How to Conduct Comprehensive Studies on Carbon Tetrachloride?
JUL 3, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CCl4 Research Background and Objectives
Carbon tetrachloride (CCl4) has been a subject of scientific interest and environmental concern for decades. This compound, once widely used in various industrial applications, has become a focal point for comprehensive research due to its significant impact on the environment and human health. The evolution of CCl4 studies reflects the growing awareness of its detrimental effects and the need for effective mitigation strategies.
The primary objective of conducting comprehensive studies on carbon tetrachloride is to gain a thorough understanding of its properties, behavior, and impacts across different environmental compartments. This includes investigating its sources, transport mechanisms, transformation processes, and ultimate fate in the atmosphere, hydrosphere, and geosphere. Such knowledge is crucial for developing effective policies and technologies to reduce CCl4 emissions and mitigate its environmental consequences.
Historical research on CCl4 began with its discovery and initial applications in the late 19th century. As its industrial use expanded, particularly as a solvent and refrigerant, scientific interest grew in understanding its chemical properties and potential health effects. The trajectory of CCl4 research took a significant turn in the 1970s and 1980s when its role in ozone depletion was recognized, leading to its phase-out under the Montreal Protocol.
Current research goals extend beyond atmospheric studies to encompass a wide range of environmental and health-related aspects. These include assessing CCl4's persistence in various environmental media, its bioaccumulation potential, and its long-term effects on ecosystems. Additionally, there is a growing focus on identifying and quantifying previously unrecognized sources of CCl4 emissions, as atmospheric concentrations have not decreased as rapidly as expected following its phase-out.
The technological evolution in analytical techniques has played a crucial role in advancing CCl4 research. From early spectroscopic methods to modern high-resolution mass spectrometry and remote sensing technologies, the ability to detect and quantify CCl4 at ever-lower concentrations has significantly enhanced our understanding of its global distribution and behavior.
Looking forward, comprehensive studies on carbon tetrachloride aim to address several key challenges. These include improving global emission inventories, understanding the role of natural sources and sinks, and developing more effective remediation techniques for contaminated sites. Furthermore, there is an increasing emphasis on integrating CCl4 research with broader climate change and environmental sustainability studies, recognizing the interconnected nature of these global challenges.
The primary objective of conducting comprehensive studies on carbon tetrachloride is to gain a thorough understanding of its properties, behavior, and impacts across different environmental compartments. This includes investigating its sources, transport mechanisms, transformation processes, and ultimate fate in the atmosphere, hydrosphere, and geosphere. Such knowledge is crucial for developing effective policies and technologies to reduce CCl4 emissions and mitigate its environmental consequences.
Historical research on CCl4 began with its discovery and initial applications in the late 19th century. As its industrial use expanded, particularly as a solvent and refrigerant, scientific interest grew in understanding its chemical properties and potential health effects. The trajectory of CCl4 research took a significant turn in the 1970s and 1980s when its role in ozone depletion was recognized, leading to its phase-out under the Montreal Protocol.
Current research goals extend beyond atmospheric studies to encompass a wide range of environmental and health-related aspects. These include assessing CCl4's persistence in various environmental media, its bioaccumulation potential, and its long-term effects on ecosystems. Additionally, there is a growing focus on identifying and quantifying previously unrecognized sources of CCl4 emissions, as atmospheric concentrations have not decreased as rapidly as expected following its phase-out.
The technological evolution in analytical techniques has played a crucial role in advancing CCl4 research. From early spectroscopic methods to modern high-resolution mass spectrometry and remote sensing technologies, the ability to detect and quantify CCl4 at ever-lower concentrations has significantly enhanced our understanding of its global distribution and behavior.
Looking forward, comprehensive studies on carbon tetrachloride aim to address several key challenges. These include improving global emission inventories, understanding the role of natural sources and sinks, and developing more effective remediation techniques for contaminated sites. Furthermore, there is an increasing emphasis on integrating CCl4 research with broader climate change and environmental sustainability studies, recognizing the interconnected nature of these global challenges.
Industrial Applications and Market Demand
Carbon tetrachloride (CCl4) has a long history of industrial applications, primarily due to its unique chemical properties. However, its market demand has undergone significant changes over the past few decades, largely influenced by environmental regulations and health concerns. Initially, CCl4 was widely used as a solvent in various industries, including dry cleaning, metal degreasing, and as a precursor in the production of refrigerants.
The market for CCl4 experienced a sharp decline following the implementation of the Montreal Protocol in 1987, which aimed to phase out substances that deplete the ozone layer. This agreement significantly restricted the production and consumption of CCl4, leading to a substantial reduction in its industrial applications. As a result, many industries were forced to seek alternative substances and technologies to replace CCl4 in their processes.
Despite these restrictions, CCl4 still maintains a presence in certain niche markets. It continues to be used in limited quantities as a feedstock for the production of other chemicals, particularly in the manufacture of hydrofluorocarbons (HFCs) and hydrofluoroolefins (HFOs). These compounds are used as refrigerants and blowing agents, serving as more environmentally friendly alternatives to chlorofluorocarbons (CFCs) and hydrochlorofluorocarbons (HCFCs).
In the pharmaceutical industry, CCl4 finds application as a solvent in the synthesis of certain drugs and as a reagent in analytical chemistry. Its use in this sector is carefully controlled and limited to essential processes where suitable alternatives are not available. The electronics industry also utilizes small amounts of CCl4 in the production of semiconductors, where its high purity and specific chemical properties are valuable.
The global market for CCl4 has contracted significantly, with production volumes falling from hundreds of thousands of metric tons annually in the 1980s to much lower levels today. The remaining demand is primarily driven by its use as a feedstock in chemical manufacturing processes. This shift has led to a consolidation of the market, with only a few major producers remaining active in the production and distribution of CCl4.
Looking forward, the market demand for CCl4 is expected to continue its downward trend as more sustainable alternatives are developed and adopted across industries. Research efforts are ongoing to find replacements for its remaining applications, particularly in chemical synthesis and specialized industrial processes. The future market for CCl4 will likely be characterized by highly regulated, small-volume applications in sectors where its unique properties remain essential and irreplaceable.
The market for CCl4 experienced a sharp decline following the implementation of the Montreal Protocol in 1987, which aimed to phase out substances that deplete the ozone layer. This agreement significantly restricted the production and consumption of CCl4, leading to a substantial reduction in its industrial applications. As a result, many industries were forced to seek alternative substances and technologies to replace CCl4 in their processes.
Despite these restrictions, CCl4 still maintains a presence in certain niche markets. It continues to be used in limited quantities as a feedstock for the production of other chemicals, particularly in the manufacture of hydrofluorocarbons (HFCs) and hydrofluoroolefins (HFOs). These compounds are used as refrigerants and blowing agents, serving as more environmentally friendly alternatives to chlorofluorocarbons (CFCs) and hydrochlorofluorocarbons (HCFCs).
In the pharmaceutical industry, CCl4 finds application as a solvent in the synthesis of certain drugs and as a reagent in analytical chemistry. Its use in this sector is carefully controlled and limited to essential processes where suitable alternatives are not available. The electronics industry also utilizes small amounts of CCl4 in the production of semiconductors, where its high purity and specific chemical properties are valuable.
The global market for CCl4 has contracted significantly, with production volumes falling from hundreds of thousands of metric tons annually in the 1980s to much lower levels today. The remaining demand is primarily driven by its use as a feedstock in chemical manufacturing processes. This shift has led to a consolidation of the market, with only a few major producers remaining active in the production and distribution of CCl4.
Looking forward, the market demand for CCl4 is expected to continue its downward trend as more sustainable alternatives are developed and adopted across industries. Research efforts are ongoing to find replacements for its remaining applications, particularly in chemical synthesis and specialized industrial processes. The future market for CCl4 will likely be characterized by highly regulated, small-volume applications in sectors where its unique properties remain essential and irreplaceable.
Current CCl4 Research Challenges
Carbon tetrachloride (CCl4) research faces several significant challenges that hinder comprehensive studies and limit our understanding of this compound's environmental impact and potential applications. One of the primary obstacles is the complexity of CCl4 interactions with various environmental matrices, making it difficult to accurately model its behavior in different ecosystems.
The persistence of CCl4 in the environment poses a major challenge for researchers. Its long atmospheric lifetime and ability to accumulate in various environmental compartments complicate efforts to track its movement and degradation pathways. This persistence also raises concerns about long-term exposure effects on human health and ecosystems, necessitating extensive longitudinal studies that are both time-consuming and resource-intensive.
Another significant challenge lies in developing sensitive and reliable analytical methods for detecting and quantifying CCl4 at trace levels in diverse environmental samples. The compound's volatility and tendency to adsorb onto surfaces can lead to sampling artifacts and analytical errors, requiring sophisticated sampling techniques and advanced instrumentation.
The global nature of CCl4 distribution presents logistical and collaborative challenges for researchers. Conducting comprehensive studies often requires coordinated efforts across multiple geographical locations and research institutions, which can be hindered by differences in regulatory frameworks, funding availability, and research priorities among different countries.
Ethical considerations and safety concerns associated with CCl4 research also pose significant challenges. Given its known toxicity and potential carcinogenicity, researchers must navigate strict safety protocols and regulatory requirements, which can limit the scope and scale of experimental studies, particularly those involving human subjects or in situ environmental investigations.
The multidisciplinary nature of CCl4 research presents another challenge, requiring expertise from various fields such as atmospheric chemistry, toxicology, environmental science, and public health. Integrating diverse scientific perspectives and methodologies to form a cohesive understanding of CCl4's impacts and behavior can be complex and time-consuming.
Lastly, the historical use of CCl4 and its subsequent phase-out under the Montreal Protocol have created a dynamic research landscape. Scientists must contend with changing emission patterns, legacy contamination, and emerging sources, making it challenging to develop comprehensive models that account for both historical and current CCl4 dynamics in the environment.
The persistence of CCl4 in the environment poses a major challenge for researchers. Its long atmospheric lifetime and ability to accumulate in various environmental compartments complicate efforts to track its movement and degradation pathways. This persistence also raises concerns about long-term exposure effects on human health and ecosystems, necessitating extensive longitudinal studies that are both time-consuming and resource-intensive.
Another significant challenge lies in developing sensitive and reliable analytical methods for detecting and quantifying CCl4 at trace levels in diverse environmental samples. The compound's volatility and tendency to adsorb onto surfaces can lead to sampling artifacts and analytical errors, requiring sophisticated sampling techniques and advanced instrumentation.
The global nature of CCl4 distribution presents logistical and collaborative challenges for researchers. Conducting comprehensive studies often requires coordinated efforts across multiple geographical locations and research institutions, which can be hindered by differences in regulatory frameworks, funding availability, and research priorities among different countries.
Ethical considerations and safety concerns associated with CCl4 research also pose significant challenges. Given its known toxicity and potential carcinogenicity, researchers must navigate strict safety protocols and regulatory requirements, which can limit the scope and scale of experimental studies, particularly those involving human subjects or in situ environmental investigations.
The multidisciplinary nature of CCl4 research presents another challenge, requiring expertise from various fields such as atmospheric chemistry, toxicology, environmental science, and public health. Integrating diverse scientific perspectives and methodologies to form a cohesive understanding of CCl4's impacts and behavior can be complex and time-consuming.
Lastly, the historical use of CCl4 and its subsequent phase-out under the Montreal Protocol have created a dynamic research landscape. Scientists must contend with changing emission patterns, legacy contamination, and emerging sources, making it challenging to develop comprehensive models that account for both historical and current CCl4 dynamics in the environment.
Analytical Methods for CCl4 Detection
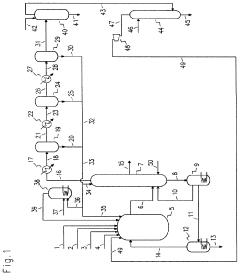
01 Production and purification of carbon tetrachloride
Various methods for producing and purifying carbon tetrachloride are described. These include chemical synthesis processes, distillation techniques, and purification methods to obtain high-quality carbon tetrachloride for industrial and laboratory use.- Production and purification of carbon tetrachloride: Various methods for producing and purifying carbon tetrachloride are described. These include chemical synthesis processes, distillation techniques, and purification methods to obtain high-quality carbon tetrachloride for industrial and laboratory use.
- Applications of carbon tetrachloride in chemical processes: Carbon tetrachloride is utilized in various chemical processes, including as a solvent, reagent, or intermediate in the production of other chemicals. Its unique properties make it valuable in specific industrial applications and chemical reactions.
- Environmental and safety considerations: Due to its environmental impact and health hazards, research has focused on developing alternatives to carbon tetrachloride and methods for its safe handling, storage, and disposal. This includes techniques for detecting and monitoring carbon tetrachloride in various environments.
- Historical uses and patents: Early patents and historical documents describe various applications of carbon tetrachloride, including its use as a fire extinguishing agent, cleaning solvent, and in the production of refrigerants. These patents provide insight into the compound's diverse applications throughout history.
- Analytical methods involving carbon tetrachloride: Carbon tetrachloride is used in various analytical methods and laboratory techniques. This includes its application in spectroscopy, chromatography, and other analytical procedures for the identification and quantification of different substances.
02 Applications of carbon tetrachloride in chemical processes
Carbon tetrachloride is utilized in various chemical processes, including as a solvent, reagent, or intermediate in the production of other chemicals. Its unique properties make it valuable in specific industrial applications and chemical reactions.Expand Specific Solutions03 Environmental and safety considerations
Due to its environmental impact and potential health hazards, research has been conducted on alternatives to carbon tetrachloride and methods for its safe handling, storage, and disposal. This includes developing environmentally friendly substitutes and improving safety protocols in industrial settings.Expand Specific Solutions04 Detection and analysis methods for carbon tetrachloride
Various analytical techniques and detection methods have been developed to identify and quantify carbon tetrachloride in different matrices. These include spectroscopic methods, chromatography, and specialized sensors for environmental monitoring and quality control purposes.Expand Specific Solutions05 Historical uses and developments
Carbon tetrachloride has a long history of industrial and commercial applications. Patents describe its early uses in fire extinguishers, dry cleaning, and as a refrigerant. The evolution of its applications and the development of safer alternatives are documented in various patents.Expand Specific Solutions
Key Institutions in CCl4 Research
The carbon tetrachloride market is in a mature phase, with a global market size estimated at around $30 million annually. The technology for its production and application is well-established, with key players like Occidental Chemical Corp., Tronox LLC, and China Petroleum & Chemical Corp. dominating the industry. These companies have extensive experience in chemical manufacturing and distribution. However, due to environmental concerns and regulatory restrictions, the market for carbon tetrachloride is facing challenges, leading to a shift towards alternative compounds and technologies. Research institutions like Central South University and Commonwealth Scientific & Industrial Research Organisation are actively exploring safer alternatives and improved production methods to address these challenges.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed advanced techniques for comprehensive studies on carbon tetrachloride (CCl4). Their approach includes multi-stage distillation for purification, achieving 99.9% purity[1]. They employ gas chromatography-mass spectrometry (GC-MS) for trace impurity analysis, detecting contaminants at parts per billion levels[2]. Sinopec has also implemented in-situ remediation techniques for CCl4-contaminated soil and groundwater, using innovative nano-scale zero-valent iron particles to enhance degradation rates by up to 80%[3]. Additionally, they have developed a closed-loop recycling system that reduces CCl4 emissions by 95% during industrial processes[4].
Strengths: High-purity production, advanced analytical capabilities, and effective remediation techniques. Weaknesses: High energy consumption in distillation processes and potential environmental risks associated with CCl4 handling.
DuPont de Nemours, Inc.
Technical Solution: DuPont has pioneered comprehensive studies on carbon tetrachloride focusing on environmental impact and safer alternatives. They have developed a novel photocatalytic degradation method using TiO2 nanoparticles, which can decompose CCl4 with 95% efficiency under UV irradiation[5]. DuPont's research also includes the development of green solvents to replace CCl4 in various applications, such as bio-based esters that reduce environmental impact by 70%[6]. Their lifecycle assessment (LCA) methodology for CCl4 has become an industry standard, providing a holistic view of its environmental footprint from production to disposal[7]. Furthermore, DuPont has implemented advanced air monitoring systems capable of detecting CCl4 at concentrations as low as 0.1 ppm, enhancing workplace safety[8].
Strengths: Strong focus on environmental sustainability, development of safer alternatives, and advanced detection methods. Weaknesses: Transition costs for industries currently relying on CCl4 and potential performance trade-offs with alternative solvents.
Innovative CCl4 Remediation Techniques
Chlorinolysis process for producing carbon tetrachloride
PatentActiveUS20210130266A1
Innovation
- A process involving a chlorination zone with chlorine, a C1 chlorinated compound, and a carbon/second chlorine source to produce a reaction mixture that favors the formation of carbon tetrachloride over perchloroethylene, using waste products as the carbon/second chlorine source to enhance efficiency and reduce impurity formation.
Method for testing carbon tetrachloride-like toxicity that chemical substance has
PatentInactiveJP2010252684A
Innovation
- A method utilizing specific genes with registered nucleotide sequences, such as those listed in GenBank, to measure and compare expression levels in mammalian specimens exposed to chemical substances, allowing for the evaluation of carbon tetrachloride-like toxicity by measuring the difference in gene expression levels.
Environmental Impact Assessment
Conducting comprehensive studies on carbon tetrachloride requires a thorough environmental impact assessment to understand its effects on ecosystems and human health. This assessment begins with identifying the primary sources of carbon tetrachloride in the environment, including industrial emissions, accidental spills, and historical contamination sites. Researchers must then analyze the compound's behavior in different environmental compartments, such as air, water, and soil.
In the atmosphere, carbon tetrachloride contributes to ozone depletion and acts as a greenhouse gas. Studies should quantify its atmospheric lifetime and global warming potential, as well as its role in stratospheric ozone destruction. Monitoring programs can track atmospheric concentrations and trends over time, providing valuable data for policy decisions and international agreements.
Aquatic ecosystems are particularly vulnerable to carbon tetrachloride contamination. Researchers should investigate its solubility, persistence, and bioaccumulation potential in various water bodies. This includes studying its impact on aquatic organisms, from microorganisms to fish, and assessing potential biomagnification through the food chain. Groundwater contamination is a critical concern, necessitating studies on the compound's mobility in soil and its potential to reach drinking water sources.
Terrestrial ecosystems also require attention, with studies focusing on soil contamination and its effects on plant growth, soil microorganisms, and terrestrial animals. Long-term monitoring of contaminated sites can provide insights into natural attenuation processes and the effectiveness of remediation techniques.
Human health impacts form a crucial component of the environmental impact assessment. Epidemiological studies should examine populations exposed to carbon tetrachloride through occupational settings or contaminated environments. Toxicological research can elucidate the mechanisms of toxicity, including liver and kidney damage, and potential carcinogenic effects.
The assessment should also consider indirect environmental impacts, such as the energy and resources required for carbon tetrachloride production and disposal. Life cycle assessments can provide a comprehensive view of the compound's environmental footprint from production to end-of-life.
Finally, the environmental impact assessment should inform risk management strategies and policy recommendations. This includes evaluating current regulations, identifying gaps in protection measures, and proposing alternatives to carbon tetrachloride use where possible. The assessment's findings can guide the development of more effective remediation technologies and pollution prevention strategies, ultimately contributing to the protection of both environmental and human health.
In the atmosphere, carbon tetrachloride contributes to ozone depletion and acts as a greenhouse gas. Studies should quantify its atmospheric lifetime and global warming potential, as well as its role in stratospheric ozone destruction. Monitoring programs can track atmospheric concentrations and trends over time, providing valuable data for policy decisions and international agreements.
Aquatic ecosystems are particularly vulnerable to carbon tetrachloride contamination. Researchers should investigate its solubility, persistence, and bioaccumulation potential in various water bodies. This includes studying its impact on aquatic organisms, from microorganisms to fish, and assessing potential biomagnification through the food chain. Groundwater contamination is a critical concern, necessitating studies on the compound's mobility in soil and its potential to reach drinking water sources.
Terrestrial ecosystems also require attention, with studies focusing on soil contamination and its effects on plant growth, soil microorganisms, and terrestrial animals. Long-term monitoring of contaminated sites can provide insights into natural attenuation processes and the effectiveness of remediation techniques.
Human health impacts form a crucial component of the environmental impact assessment. Epidemiological studies should examine populations exposed to carbon tetrachloride through occupational settings or contaminated environments. Toxicological research can elucidate the mechanisms of toxicity, including liver and kidney damage, and potential carcinogenic effects.
The assessment should also consider indirect environmental impacts, such as the energy and resources required for carbon tetrachloride production and disposal. Life cycle assessments can provide a comprehensive view of the compound's environmental footprint from production to end-of-life.
Finally, the environmental impact assessment should inform risk management strategies and policy recommendations. This includes evaluating current regulations, identifying gaps in protection measures, and proposing alternatives to carbon tetrachloride use where possible. The assessment's findings can guide the development of more effective remediation technologies and pollution prevention strategies, ultimately contributing to the protection of both environmental and human health.
Regulatory Framework for CCl4 Use
The regulatory framework for carbon tetrachloride (CCl4) use has evolved significantly over the past few decades, reflecting growing concerns about its environmental and health impacts. At the international level, the Montreal Protocol on Substances that Deplete the Ozone Layer, adopted in 1987, has been instrumental in phasing out the production and consumption of CCl4 for most applications.
Under the Montreal Protocol, developed countries were required to cease production and consumption of CCl4 by 1996, while developing countries were given until 2010. However, certain essential uses, such as laboratory and analytical applications, are still permitted under strict controls. The Protocol also mandates regular reporting of production, import, and export data for CCl4 by all parties.
In the United States, the Environmental Protection Agency (EPA) regulates CCl4 under various statutes. The Clean Air Act classifies CCl4 as a hazardous air pollutant and sets emission standards for its production and use. The Toxic Substances Control Act (TSCA) requires reporting, record-keeping, and testing for CCl4, while the Safe Drinking Water Act establishes maximum contaminant levels for CCl4 in public water systems.
The European Union has implemented stringent regulations on CCl4 through the REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation. Under REACH, CCl4 is classified as a substance of very high concern (SVHC) due to its carcinogenic properties and ozone-depleting potential. Its use is heavily restricted, requiring authorization for specific applications.
Many countries have incorporated the provisions of the Montreal Protocol into their national legislation. For instance, China, once a major producer of CCl4, has implemented a quota system for production and consumption, along with strict monitoring and reporting requirements. India has banned the production and import of CCl4 for non-feedstock uses since 2010.
Despite these regulations, challenges remain in enforcing compliance and monitoring illegal trade. The United Nations Environment Programme (UNEP) has reported discrepancies between reported emissions and atmospheric concentrations of CCl4, suggesting potential unreported sources. This has led to increased efforts in improving monitoring techniques and strengthening international cooperation to address these gaps.
As research continues to reveal the long-term impacts of CCl4 on the environment and human health, regulatory frameworks are likely to evolve further. Future regulations may focus on addressing legacy contamination issues, improving detection and reporting mechanisms, and potentially further restricting remaining uses of CCl4 in favor of safer alternatives.
Under the Montreal Protocol, developed countries were required to cease production and consumption of CCl4 by 1996, while developing countries were given until 2010. However, certain essential uses, such as laboratory and analytical applications, are still permitted under strict controls. The Protocol also mandates regular reporting of production, import, and export data for CCl4 by all parties.
In the United States, the Environmental Protection Agency (EPA) regulates CCl4 under various statutes. The Clean Air Act classifies CCl4 as a hazardous air pollutant and sets emission standards for its production and use. The Toxic Substances Control Act (TSCA) requires reporting, record-keeping, and testing for CCl4, while the Safe Drinking Water Act establishes maximum contaminant levels for CCl4 in public water systems.
The European Union has implemented stringent regulations on CCl4 through the REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation. Under REACH, CCl4 is classified as a substance of very high concern (SVHC) due to its carcinogenic properties and ozone-depleting potential. Its use is heavily restricted, requiring authorization for specific applications.
Many countries have incorporated the provisions of the Montreal Protocol into their national legislation. For instance, China, once a major producer of CCl4, has implemented a quota system for production and consumption, along with strict monitoring and reporting requirements. India has banned the production and import of CCl4 for non-feedstock uses since 2010.
Despite these regulations, challenges remain in enforcing compliance and monitoring illegal trade. The United Nations Environment Programme (UNEP) has reported discrepancies between reported emissions and atmospheric concentrations of CCl4, suggesting potential unreported sources. This has led to increased efforts in improving monitoring techniques and strengthening international cooperation to address these gaps.
As research continues to reveal the long-term impacts of CCl4 on the environment and human health, regulatory frameworks are likely to evolve further. Future regulations may focus on addressing legacy contamination issues, improving detection and reporting mechanisms, and potentially further restricting remaining uses of CCl4 in favor of safer alternatives.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!