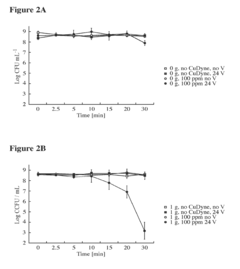
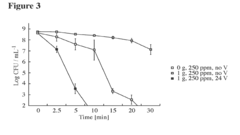
How to Expand Knowledge of Carbon Tetrachloride Properties?
JUL 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CCl4 Research Background and Objectives
Carbon tetrachloride (CCl4) has been a subject of scientific interest and industrial application for over a century. This compound, first synthesized in the 1840s, has played a significant role in various fields, including chemistry, industry, and environmental science. The evolution of our understanding of CCl4 properties has been closely tied to advancements in analytical techniques and the growing awareness of its environmental impact.
The primary objective of expanding knowledge about carbon tetrachloride properties is to address the complex challenges associated with its use and presence in the environment. Despite its ban in consumer products due to ozone depletion concerns, CCl4 remains relevant in industrial processes and as a subject of environmental remediation efforts. Comprehensive understanding of its properties is crucial for developing effective strategies for its detection, containment, and potential alternatives.
Recent technological advancements have opened new avenues for investigating CCl4 properties at molecular and atomic levels. High-resolution spectroscopy, advanced computational modeling, and sophisticated environmental monitoring techniques have enabled researchers to delve deeper into the compound's behavior under various conditions. These developments have shed light on previously unknown aspects of CCl4's reactivity, transport mechanisms in different media, and its interactions with other substances.
The global scientific community has recognized the need for a multidisciplinary approach to expand knowledge of CCl4 properties. This involves collaboration among chemists, environmental scientists, toxicologists, and materials engineers. The goal is to create a comprehensive profile of CCl4, encompassing its physical, chemical, and biological properties, as well as its environmental fate and transport characteristics.
One of the key focus areas in current research is the investigation of CCl4's behavior in complex environmental systems. This includes studying its interactions with soil particles, its migration through groundwater, and its transformation in the atmosphere. Understanding these processes is vital for predicting the long-term environmental impact of historical CCl4 releases and developing more effective remediation strategies.
Another important aspect of expanding knowledge about CCl4 properties is the exploration of its potential applications in emerging fields. While its use as a solvent and cleaning agent has been phased out, researchers are investigating its properties for possible applications in advanced materials science and nanotechnology. This requires a deeper understanding of its molecular structure and reactivity under controlled conditions.
The technological trajectory for CCl4 research is expected to leverage cutting-edge analytical tools and methodologies. This includes the use of synchrotron radiation facilities for detailed structural analysis, advanced mass spectrometry for trace detection, and machine learning algorithms for predicting its behavior in complex systems. These approaches aim to fill the gaps in our current knowledge and provide a more nuanced understanding of CCl4 properties.
The primary objective of expanding knowledge about carbon tetrachloride properties is to address the complex challenges associated with its use and presence in the environment. Despite its ban in consumer products due to ozone depletion concerns, CCl4 remains relevant in industrial processes and as a subject of environmental remediation efforts. Comprehensive understanding of its properties is crucial for developing effective strategies for its detection, containment, and potential alternatives.
Recent technological advancements have opened new avenues for investigating CCl4 properties at molecular and atomic levels. High-resolution spectroscopy, advanced computational modeling, and sophisticated environmental monitoring techniques have enabled researchers to delve deeper into the compound's behavior under various conditions. These developments have shed light on previously unknown aspects of CCl4's reactivity, transport mechanisms in different media, and its interactions with other substances.
The global scientific community has recognized the need for a multidisciplinary approach to expand knowledge of CCl4 properties. This involves collaboration among chemists, environmental scientists, toxicologists, and materials engineers. The goal is to create a comprehensive profile of CCl4, encompassing its physical, chemical, and biological properties, as well as its environmental fate and transport characteristics.
One of the key focus areas in current research is the investigation of CCl4's behavior in complex environmental systems. This includes studying its interactions with soil particles, its migration through groundwater, and its transformation in the atmosphere. Understanding these processes is vital for predicting the long-term environmental impact of historical CCl4 releases and developing more effective remediation strategies.
Another important aspect of expanding knowledge about CCl4 properties is the exploration of its potential applications in emerging fields. While its use as a solvent and cleaning agent has been phased out, researchers are investigating its properties for possible applications in advanced materials science and nanotechnology. This requires a deeper understanding of its molecular structure and reactivity under controlled conditions.
The technological trajectory for CCl4 research is expected to leverage cutting-edge analytical tools and methodologies. This includes the use of synchrotron radiation facilities for detailed structural analysis, advanced mass spectrometry for trace detection, and machine learning algorithms for predicting its behavior in complex systems. These approaches aim to fill the gaps in our current knowledge and provide a more nuanced understanding of CCl4 properties.
Industrial Applications and Market Demand
Carbon tetrachloride, a versatile chemical compound, has found extensive applications across various industries due to its unique properties. The market demand for this substance has been primarily driven by its use as a solvent, cleaning agent, and intermediate in chemical synthesis. In the past, carbon tetrachloride was widely employed as a dry cleaning solvent and fire extinguishing agent. However, environmental concerns and health risks associated with its use have led to significant changes in its industrial applications and market dynamics.
The pharmaceutical industry continues to be a major consumer of carbon tetrachloride, utilizing it as a solvent in drug manufacturing processes. Its ability to dissolve a wide range of organic compounds makes it valuable in the extraction and purification of pharmaceutical ingredients. Additionally, the chemical industry relies on carbon tetrachloride as a feedstock for the production of chlorofluorocarbons (CFCs) and their alternatives, although this application has diminished due to the phase-out of ozone-depleting substances.
In the agrochemical sector, carbon tetrachloride serves as a precursor in the synthesis of various pesticides and herbicides. Its reactivity and stability make it an essential component in the production of these agricultural chemicals. The electronics industry also utilizes carbon tetrachloride in the manufacturing of semiconductors and as a cleaning agent for electronic components, albeit in limited quantities due to environmental regulations.
Despite its industrial utility, the market demand for carbon tetrachloride has experienced a significant decline in recent years. This downward trend can be attributed to increased awareness of its environmental impact and the implementation of stringent regulations aimed at reducing its use and emissions. The Montreal Protocol, an international treaty designed to protect the ozone layer, has played a crucial role in phasing out the production and consumption of carbon tetrachloride for non-feedstock applications.
As a result of these regulatory pressures, industries have been actively seeking alternatives to carbon tetrachloride. This shift has led to the development of new technologies and substitute chemicals that offer similar functionalities with reduced environmental and health risks. The market has witnessed a growing demand for these alternatives, particularly in sectors such as refrigeration, fire suppression, and industrial cleaning.
Despite the overall decline, there remains a niche market for carbon tetrachloride in specific applications where suitable alternatives are yet to be developed or implemented. Research and development efforts continue to focus on expanding the knowledge of carbon tetrachloride properties to identify potential new applications or improve existing ones while minimizing environmental impact. This ongoing research may uncover novel uses for carbon tetrachloride in emerging technologies or specialized industrial processes, potentially creating new market opportunities.
The pharmaceutical industry continues to be a major consumer of carbon tetrachloride, utilizing it as a solvent in drug manufacturing processes. Its ability to dissolve a wide range of organic compounds makes it valuable in the extraction and purification of pharmaceutical ingredients. Additionally, the chemical industry relies on carbon tetrachloride as a feedstock for the production of chlorofluorocarbons (CFCs) and their alternatives, although this application has diminished due to the phase-out of ozone-depleting substances.
In the agrochemical sector, carbon tetrachloride serves as a precursor in the synthesis of various pesticides and herbicides. Its reactivity and stability make it an essential component in the production of these agricultural chemicals. The electronics industry also utilizes carbon tetrachloride in the manufacturing of semiconductors and as a cleaning agent for electronic components, albeit in limited quantities due to environmental regulations.
Despite its industrial utility, the market demand for carbon tetrachloride has experienced a significant decline in recent years. This downward trend can be attributed to increased awareness of its environmental impact and the implementation of stringent regulations aimed at reducing its use and emissions. The Montreal Protocol, an international treaty designed to protect the ozone layer, has played a crucial role in phasing out the production and consumption of carbon tetrachloride for non-feedstock applications.
As a result of these regulatory pressures, industries have been actively seeking alternatives to carbon tetrachloride. This shift has led to the development of new technologies and substitute chemicals that offer similar functionalities with reduced environmental and health risks. The market has witnessed a growing demand for these alternatives, particularly in sectors such as refrigeration, fire suppression, and industrial cleaning.
Despite the overall decline, there remains a niche market for carbon tetrachloride in specific applications where suitable alternatives are yet to be developed or implemented. Research and development efforts continue to focus on expanding the knowledge of carbon tetrachloride properties to identify potential new applications or improve existing ones while minimizing environmental impact. This ongoing research may uncover novel uses for carbon tetrachloride in emerging technologies or specialized industrial processes, potentially creating new market opportunities.
Current Understanding and Challenges
Carbon tetrachloride (CCl4) has been extensively studied due to its historical industrial applications and environmental impact. Current understanding of its properties is comprehensive, yet challenges remain in expanding this knowledge further.
The physical properties of carbon tetrachloride are well-established. It is a colorless liquid at room temperature with a characteristic sweet odor. Its boiling point is 76.72°C, and it has a density of 1.594 g/cm³ at 20°C. These properties have been accurately measured and documented in numerous scientific publications and databases.
Chemically, carbon tetrachloride is known for its stability and low reactivity under normal conditions. It is non-flammable and resistant to hydrolysis, making it historically useful as a solvent and fire extinguishing agent. However, its reactivity increases under certain conditions, such as high temperatures or in the presence of strong oxidizing agents.
The toxicological profile of carbon tetrachloride has been extensively studied. It is recognized as a potent hepatotoxin, capable of causing severe liver damage through the formation of free radicals. Its carcinogenic potential has also been established, leading to its classification as a Group 2B carcinogen by the International Agency for Research on Cancer (IARC).
Environmental behavior of carbon tetrachloride is another area of significant research. Its long atmospheric lifetime and ozone-depleting potential have been well-documented, leading to its regulation under the Montreal Protocol. Studies have also focused on its persistence in soil and groundwater, as well as its bioaccumulation potential in aquatic ecosystems.
Despite this wealth of knowledge, several challenges persist in expanding our understanding of carbon tetrachloride properties. One major challenge is the need for more accurate and comprehensive data on its behavior under extreme conditions, such as high pressures or temperatures encountered in deep geological formations. This information is crucial for predicting its long-term fate in the environment and developing more effective remediation strategies.
Another challenge lies in fully elucidating the mechanisms of carbon tetrachloride toxicity at the molecular level. While the general pathways of liver damage are known, a more detailed understanding of its interactions with cellular components and signaling pathways could lead to improved treatment strategies for exposure cases.
Furthermore, there is a need for more refined models of carbon tetrachloride's atmospheric chemistry and its interactions with other pollutants. This could enhance our ability to predict its impact on climate change and stratospheric ozone depletion more accurately.
Lastly, the development of more sensitive and specific analytical techniques for detecting and quantifying carbon tetrachloride at trace levels in various environmental matrices remains an ongoing challenge. Improving these methods could lead to better monitoring of environmental contamination and more effective cleanup efforts.
The physical properties of carbon tetrachloride are well-established. It is a colorless liquid at room temperature with a characteristic sweet odor. Its boiling point is 76.72°C, and it has a density of 1.594 g/cm³ at 20°C. These properties have been accurately measured and documented in numerous scientific publications and databases.
Chemically, carbon tetrachloride is known for its stability and low reactivity under normal conditions. It is non-flammable and resistant to hydrolysis, making it historically useful as a solvent and fire extinguishing agent. However, its reactivity increases under certain conditions, such as high temperatures or in the presence of strong oxidizing agents.
The toxicological profile of carbon tetrachloride has been extensively studied. It is recognized as a potent hepatotoxin, capable of causing severe liver damage through the formation of free radicals. Its carcinogenic potential has also been established, leading to its classification as a Group 2B carcinogen by the International Agency for Research on Cancer (IARC).
Environmental behavior of carbon tetrachloride is another area of significant research. Its long atmospheric lifetime and ozone-depleting potential have been well-documented, leading to its regulation under the Montreal Protocol. Studies have also focused on its persistence in soil and groundwater, as well as its bioaccumulation potential in aquatic ecosystems.
Despite this wealth of knowledge, several challenges persist in expanding our understanding of carbon tetrachloride properties. One major challenge is the need for more accurate and comprehensive data on its behavior under extreme conditions, such as high pressures or temperatures encountered in deep geological formations. This information is crucial for predicting its long-term fate in the environment and developing more effective remediation strategies.
Another challenge lies in fully elucidating the mechanisms of carbon tetrachloride toxicity at the molecular level. While the general pathways of liver damage are known, a more detailed understanding of its interactions with cellular components and signaling pathways could lead to improved treatment strategies for exposure cases.
Furthermore, there is a need for more refined models of carbon tetrachloride's atmospheric chemistry and its interactions with other pollutants. This could enhance our ability to predict its impact on climate change and stratospheric ozone depletion more accurately.
Lastly, the development of more sensitive and specific analytical techniques for detecting and quantifying carbon tetrachloride at trace levels in various environmental matrices remains an ongoing challenge. Improving these methods could lead to better monitoring of environmental contamination and more effective cleanup efforts.
Existing Methodologies for CCl4 Study
01 Physical properties of carbon tetrachloride
Carbon tetrachloride is a colorless, volatile liquid with a characteristic odor. It has a high density and low boiling point, making it useful in various industrial applications. Its physical properties, such as its non-flammability and ability to dissolve many organic compounds, have made it a popular solvent in the past.- Physical properties of carbon tetrachloride: Carbon tetrachloride is a colorless, volatile liquid with a characteristic odor. It has a high density and low boiling point, making it useful in various industrial applications. Its non-flammable nature and ability to dissolve many organic compounds contribute to its historical use as a solvent and cleaning agent.
- Chemical properties and reactions: Carbon tetrachloride is a relatively inert compound but can undergo various chemical reactions under specific conditions. It can be used as a source of chlorine in organic synthesis and can participate in free radical reactions. Its stability and reactivity properties make it valuable in certain chemical processes and analytical methods.
- Environmental impact and toxicity: Carbon tetrachloride is known for its negative environmental impact and toxicity. It is a potent ozone-depleting substance and has been phased out in many applications due to environmental concerns. Exposure to carbon tetrachloride can cause liver and kidney damage, and it is classified as a potential carcinogen.
- Industrial applications and alternatives: Historically, carbon tetrachloride was widely used in fire extinguishers, as a refrigerant, and in the production of chlorofluorocarbons. Due to its harmful effects, many industries have sought alternatives. Current research focuses on developing safer substitutes and improving processes to eliminate or reduce the use of carbon tetrachloride.
- Detection and analysis methods: Various analytical techniques have been developed for the detection and quantification of carbon tetrachloride in environmental and industrial samples. These methods include gas chromatography, mass spectrometry, and spectrophotometric techniques. Accurate detection is crucial for monitoring environmental contamination and ensuring workplace safety.
02 Chemical properties and reactions
Carbon tetrachloride is a highly stable compound due to its symmetrical tetrahedral structure. It is non-polar and inert to many chemical reactions. However, it can undergo reactions under specific conditions, such as hydrolysis in the presence of strong bases or reduction to form chloroform. Its chemical properties have been utilized in various industrial processes and organic synthesis.Expand Specific Solutions03 Environmental impact and toxicity
Carbon tetrachloride has been recognized as an environmental pollutant and a health hazard. It is known to deplete the ozone layer and contribute to global warming. Exposure to carbon tetrachloride can cause liver and kidney damage, as well as central nervous system depression. Due to these concerns, its use has been heavily restricted or banned in many countries.Expand Specific Solutions04 Industrial applications and alternatives
Historically, carbon tetrachloride was widely used as a solvent, cleaning agent, and in the production of refrigerants. However, due to its environmental and health risks, many industries have sought alternatives. Modern applications focus on finding safer substitutes or developing processes that minimize its use while maintaining effectiveness in specific industrial processes.Expand Specific Solutions05 Detection and analysis methods
Various analytical techniques have been developed to detect and quantify carbon tetrachloride in environmental samples and industrial products. These methods include gas chromatography, mass spectrometry, and spectrophotometric techniques. Accurate detection is crucial for monitoring environmental contamination and ensuring compliance with regulations limiting its use and release.Expand Specific Solutions
Key Research Institutions and Companies
The carbon tetrachloride properties market is in a mature stage, with a relatively stable global market size estimated around $300-400 million annually. The technology for producing and utilizing carbon tetrachloride is well-established, with key players like Occidental Chemical Corp., Tronox LLC, and Jiangsu Lee & Man Chemical Ltd. having extensive experience in chlorinated solvents. Research institutions such as Central South University and Zhejiang University of Technology continue to explore new applications and improved production methods. However, due to environmental concerns and regulations, the market focus has shifted towards finding safer alternatives and optimizing existing processes rather than expanding production capacity.
Tronox LLC
Technical Solution: Tronox LLC has developed advanced spectroscopic techniques to expand knowledge of carbon tetrachloride properties. Their approach combines Raman spectroscopy and infrared spectroscopy to provide detailed information about the molecular structure and vibrational modes of carbon tetrachloride[1]. This method allows for the identification of impurities and structural changes under various conditions. Additionally, Tronox has implemented high-pressure studies to investigate the behavior of carbon tetrachloride at extreme conditions, revealing phase transitions and changes in intermolecular interactions[3]. The company has also invested in computational chemistry tools to model and predict carbon tetrachloride properties in different environments, enhancing their understanding of its behavior in industrial processes[5].
Strengths: Comprehensive spectroscopic analysis, high-pressure experimental capabilities, and computational modeling. Weaknesses: Limited focus on environmental impact and potential alternatives to carbon tetrachloride usage.
Occidental Chemical Corp.
Technical Solution: Occidental Chemical Corp. has focused on expanding knowledge of carbon tetrachloride properties through innovative analytical techniques and environmental fate studies. They have developed a novel gas chromatography-mass spectrometry (GC-MS) method for ultra-trace analysis of carbon tetrachloride in various matrices, including water and soil samples[2]. This technique allows for the detection of carbon tetrachloride at concentrations as low as 0.1 parts per billion, significantly improving environmental monitoring capabilities. Additionally, Occidental has conducted extensive research on the degradation pathways of carbon tetrachloride in natural environments, including studies on its transformation in groundwater and soil systems[4]. Their research has also explored the potential for bioremediation of carbon tetrachloride contamination, investigating microbial communities capable of degrading this compound under anaerobic conditions[6].
Strengths: Advanced analytical capabilities for environmental monitoring and comprehensive understanding of environmental fate. Weaknesses: Limited focus on industrial applications and physical property characterization.
Critical Discoveries in CCl4 Properties
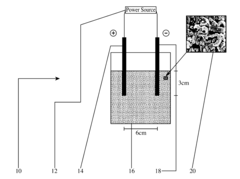
Electrochemical decontamination cells
PatentActiveUS20170029298A1
Innovation
- A method involving a filter material with a porous carbon support layer and silicate/glass wool, where an electric current is passed through to generate reductive and oxidative chemical species, allowing halogens or halide salts to distribute within the material, and contaminants are adsorbed and removed from the fluid stream onto the filter material.
Odor-reduction system and materials
PatentActiveUS20180334395A1
Innovation
- A method involving a filter material with a porous carbon support layer and silicate material, where an electric current is passed through to generate reductive and oxidative chemical species, allowing for the disinfection and removal of contaminants by distributing halogens or halides within the filter material, thereby regenerating the activated carbon.
Environmental Impact and Regulations
Carbon tetrachloride, once widely used in various industrial applications, has been recognized as a significant environmental pollutant with severe impacts on both human health and ecosystems. The environmental impact of carbon tetrachloride is primarily due to its persistence in the atmosphere and its role in ozone depletion. When released into the air, it can remain for up to 50 years, contributing to the destruction of the ozone layer and potentially exacerbating global warming.
In aquatic environments, carbon tetrachloride can accumulate in sediments and bioaccumulate in marine organisms, posing risks to aquatic ecosystems and potentially entering the food chain. Its presence in soil can lead to groundwater contamination, affecting drinking water sources and agricultural productivity. The compound's toxicity to plants and animals further compounds its environmental impact, potentially disrupting biodiversity and ecosystem balance.
Recognizing these severe environmental consequences, numerous international regulations and agreements have been implemented to control the production, use, and disposal of carbon tetrachloride. The Montreal Protocol, signed in 1987 and subsequently amended, has been instrumental in phasing out the production and consumption of ozone-depleting substances, including carbon tetrachloride. This global effort has resulted in a significant reduction in atmospheric concentrations of the compound.
In the United States, the Environmental Protection Agency (EPA) has classified carbon tetrachloride as a hazardous air pollutant under the Clean Air Act and a priority pollutant under the Clean Water Act. The Toxic Substances Control Act (TSCA) also regulates its manufacture, import, and use. Similar regulations exist in the European Union under the REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) framework, which aims to protect human health and the environment from chemical risks.
Despite these regulatory efforts, challenges remain in addressing the legacy of past carbon tetrachloride use and preventing its release from existing sources. Ongoing research focuses on developing more effective remediation techniques for contaminated sites and improving detection methods to monitor environmental levels. Additionally, there is a growing emphasis on finding safer alternatives to carbon tetrachloride in industrial processes where its use has not been completely phased out.
As our understanding of carbon tetrachloride's environmental impact continues to evolve, regulations are likely to become more stringent. Future policy directions may include stricter emission controls, enhanced monitoring programs, and increased support for research into eco-friendly substitutes. The global community's commitment to addressing the environmental challenges posed by carbon tetrachloride underscores the importance of continued scientific investigation and regulatory vigilance in protecting our planet's ecosystems and human health.
In aquatic environments, carbon tetrachloride can accumulate in sediments and bioaccumulate in marine organisms, posing risks to aquatic ecosystems and potentially entering the food chain. Its presence in soil can lead to groundwater contamination, affecting drinking water sources and agricultural productivity. The compound's toxicity to plants and animals further compounds its environmental impact, potentially disrupting biodiversity and ecosystem balance.
Recognizing these severe environmental consequences, numerous international regulations and agreements have been implemented to control the production, use, and disposal of carbon tetrachloride. The Montreal Protocol, signed in 1987 and subsequently amended, has been instrumental in phasing out the production and consumption of ozone-depleting substances, including carbon tetrachloride. This global effort has resulted in a significant reduction in atmospheric concentrations of the compound.
In the United States, the Environmental Protection Agency (EPA) has classified carbon tetrachloride as a hazardous air pollutant under the Clean Air Act and a priority pollutant under the Clean Water Act. The Toxic Substances Control Act (TSCA) also regulates its manufacture, import, and use. Similar regulations exist in the European Union under the REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) framework, which aims to protect human health and the environment from chemical risks.
Despite these regulatory efforts, challenges remain in addressing the legacy of past carbon tetrachloride use and preventing its release from existing sources. Ongoing research focuses on developing more effective remediation techniques for contaminated sites and improving detection methods to monitor environmental levels. Additionally, there is a growing emphasis on finding safer alternatives to carbon tetrachloride in industrial processes where its use has not been completely phased out.
As our understanding of carbon tetrachloride's environmental impact continues to evolve, regulations are likely to become more stringent. Future policy directions may include stricter emission controls, enhanced monitoring programs, and increased support for research into eco-friendly substitutes. The global community's commitment to addressing the environmental challenges posed by carbon tetrachloride underscores the importance of continued scientific investigation and regulatory vigilance in protecting our planet's ecosystems and human health.
Safety Protocols and Handling Guidelines
Expanding knowledge of carbon tetrachloride properties requires strict adherence to safety protocols and handling guidelines. Carbon tetrachloride is a highly toxic and potentially carcinogenic substance, necessitating careful management in laboratory and industrial settings. Personal protective equipment (PPE) is essential when working with this compound. This includes wearing chemical-resistant gloves, safety goggles, and a lab coat. In cases where exposure to vapors is possible, a properly fitted respirator with appropriate cartridges should be used.
Proper ventilation is crucial when handling carbon tetrachloride. All work should be conducted in a fume hood or well-ventilated area to minimize inhalation risks. Storage of carbon tetrachloride requires special consideration. It should be kept in tightly sealed containers made of materials resistant to its corrosive properties, such as glass or certain plastics. These containers must be stored in a cool, dry place away from direct sunlight and sources of heat or ignition.
Spill response procedures are vital for safe handling. Small spills can be contained using absorbent materials specifically designed for chemical spills. Larger spills may require professional hazardous material handling teams. In all cases, proper disposal of contaminated materials is essential, following local, state, and federal regulations for hazardous waste management.
Training is a critical component of safety protocols. All personnel working with carbon tetrachloride should receive comprehensive training on its properties, hazards, and proper handling techniques. This training should be regularly updated to reflect the latest safety guidelines and research findings. Emergency response plans must be in place and regularly practiced, including procedures for chemical exposure, spills, and fires.
When conducting experiments or industrial processes involving carbon tetrachloride, it is important to minimize the quantity used and explore safer alternatives whenever possible. Closed-system operations and automated handling equipment can significantly reduce exposure risks. Regular monitoring of air quality in work areas where carbon tetrachloride is used is essential to ensure that exposure levels remain below established safety thresholds.
Documentation and record-keeping are crucial aspects of safety protocols. Detailed logs of carbon tetrachloride usage, storage, and disposal should be maintained. Safety data sheets (SDS) must be readily available to all personnel, providing quick access to critical safety information. Regular safety audits and inspections should be conducted to ensure compliance with established protocols and to identify areas for improvement in handling practices.
Proper ventilation is crucial when handling carbon tetrachloride. All work should be conducted in a fume hood or well-ventilated area to minimize inhalation risks. Storage of carbon tetrachloride requires special consideration. It should be kept in tightly sealed containers made of materials resistant to its corrosive properties, such as glass or certain plastics. These containers must be stored in a cool, dry place away from direct sunlight and sources of heat or ignition.
Spill response procedures are vital for safe handling. Small spills can be contained using absorbent materials specifically designed for chemical spills. Larger spills may require professional hazardous material handling teams. In all cases, proper disposal of contaminated materials is essential, following local, state, and federal regulations for hazardous waste management.
Training is a critical component of safety protocols. All personnel working with carbon tetrachloride should receive comprehensive training on its properties, hazards, and proper handling techniques. This training should be regularly updated to reflect the latest safety guidelines and research findings. Emergency response plans must be in place and regularly practiced, including procedures for chemical exposure, spills, and fires.
When conducting experiments or industrial processes involving carbon tetrachloride, it is important to minimize the quantity used and explore safer alternatives whenever possible. Closed-system operations and automated handling equipment can significantly reduce exposure risks. Regular monitoring of air quality in work areas where carbon tetrachloride is used is essential to ensure that exposure levels remain below established safety thresholds.
Documentation and record-keeping are crucial aspects of safety protocols. Detailed logs of carbon tetrachloride usage, storage, and disposal should be maintained. Safety data sheets (SDS) must be readily available to all personnel, providing quick access to critical safety information. Regular safety audits and inspections should be conducted to ensure compliance with established protocols and to identify areas for improvement in handling practices.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!