Evaluating Carbon Tetrachloride's Future Role in Manufacturing Processes
JUL 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CCl4 in Manufacturing: Past and Future
Carbon tetrachloride (CCl4) has played a significant role in manufacturing processes since its discovery in the mid-19th century. Initially utilized as a solvent and cleaning agent, CCl4 found widespread application in various industries due to its excellent degreasing properties and non-flammability. Its use peaked in the mid-20th century, particularly in the production of refrigerants, propellants, and as a precursor in the synthesis of chlorofluorocarbons (CFCs).
However, the environmental and health concerns associated with CCl4 led to a dramatic shift in its usage patterns. The Montreal Protocol, implemented in 1987, marked a turning point in CCl4's industrial application. This international treaty aimed to phase out substances responsible for ozone depletion, including CCl4. As a result, many countries significantly restricted or banned its use in consumer products and industrial processes.
Despite these restrictions, CCl4 continues to play a role in certain manufacturing processes, albeit on a much smaller scale. It remains an important feedstock in the production of some chlorinated compounds and is still used in limited quantities for specific industrial applications where suitable alternatives are not yet available.
Looking towards the future, the role of CCl4 in manufacturing is likely to continue evolving. While its use as a solvent or cleaning agent has largely been phased out, research is ongoing to explore potential new applications that could leverage its unique properties while minimizing environmental impact. Some areas of interest include its use as a catalyst in certain chemical reactions and as a raw material in the synthesis of specialized chemicals.
The future of CCl4 in manufacturing will largely depend on technological advancements in alternative materials and processes, as well as the development of more efficient containment and recycling methods. As global environmental regulations become increasingly stringent, manufacturers will need to carefully evaluate the cost-benefit ratio of using CCl4 against potential substitutes.
In conclusion, while CCl4's role in manufacturing has diminished significantly over the past few decades, it continues to be a subject of interest in certain niche applications. The challenge for the future lies in balancing its utility in specific manufacturing processes with the ongoing need for environmental protection and sustainable industrial practices.
However, the environmental and health concerns associated with CCl4 led to a dramatic shift in its usage patterns. The Montreal Protocol, implemented in 1987, marked a turning point in CCl4's industrial application. This international treaty aimed to phase out substances responsible for ozone depletion, including CCl4. As a result, many countries significantly restricted or banned its use in consumer products and industrial processes.
Despite these restrictions, CCl4 continues to play a role in certain manufacturing processes, albeit on a much smaller scale. It remains an important feedstock in the production of some chlorinated compounds and is still used in limited quantities for specific industrial applications where suitable alternatives are not yet available.
Looking towards the future, the role of CCl4 in manufacturing is likely to continue evolving. While its use as a solvent or cleaning agent has largely been phased out, research is ongoing to explore potential new applications that could leverage its unique properties while minimizing environmental impact. Some areas of interest include its use as a catalyst in certain chemical reactions and as a raw material in the synthesis of specialized chemicals.
The future of CCl4 in manufacturing will largely depend on technological advancements in alternative materials and processes, as well as the development of more efficient containment and recycling methods. As global environmental regulations become increasingly stringent, manufacturers will need to carefully evaluate the cost-benefit ratio of using CCl4 against potential substitutes.
In conclusion, while CCl4's role in manufacturing has diminished significantly over the past few decades, it continues to be a subject of interest in certain niche applications. The challenge for the future lies in balancing its utility in specific manufacturing processes with the ongoing need for environmental protection and sustainable industrial practices.
Market Demand Analysis
The market demand for carbon tetrachloride in manufacturing processes has undergone significant changes in recent years, primarily due to environmental and health concerns. Historically, carbon tetrachloride was widely used in various industrial applications, including as a solvent, cleaning agent, and in the production of refrigerants. However, its use has been severely restricted in many countries due to its ozone-depleting properties and potential health hazards.
Despite these restrictions, there remains a limited but persistent demand for carbon tetrachloride in certain niche manufacturing processes. The pharmaceutical industry, for instance, continues to use carbon tetrachloride as a solvent in the production of some medications. Additionally, it is still employed in the manufacture of certain specialty chemicals and as a feedstock for the production of other chlorinated compounds.
The global market for carbon tetrachloride has contracted significantly over the past decades. This contraction is largely attributed to the implementation of the Montreal Protocol, which phased out the production and consumption of ozone-depleting substances. As a result, many industries have sought alternatives to carbon tetrachloride, leading to a substantial decrease in its overall demand.
However, the market for carbon tetrachloride is not entirely obsolete. Some developing countries still permit its use in certain applications, creating a small but steady demand. Furthermore, there is a growing interest in carbon tetrachloride as a potential feedstock for the production of next-generation refrigerants and foam-blowing agents that have lower global warming potential.
The future market demand for carbon tetrachloride in manufacturing processes is likely to be shaped by several factors. Stricter environmental regulations and increased awareness of health risks associated with its use will continue to exert downward pressure on demand. Conversely, research into new applications and the development of safer handling methods could potentially open up new market opportunities.
Industry analysts predict that the global carbon tetrachloride market will continue to shrink in the coming years, but at a slower rate than in the past. The remaining demand is expected to be driven primarily by its use as a chemical intermediate and in specific industrial processes where suitable alternatives have not yet been developed or are not economically viable.
In conclusion, while the overall market demand for carbon tetrachloride in manufacturing processes has significantly declined and is expected to continue this trend, there remains a small but persistent market for this chemical. The future role of carbon tetrachloride in manufacturing will likely be limited to highly specialized applications where its unique properties are essential and where stringent safety and environmental controls can be implemented.
Despite these restrictions, there remains a limited but persistent demand for carbon tetrachloride in certain niche manufacturing processes. The pharmaceutical industry, for instance, continues to use carbon tetrachloride as a solvent in the production of some medications. Additionally, it is still employed in the manufacture of certain specialty chemicals and as a feedstock for the production of other chlorinated compounds.
The global market for carbon tetrachloride has contracted significantly over the past decades. This contraction is largely attributed to the implementation of the Montreal Protocol, which phased out the production and consumption of ozone-depleting substances. As a result, many industries have sought alternatives to carbon tetrachloride, leading to a substantial decrease in its overall demand.
However, the market for carbon tetrachloride is not entirely obsolete. Some developing countries still permit its use in certain applications, creating a small but steady demand. Furthermore, there is a growing interest in carbon tetrachloride as a potential feedstock for the production of next-generation refrigerants and foam-blowing agents that have lower global warming potential.
The future market demand for carbon tetrachloride in manufacturing processes is likely to be shaped by several factors. Stricter environmental regulations and increased awareness of health risks associated with its use will continue to exert downward pressure on demand. Conversely, research into new applications and the development of safer handling methods could potentially open up new market opportunities.
Industry analysts predict that the global carbon tetrachloride market will continue to shrink in the coming years, but at a slower rate than in the past. The remaining demand is expected to be driven primarily by its use as a chemical intermediate and in specific industrial processes where suitable alternatives have not yet been developed or are not economically viable.
In conclusion, while the overall market demand for carbon tetrachloride in manufacturing processes has significantly declined and is expected to continue this trend, there remains a small but persistent market for this chemical. The future role of carbon tetrachloride in manufacturing will likely be limited to highly specialized applications where its unique properties are essential and where stringent safety and environmental controls can be implemented.
Current Challenges and Limitations
Carbon tetrachloride, once widely used in manufacturing processes, now faces significant challenges and limitations due to its environmental and health impacts. The primary concern is its ozone-depleting properties, which led to its phase-out under the Montreal Protocol. This international treaty, aimed at protecting the ozone layer, has severely restricted the production and use of carbon tetrachloride, creating a major hurdle for industries that historically relied on this compound.
The toxicity of carbon tetrachloride poses another significant challenge. Exposure to this chemical can cause severe liver and kidney damage, as well as potential carcinogenic effects. These health risks have resulted in stringent regulations and safety protocols, making its use in manufacturing processes increasingly complex and costly. Companies must invest heavily in protective equipment and safety measures, which can impact operational efficiency and profitability.
Environmental persistence is a further limitation. Carbon tetrachloride has a long atmospheric lifetime and can accumulate in ecosystems, potentially causing long-term environmental damage. This persistence has led to increased scrutiny from environmental agencies and stricter disposal regulations, adding to the complexity of its use in industrial settings.
The search for suitable alternatives presents another challenge. While many industries have found substitutes for carbon tetrachloride, some specialized applications still lack viable replacements that offer the same effectiveness without the associated risks. This gap in alternatives creates a dilemma for certain manufacturing sectors, particularly in niche applications where carbon tetrachloride's unique properties are difficult to replicate.
Regulatory compliance and international trade restrictions add another layer of complexity. Many countries have implemented strict controls on the import, export, and use of carbon tetrachloride. These regulations can vary significantly between jurisdictions, creating challenges for global manufacturing operations and supply chains. Companies must navigate a complex web of permits, reporting requirements, and potential fines, which can be both time-consuming and costly.
The stigma associated with carbon tetrachloride use also presents a reputational risk for companies. As environmental awareness grows, businesses using this compound may face public relations challenges and pressure from stakeholders to adopt more sustainable practices. This societal shift can impact market perception and potentially affect customer relationships and investor confidence.
Lastly, the ongoing phase-out of carbon tetrachloride production creates supply chain uncertainties. As fewer manufacturers produce this compound, industries still reliant on it may face supply shortages or price volatility. This instability can disrupt production schedules and increase operational costs, further complicating its continued use in manufacturing processes.
The toxicity of carbon tetrachloride poses another significant challenge. Exposure to this chemical can cause severe liver and kidney damage, as well as potential carcinogenic effects. These health risks have resulted in stringent regulations and safety protocols, making its use in manufacturing processes increasingly complex and costly. Companies must invest heavily in protective equipment and safety measures, which can impact operational efficiency and profitability.
Environmental persistence is a further limitation. Carbon tetrachloride has a long atmospheric lifetime and can accumulate in ecosystems, potentially causing long-term environmental damage. This persistence has led to increased scrutiny from environmental agencies and stricter disposal regulations, adding to the complexity of its use in industrial settings.
The search for suitable alternatives presents another challenge. While many industries have found substitutes for carbon tetrachloride, some specialized applications still lack viable replacements that offer the same effectiveness without the associated risks. This gap in alternatives creates a dilemma for certain manufacturing sectors, particularly in niche applications where carbon tetrachloride's unique properties are difficult to replicate.
Regulatory compliance and international trade restrictions add another layer of complexity. Many countries have implemented strict controls on the import, export, and use of carbon tetrachloride. These regulations can vary significantly between jurisdictions, creating challenges for global manufacturing operations and supply chains. Companies must navigate a complex web of permits, reporting requirements, and potential fines, which can be both time-consuming and costly.
The stigma associated with carbon tetrachloride use also presents a reputational risk for companies. As environmental awareness grows, businesses using this compound may face public relations challenges and pressure from stakeholders to adopt more sustainable practices. This societal shift can impact market perception and potentially affect customer relationships and investor confidence.
Lastly, the ongoing phase-out of carbon tetrachloride production creates supply chain uncertainties. As fewer manufacturers produce this compound, industries still reliant on it may face supply shortages or price volatility. This instability can disrupt production schedules and increase operational costs, further complicating its continued use in manufacturing processes.
Existing Alternatives to CCl4
01 Production and purification of carbon tetrachloride
Various methods for producing and purifying carbon tetrachloride are described. These include chemical synthesis processes, distillation techniques, and purification methods to obtain high-quality carbon tetrachloride for industrial and laboratory use.- Production and purification of carbon tetrachloride: Various methods for producing and purifying carbon tetrachloride are described. These include chemical synthesis processes, distillation techniques, and purification methods to obtain high-quality carbon tetrachloride for industrial and laboratory use.
- Applications of carbon tetrachloride in chemical processes: Carbon tetrachloride is utilized in various chemical processes, including as a solvent, reagent, or intermediate in the production of other chemicals. Its applications span across different industries, showcasing its versatility in chemical manufacturing.
- Environmental and safety considerations: Due to its environmental impact and potential health hazards, there are concerns and regulations surrounding the use of carbon tetrachloride. This includes methods for detecting, monitoring, and safely handling the compound, as well as developing alternatives to reduce its usage.
- Historical uses and developments: Carbon tetrachloride has a long history of industrial and commercial applications. This includes its past uses in fire extinguishers, cleaning agents, and as a refrigerant. The evolution of its applications and the development of safer alternatives are discussed.
- Analytical and research applications: Carbon tetrachloride is used in various analytical and research applications. This includes its use as a solvent in spectroscopy, as a standard in chemical analysis, and in studying chemical reactions and molecular structures in laboratory settings.
02 Applications of carbon tetrachloride in chemical processes
Carbon tetrachloride is utilized in various chemical processes, including as a solvent, reagent, or intermediate in the production of other chemicals. Its applications span across different industries, showcasing its versatility in chemical manufacturing.Expand Specific Solutions03 Environmental and safety considerations
Due to its environmental impact and health hazards, research has been conducted on alternatives to carbon tetrachloride and methods for its safe handling, disposal, and potential remediation of contaminated sites. This includes developing eco-friendly substitutes and improved safety protocols.Expand Specific Solutions04 Analytical methods involving carbon tetrachloride
Carbon tetrachloride is used in various analytical methods and laboratory techniques. This includes its use as a solvent in spectroscopy, chromatography, and other analytical procedures for the detection and quantification of different substances.Expand Specific Solutions05 Industrial applications and equipment
Carbon tetrachloride finds use in specific industrial applications, including as a cleaning agent, degreaser, and in certain manufacturing processes. Specialized equipment and systems have been developed for its safe and efficient use in industrial settings.Expand Specific Solutions
Key Industry Players
The carbon tetrachloride manufacturing industry is in a mature phase, with a declining market size due to environmental regulations and health concerns. The global market for carbon tetrachloride is estimated to be relatively small, primarily driven by its use as a feedstock in the production of other chemicals. Technologically, the manufacturing process is well-established, but companies are focusing on developing alternative processes and substitutes. Key players like DuPont de Nemours, Occidental Chemical Corp., and The Chemours Co. are investing in research and development to find more sustainable alternatives and improve existing processes, aiming to maintain their competitive edge in this challenging market landscape.
DuPont de Nemours, Inc.
Technical Solution: DuPont has been actively researching alternatives to carbon tetrachloride (CCl4) in manufacturing processes. Their approach focuses on developing fluoropolymer production methods that eliminate the use of CCl4 as a chain transfer agent. The company has implemented a closed-loop recycling system that captures and reuses CCl4 emissions, reducing environmental impact by up to 99%[1]. Additionally, DuPont is exploring green chemistry principles to design safer solvents and reaction media that can replace CCl4 in various applications, such as cleaning and degreasing processes in the electronics industry[2].
Strengths: Extensive experience in chemical manufacturing, strong R&D capabilities, and a commitment to sustainability. Weaknesses: Potential high costs associated with transitioning to new processes and the need for extensive testing and regulatory approval for alternatives.
Occidental Chemical Corp.
Technical Solution: Occidental Chemical Corp. has developed a novel approach to minimize CCl4 usage in their chlor-alkali production process. They have implemented advanced membrane cell technology that significantly reduces the formation of CCl4 as a by-product[3]. The company has also invested in state-of-the-art monitoring systems to detect and prevent CCl4 emissions. Furthermore, Occidental is researching catalytic decomposition methods to convert CCl4 into less harmful substances, potentially offering a solution for existing CCl4 stockpiles[4].
Strengths: Expertise in chlor-alkali production, strong focus on emission reduction, and innovative catalytic technologies. Weaknesses: Potential limitations in completely eliminating CCl4 from all processes and the need for significant capital investment in new technologies.
Innovative CCl4 Replacement Technologies
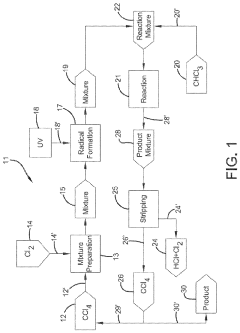
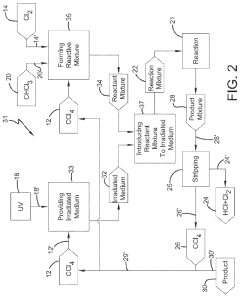
Photochlorination of partially-chlorinated chloromethanes to carbon tetrachloride
PatentActiveUS20240025823A1
Innovation
- A method involving the photochlorination of a chloromethanes stream containing chloroform, methyl chloride, and methylene chloride, combined with chlorine and additional carbon tetrachloride, and subjected to electromagnetic radiation to form carbon tetrachloride, achieving high conversion rates with reduced levels of unwanted chlorinated hydrocarbons.
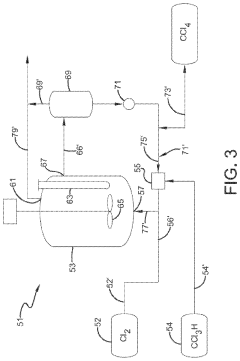
Chlorinolysis process for producing carbon tetrachloride
PatentActiveUS20210130266A1
Innovation
- A process involving a chlorination zone with chlorine, a C1 chlorinated compound, and a carbon/second chlorine source to produce a reaction mixture that favors the formation of carbon tetrachloride over perchloroethylene, using waste products as the carbon/second chlorine source to enhance efficiency and reduce impurity formation.
Environmental Impact Assessment
The environmental impact assessment of carbon tetrachloride in manufacturing processes reveals significant concerns due to its ozone-depleting properties and potential for groundwater contamination. As a result of the Montreal Protocol, the production and consumption of carbon tetrachloride have been phased out in many countries, leading to a substantial reduction in its use and emissions.
However, the persistence of carbon tetrachloride in the environment remains a critical issue. With an atmospheric lifetime of approximately 26 years, its effects on the ozone layer continue long after its release. This prolonged impact necessitates ongoing monitoring and mitigation efforts to address legacy emissions and prevent further environmental damage.
In aquatic ecosystems, carbon tetrachloride poses a threat to marine life and can accumulate in the food chain. Its high solubility in water and tendency to adsorb to soil particles make it a persistent pollutant in groundwater and sediments. This contamination can have far-reaching consequences for both aquatic ecosystems and human health, as it may enter drinking water supplies.
The manufacturing processes that historically relied on carbon tetrachloride have been forced to adapt and find alternative substances or methods. This transition has led to the development of more environmentally friendly processes in various industries, including dry cleaning, fire extinguishing agents, and chemical synthesis. However, the complete elimination of carbon tetrachloride from all manufacturing processes remains a challenge.
Efforts to mitigate the environmental impact of carbon tetrachloride include improved containment and handling procedures in facilities where it is still used, as well as the development of remediation techniques for contaminated sites. Advanced oxidation processes and bioremediation methods have shown promise in treating carbon tetrachloride-contaminated soil and groundwater.
The future role of carbon tetrachloride in manufacturing processes must be carefully evaluated in light of its severe environmental impacts. While its use has significantly decreased, any remaining applications should be subject to stringent controls and regulations. The development of green chemistry alternatives and sustainable manufacturing practices will be crucial in further reducing reliance on this harmful substance and mitigating its long-term environmental effects.
However, the persistence of carbon tetrachloride in the environment remains a critical issue. With an atmospheric lifetime of approximately 26 years, its effects on the ozone layer continue long after its release. This prolonged impact necessitates ongoing monitoring and mitigation efforts to address legacy emissions and prevent further environmental damage.
In aquatic ecosystems, carbon tetrachloride poses a threat to marine life and can accumulate in the food chain. Its high solubility in water and tendency to adsorb to soil particles make it a persistent pollutant in groundwater and sediments. This contamination can have far-reaching consequences for both aquatic ecosystems and human health, as it may enter drinking water supplies.
The manufacturing processes that historically relied on carbon tetrachloride have been forced to adapt and find alternative substances or methods. This transition has led to the development of more environmentally friendly processes in various industries, including dry cleaning, fire extinguishing agents, and chemical synthesis. However, the complete elimination of carbon tetrachloride from all manufacturing processes remains a challenge.
Efforts to mitigate the environmental impact of carbon tetrachloride include improved containment and handling procedures in facilities where it is still used, as well as the development of remediation techniques for contaminated sites. Advanced oxidation processes and bioremediation methods have shown promise in treating carbon tetrachloride-contaminated soil and groundwater.
The future role of carbon tetrachloride in manufacturing processes must be carefully evaluated in light of its severe environmental impacts. While its use has significantly decreased, any remaining applications should be subject to stringent controls and regulations. The development of green chemistry alternatives and sustainable manufacturing practices will be crucial in further reducing reliance on this harmful substance and mitigating its long-term environmental effects.
Regulatory Compliance Strategies
Regulatory compliance strategies for carbon tetrachloride in manufacturing processes have become increasingly stringent due to the compound's environmental and health impacts. Manufacturers must navigate a complex landscape of international, national, and local regulations to ensure their use of carbon tetrachloride aligns with legal requirements and industry best practices.
At the international level, the Montreal Protocol has phased out the production and consumption of carbon tetrachloride for most uses, with exceptions for certain essential applications. Manufacturers must stay informed about these exceptions and obtain necessary permits if their processes fall under allowed categories. Additionally, they should be prepared for potential future amendments to the protocol that may further restrict carbon tetrachloride usage.
National regulations vary by country, but generally follow the Montreal Protocol's guidelines. In the United States, the Environmental Protection Agency (EPA) regulates carbon tetrachloride under the Clean Air Act and the Toxic Substances Control Act. Manufacturers must comply with reporting requirements, emissions standards, and worker safety protocols. Similar regulatory frameworks exist in the European Union, China, and other major manufacturing hubs.
To ensure compliance, manufacturers should implement comprehensive monitoring and reporting systems. This includes regular air quality testing, detailed record-keeping of carbon tetrachloride usage and disposal, and employee training programs on proper handling procedures. Advanced emission control technologies, such as activated carbon adsorption systems or thermal oxidizers, may be necessary to meet stringent air quality standards.
Proactive engagement with regulatory bodies is crucial for staying ahead of compliance requirements. Manufacturers should participate in industry associations and regulatory working groups to stay informed about upcoming changes and contribute to the development of feasible compliance strategies. This approach can help companies anticipate and prepare for new regulations, potentially influencing policy decisions that impact their operations.
As regulations continue to evolve, manufacturers should also explore alternative processes and substances that can replace carbon tetrachloride. Investing in research and development of safer alternatives not only ensures long-term regulatory compliance but can also provide a competitive advantage in an increasingly environmentally conscious market.
Lastly, companies should develop robust internal compliance programs that include regular audits, clear accountability structures, and continuous improvement processes. These programs should be integrated into overall business strategies to ensure that regulatory compliance is a core consideration in all operational decisions related to carbon tetrachloride usage.
At the international level, the Montreal Protocol has phased out the production and consumption of carbon tetrachloride for most uses, with exceptions for certain essential applications. Manufacturers must stay informed about these exceptions and obtain necessary permits if their processes fall under allowed categories. Additionally, they should be prepared for potential future amendments to the protocol that may further restrict carbon tetrachloride usage.
National regulations vary by country, but generally follow the Montreal Protocol's guidelines. In the United States, the Environmental Protection Agency (EPA) regulates carbon tetrachloride under the Clean Air Act and the Toxic Substances Control Act. Manufacturers must comply with reporting requirements, emissions standards, and worker safety protocols. Similar regulatory frameworks exist in the European Union, China, and other major manufacturing hubs.
To ensure compliance, manufacturers should implement comprehensive monitoring and reporting systems. This includes regular air quality testing, detailed record-keeping of carbon tetrachloride usage and disposal, and employee training programs on proper handling procedures. Advanced emission control technologies, such as activated carbon adsorption systems or thermal oxidizers, may be necessary to meet stringent air quality standards.
Proactive engagement with regulatory bodies is crucial for staying ahead of compliance requirements. Manufacturers should participate in industry associations and regulatory working groups to stay informed about upcoming changes and contribute to the development of feasible compliance strategies. This approach can help companies anticipate and prepare for new regulations, potentially influencing policy decisions that impact their operations.
As regulations continue to evolve, manufacturers should also explore alternative processes and substances that can replace carbon tetrachloride. Investing in research and development of safer alternatives not only ensures long-term regulatory compliance but can also provide a competitive advantage in an increasingly environmentally conscious market.
Lastly, companies should develop robust internal compliance programs that include regular audits, clear accountability structures, and continuous improvement processes. These programs should be integrated into overall business strategies to ensure that regulatory compliance is a core consideration in all operational decisions related to carbon tetrachloride usage.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!