter sweeps and optimization studies. Container technologies facilitate reproducible simulation environments, while workflow management tools enable automated simulation campaigns across distributed computing resources.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!