Carbon Tetrachloride's Role in Industrial Chemical Processes
JUL 2, 20258 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CCl4 in Industry: History and Objectives
Carbon tetrachloride (CCl4) has played a significant role in industrial chemical processes since its discovery in the mid-19th century. Initially synthesized by French chemist Henri Victor Regnault in 1839, CCl4 quickly gained prominence due to its unique properties and versatile applications. The compound's non-flammability and excellent solvency made it an attractive option for various industrial uses.
Throughout the early 20th century, CCl4 found widespread application as a cleaning agent, degreaser, and fire extinguishing agent. Its ability to dissolve a wide range of organic compounds made it particularly valuable in the textile and metal industries. The chemical's low boiling point and high density also led to its use as a refrigerant and in the production of chlorofluorocarbons (CFCs).
However, the 1970s marked a turning point in the history of CCl4 usage. Growing awareness of its environmental impact and potential health hazards led to increased scrutiny and regulation. The Montreal Protocol, signed in 1987, began the phase-out of CCl4 production and consumption due to its ozone-depleting properties.
Despite these restrictions, CCl4 continued to play a role in certain industrial processes, particularly as a feedstock for the production of other chemicals. Its use in the manufacture of chlorofluorocarbons (CFCs) and their replacements, hydrochlorofluorocarbons (HCFCs), remained significant until further regulations were implemented.
The primary objective in studying CCl4's role in industrial chemical processes is to understand its historical significance, current applications, and potential future uses. This includes examining alternative compounds that can replace CCl4 in various processes while maintaining efficiency and minimizing environmental impact.
Another crucial goal is to explore innovative methods for the safe handling, use, and disposal of CCl4 in essential applications where substitutes are not yet available. This involves developing advanced containment systems, improving recycling techniques, and implementing stricter safety protocols to minimize environmental release and worker exposure.
Furthermore, research aims to uncover any remaining beneficial properties of CCl4 that could be harnessed in new, environmentally friendly ways. This includes investigating its potential role in emerging technologies or as a precursor for novel materials with reduced environmental impact.
Ultimately, the study of CCl4 in industry serves as a case study in the evolution of chemical use, highlighting the importance of balancing technological progress with environmental stewardship and human health considerations. It underscores the need for continuous innovation in developing sustainable alternatives and improving industrial processes to meet global environmental standards.
Throughout the early 20th century, CCl4 found widespread application as a cleaning agent, degreaser, and fire extinguishing agent. Its ability to dissolve a wide range of organic compounds made it particularly valuable in the textile and metal industries. The chemical's low boiling point and high density also led to its use as a refrigerant and in the production of chlorofluorocarbons (CFCs).
However, the 1970s marked a turning point in the history of CCl4 usage. Growing awareness of its environmental impact and potential health hazards led to increased scrutiny and regulation. The Montreal Protocol, signed in 1987, began the phase-out of CCl4 production and consumption due to its ozone-depleting properties.
Despite these restrictions, CCl4 continued to play a role in certain industrial processes, particularly as a feedstock for the production of other chemicals. Its use in the manufacture of chlorofluorocarbons (CFCs) and their replacements, hydrochlorofluorocarbons (HCFCs), remained significant until further regulations were implemented.
The primary objective in studying CCl4's role in industrial chemical processes is to understand its historical significance, current applications, and potential future uses. This includes examining alternative compounds that can replace CCl4 in various processes while maintaining efficiency and minimizing environmental impact.
Another crucial goal is to explore innovative methods for the safe handling, use, and disposal of CCl4 in essential applications where substitutes are not yet available. This involves developing advanced containment systems, improving recycling techniques, and implementing stricter safety protocols to minimize environmental release and worker exposure.
Furthermore, research aims to uncover any remaining beneficial properties of CCl4 that could be harnessed in new, environmentally friendly ways. This includes investigating its potential role in emerging technologies or as a precursor for novel materials with reduced environmental impact.
Ultimately, the study of CCl4 in industry serves as a case study in the evolution of chemical use, highlighting the importance of balancing technological progress with environmental stewardship and human health considerations. It underscores the need for continuous innovation in developing sustainable alternatives and improving industrial processes to meet global environmental standards.
Market Analysis for CCl4 Applications
Carbon tetrachloride (CCl4) has been a significant player in various industrial chemical processes for decades. The global market for CCl4 applications has undergone substantial changes due to environmental regulations and shifting industrial needs. Currently, the primary markets for CCl4 are in the production of chlorofluorocarbons (CFCs) and their alternatives, as well as in specific niche applications.
The largest market segment for CCl4 is in the manufacturing of chlorofluorocarbons and hydrochlorofluorocarbons (HCFCs), particularly HCFC-22, which is used as a refrigerant and a feedstock for fluoropolymers. However, this market is in decline due to the phase-out of ozone-depleting substances under the Montreal Protocol. As a result, the demand for CCl4 in this sector is expected to decrease significantly over the coming years.
Despite the overall decline, CCl4 still finds applications in various industrial processes. It is used as a solvent in the production of agrochemicals, pharmaceuticals, and in the processing of silicone and other specialty chemicals. The chemical industry continues to utilize CCl4 in controlled environments for specific reactions and as a chain transfer agent in polymer production.
In the pharmaceutical sector, CCl4 serves as a reagent in organic synthesis and as a solvent in drug manufacturing processes. While alternatives are being sought, certain niche applications still rely on CCl4 due to its unique properties and established protocols.
The market for CCl4 in laboratory and analytical applications remains stable, albeit small. It is used as a solvent in spectroscopy and as a standard in environmental testing. This segment, while not large in volume, provides a consistent demand for high-purity CCl4.
Geographically, the market for CCl4 applications is shifting. With stricter regulations in developed countries, particularly in North America and Europe, the demand has moved towards emerging economies in Asia and Latin America. China, in particular, has become a significant consumer and producer of CCl4 for various industrial applications.
The future market for CCl4 applications is likely to be shaped by ongoing research into safer alternatives and the development of more environmentally friendly processes. As industries adapt to regulatory pressures and sustainability goals, the market for CCl4 is expected to continue its gradual contraction, with potential growth only in highly specialized applications where substitutes are not yet viable.
The largest market segment for CCl4 is in the manufacturing of chlorofluorocarbons and hydrochlorofluorocarbons (HCFCs), particularly HCFC-22, which is used as a refrigerant and a feedstock for fluoropolymers. However, this market is in decline due to the phase-out of ozone-depleting substances under the Montreal Protocol. As a result, the demand for CCl4 in this sector is expected to decrease significantly over the coming years.
Despite the overall decline, CCl4 still finds applications in various industrial processes. It is used as a solvent in the production of agrochemicals, pharmaceuticals, and in the processing of silicone and other specialty chemicals. The chemical industry continues to utilize CCl4 in controlled environments for specific reactions and as a chain transfer agent in polymer production.
In the pharmaceutical sector, CCl4 serves as a reagent in organic synthesis and as a solvent in drug manufacturing processes. While alternatives are being sought, certain niche applications still rely on CCl4 due to its unique properties and established protocols.
The market for CCl4 in laboratory and analytical applications remains stable, albeit small. It is used as a solvent in spectroscopy and as a standard in environmental testing. This segment, while not large in volume, provides a consistent demand for high-purity CCl4.
Geographically, the market for CCl4 applications is shifting. With stricter regulations in developed countries, particularly in North America and Europe, the demand has moved towards emerging economies in Asia and Latin America. China, in particular, has become a significant consumer and producer of CCl4 for various industrial applications.
The future market for CCl4 applications is likely to be shaped by ongoing research into safer alternatives and the development of more environmentally friendly processes. As industries adapt to regulatory pressures and sustainability goals, the market for CCl4 is expected to continue its gradual contraction, with potential growth only in highly specialized applications where substitutes are not yet viable.
CCl4 Usage: Current Status and Challenges
Carbon tetrachloride (CCl4) has long been a significant chemical in various industrial processes. However, its usage has faced substantial challenges and restrictions in recent years due to environmental and health concerns. Currently, the primary applications of CCl4 are limited to specific industrial processes where suitable alternatives are not readily available.
In the chemical industry, CCl4 continues to serve as a feedstock for the production of certain chlorinated compounds, particularly hydrofluorocarbons (HFCs) and hydrofluoroolefins (HFOs). These substances are used in refrigeration and air conditioning systems as replacements for ozone-depleting chlorofluorocarbons (CFCs). The controlled use of CCl4 in this capacity is permitted under international agreements, such as the Montreal Protocol, which aims to phase out ozone-depleting substances.
Despite its continued use in some sectors, the overall consumption of CCl4 has significantly decreased globally. This reduction is primarily due to stringent regulations and the development of alternative substances and processes. Many countries have implemented bans or severe restrictions on CCl4 use in consumer products and non-essential industrial applications.
One of the main challenges facing the current use of CCl4 is its classification as an ozone-depleting substance and a potential carcinogen. This designation has led to increased scrutiny and regulatory pressure, making it difficult for industries to justify its continued use where alternatives exist. Additionally, the management and disposal of CCl4 pose significant environmental risks, requiring specialized handling and treatment procedures.
Another challenge is the ongoing search for suitable replacements in processes where CCl4 is still considered essential. While progress has been made in many areas, some niche applications continue to rely on CCl4 due to its unique chemical properties. This dependency creates a complex situation where the need for the chemical must be balanced against its environmental impact.
The scientific community and industry stakeholders are actively working to address these challenges. Research efforts are focused on developing more environmentally friendly alternatives and improving existing processes to reduce or eliminate CCl4 usage. However, the transition away from CCl4 in certain applications remains a gradual process, constrained by technical, economic, and regulatory factors.
In conclusion, while CCl4 usage has dramatically decreased, it continues to play a role in specific industrial chemical processes. The current status is characterized by limited, controlled use in essential applications, coupled with ongoing efforts to further reduce its consumption. The challenges of environmental impact, health risks, and the need for viable alternatives continue to shape the future of CCl4 in industrial settings.
In the chemical industry, CCl4 continues to serve as a feedstock for the production of certain chlorinated compounds, particularly hydrofluorocarbons (HFCs) and hydrofluoroolefins (HFOs). These substances are used in refrigeration and air conditioning systems as replacements for ozone-depleting chlorofluorocarbons (CFCs). The controlled use of CCl4 in this capacity is permitted under international agreements, such as the Montreal Protocol, which aims to phase out ozone-depleting substances.
Despite its continued use in some sectors, the overall consumption of CCl4 has significantly decreased globally. This reduction is primarily due to stringent regulations and the development of alternative substances and processes. Many countries have implemented bans or severe restrictions on CCl4 use in consumer products and non-essential industrial applications.
One of the main challenges facing the current use of CCl4 is its classification as an ozone-depleting substance and a potential carcinogen. This designation has led to increased scrutiny and regulatory pressure, making it difficult for industries to justify its continued use where alternatives exist. Additionally, the management and disposal of CCl4 pose significant environmental risks, requiring specialized handling and treatment procedures.
Another challenge is the ongoing search for suitable replacements in processes where CCl4 is still considered essential. While progress has been made in many areas, some niche applications continue to rely on CCl4 due to its unique chemical properties. This dependency creates a complex situation where the need for the chemical must be balanced against its environmental impact.
The scientific community and industry stakeholders are actively working to address these challenges. Research efforts are focused on developing more environmentally friendly alternatives and improving existing processes to reduce or eliminate CCl4 usage. However, the transition away from CCl4 in certain applications remains a gradual process, constrained by technical, economic, and regulatory factors.
In conclusion, while CCl4 usage has dramatically decreased, it continues to play a role in specific industrial chemical processes. The current status is characterized by limited, controlled use in essential applications, coupled with ongoing efforts to further reduce its consumption. The challenges of environmental impact, health risks, and the need for viable alternatives continue to shape the future of CCl4 in industrial settings.
Current CCl4 Utilization Methods
01 Production and purification of carbon tetrachloride
Various methods for producing and purifying carbon tetrachloride are described. These include chemical synthesis processes, distillation techniques, and purification methods to obtain high-quality carbon tetrachloride for industrial and laboratory use.- Production and purification of carbon tetrachloride: Various methods for producing and purifying carbon tetrachloride are described. These include chemical synthesis processes, distillation techniques, and purification methods to obtain high-quality carbon tetrachloride for industrial and laboratory use.
- Applications of carbon tetrachloride in chemical processes: Carbon tetrachloride is utilized in various chemical processes, including as a solvent, reagent, or intermediate in the production of other chemicals. Its applications span across different industries, showcasing its versatility in chemical manufacturing.
- Environmental and safety considerations: Due to its environmental impact and health hazards, research focuses on developing alternatives to carbon tetrachloride and methods for its safe handling, disposal, and remediation. This includes studies on its effects on the ozone layer and strategies for reducing its use in industrial processes.
- Detection and analysis methods: Various techniques and apparatus are developed for detecting and analyzing carbon tetrachloride in different environments. These include spectroscopic methods, chromatography, and specialized sensors designed to measure carbon tetrachloride concentrations in air, water, or other media.
- Historical uses and patents: Early patents and historical documents reveal the diverse applications of carbon tetrachloride in the past, including its use in fire extinguishers, dry cleaning, and as a degreasing agent. These historical patents provide insight into the evolution of carbon tetrachloride's industrial applications over time.
02 Applications of carbon tetrachloride in chemical processes
Carbon tetrachloride is utilized in various chemical processes as a solvent, reagent, or intermediate. It finds applications in organic synthesis, extraction processes, and as a precursor for other chlorinated compounds.Expand Specific Solutions03 Environmental and safety considerations
Due to its environmental impact and health hazards, research focuses on developing alternatives to carbon tetrachloride and methods for its safe handling, storage, and disposal. This includes techniques for detecting and monitoring carbon tetrachloride in various environments.Expand Specific Solutions04 Carbon tetrachloride in analytical chemistry
Carbon tetrachloride is used in various analytical techniques and procedures. It serves as a solvent in spectroscopy, chromatography, and other analytical methods for the identification and quantification of chemical compounds.Expand Specific Solutions05 Historical uses and industrial applications
Carbon tetrachloride has been used historically in various industrial applications, including as a refrigerant, fire extinguishing agent, and cleaning solvent. While many of these uses have been phased out due to environmental concerns, understanding its historical applications is important for legacy issues and potential remediation efforts.Expand Specific Solutions
Key Industrial Players and Competitors
The carbon tetrachloride market is in a mature phase, with a relatively stable global demand. The market size is estimated to be in the range of hundreds of millions of dollars annually. Technologically, carbon tetrachloride production is well-established, with major players like Occidental Chemical Corp., Norsk Hydro ASA, and Bayer AG having refined their processes over decades. However, due to environmental concerns and regulatory restrictions, the industry is shifting towards alternative compounds and greener technologies. Companies such as DuPont de Nemours, Inc. and The Chemours Co. are investing in research and development to find more sustainable solutions, indicating a potential technological transition in the sector.
Occidental Chemical Corp.
Technical Solution: Occidental Chemical Corp. has developed an innovative process for the production of chlorinated hydrocarbons using carbon tetrachloride as a key intermediate. Their method involves a controlled reaction between methane and chlorine in the presence of a catalyst, producing carbon tetrachloride with high efficiency. This carbon tetrachloride is then used in a subsequent step to produce other chlorinated compounds such as perchloroethylene and trichloroethylene[1]. The process incorporates advanced reactor designs and precise temperature control to maximize yield and minimize unwanted by-products. Additionally, Occidental has implemented a closed-loop system that captures and recycles unreacted carbon tetrachloride, significantly reducing waste and improving overall process economics[3].
Strengths: High efficiency, reduced waste through recycling, versatile production of multiple chlorinated compounds. Weaknesses: Potential environmental concerns due to carbon tetrachloride use, reliance on methane as a feedstock.
Bayer AG
Technical Solution: Bayer AG has developed a novel approach to utilizing carbon tetrachloride in the production of pharmaceuticals and agrochemicals. Their process involves using carbon tetrachloride as a chlorinating agent in the synthesis of complex organic molecules. By carefully controlling reaction conditions and using proprietary catalysts, Bayer has achieved high selectivity and yield in these chlorination reactions[2]. The company has also implemented advanced safety measures to mitigate the risks associated with carbon tetrachloride handling. Furthermore, Bayer has invested in research to develop alternative chlorinating agents that could potentially replace carbon tetrachloride in some applications, demonstrating their commitment to sustainable chemistry[4]. Their process also incorporates real-time monitoring and advanced process control systems to ensure consistent product quality and minimize environmental impact.
Strengths: High selectivity in chlorination reactions, advanced safety measures, ongoing research into alternatives. Weaknesses: Continued reliance on a potentially hazardous substance, potential regulatory challenges.
CCl4 Patents and Technical Literature
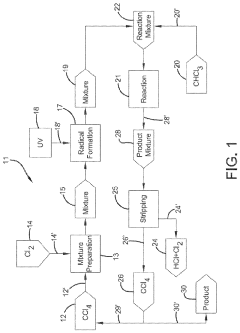
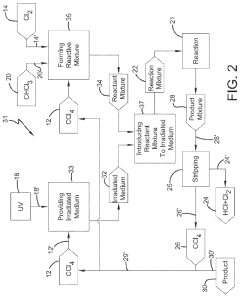
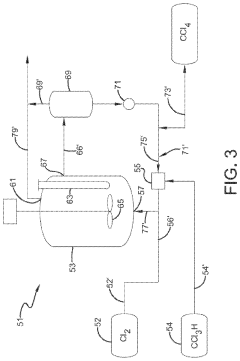
Photochlorination of partially-chlorinated chloromethanes to carbon tetrachloride
PatentActiveUS20240025823A1
Innovation
- A method involving the photochlorination of a chloromethanes stream containing chloroform, methyl chloride, and methylene chloride, combined with chlorine and additional carbon tetrachloride, and subjected to electromagnetic radiation to form carbon tetrachloride, achieving high conversion rates with reduced levels of unwanted chlorinated hydrocarbons.
Chlorinolysis process for producing carbon tetrachloride
PatentActiveUS20210130266A1
Innovation
- A process involving a chlorination zone with chlorine, a C1 chlorinated compound, and a carbon/second chlorine source to produce a reaction mixture that favors the formation of carbon tetrachloride over perchloroethylene, using waste products as the carbon/second chlorine source to enhance efficiency and reduce impurity formation.
Environmental Impact of CCl4 Use
Carbon tetrachloride (CCl4) has long been used in various industrial chemical processes due to its unique properties. However, its environmental impact has become a significant concern, leading to strict regulations and efforts to phase out its use in many applications.
The release of CCl4 into the atmosphere has been identified as a major contributor to ozone depletion. As a chlorinated compound, it can persist in the atmosphere for decades, slowly breaking down and releasing chlorine atoms that catalyze the destruction of ozone molecules. This process weakens the Earth's protective ozone layer, increasing the amount of harmful ultraviolet radiation reaching the surface.
In aquatic environments, CCl4 contamination poses serious risks to ecosystems and human health. Its high stability and low water solubility allow it to persist in groundwater and surface water for extended periods. Aquatic organisms exposed to CCl4 may suffer from various toxic effects, including liver and kidney damage. Bioaccumulation of CCl4 in the food chain can lead to increased concentrations in higher-level predators, potentially affecting entire ecosystems.
Soil contamination by CCl4 is another significant environmental concern. When released into soil, it can volatilize and contaminate the air or leach into groundwater. This contamination can persist for years, affecting soil microorganisms, plant growth, and potentially entering the food chain through agricultural products.
The production and use of CCl4 also contribute to air pollution and the formation of photochemical smog. When released into the air, it can react with other pollutants in the presence of sunlight, forming harmful secondary pollutants that negatively impact air quality and human health.
Recognizing these environmental impacts, international agreements such as the Montreal Protocol have targeted the phase-out of CCl4 production and use. Many countries have implemented strict regulations on its handling, storage, and disposal. Industries have been encouraged to develop and adopt alternative substances and processes that are less harmful to the environment.
Efforts to mitigate the environmental impact of CCl4 include improved containment and handling practices in industrial settings, development of more efficient recycling and destruction technologies, and increased monitoring of environmental levels. Research into bioremediation techniques and advanced oxidation processes for treating CCl4-contaminated sites has shown promise in reducing its environmental persistence.
As awareness of the environmental consequences of CCl4 use has grown, there has been a shift towards greener alternatives in industrial processes. This transition not only addresses the immediate environmental concerns but also promotes the development of more sustainable chemical technologies for the future.
The release of CCl4 into the atmosphere has been identified as a major contributor to ozone depletion. As a chlorinated compound, it can persist in the atmosphere for decades, slowly breaking down and releasing chlorine atoms that catalyze the destruction of ozone molecules. This process weakens the Earth's protective ozone layer, increasing the amount of harmful ultraviolet radiation reaching the surface.
In aquatic environments, CCl4 contamination poses serious risks to ecosystems and human health. Its high stability and low water solubility allow it to persist in groundwater and surface water for extended periods. Aquatic organisms exposed to CCl4 may suffer from various toxic effects, including liver and kidney damage. Bioaccumulation of CCl4 in the food chain can lead to increased concentrations in higher-level predators, potentially affecting entire ecosystems.
Soil contamination by CCl4 is another significant environmental concern. When released into soil, it can volatilize and contaminate the air or leach into groundwater. This contamination can persist for years, affecting soil microorganisms, plant growth, and potentially entering the food chain through agricultural products.
The production and use of CCl4 also contribute to air pollution and the formation of photochemical smog. When released into the air, it can react with other pollutants in the presence of sunlight, forming harmful secondary pollutants that negatively impact air quality and human health.
Recognizing these environmental impacts, international agreements such as the Montreal Protocol have targeted the phase-out of CCl4 production and use. Many countries have implemented strict regulations on its handling, storage, and disposal. Industries have been encouraged to develop and adopt alternative substances and processes that are less harmful to the environment.
Efforts to mitigate the environmental impact of CCl4 include improved containment and handling practices in industrial settings, development of more efficient recycling and destruction technologies, and increased monitoring of environmental levels. Research into bioremediation techniques and advanced oxidation processes for treating CCl4-contaminated sites has shown promise in reducing its environmental persistence.
As awareness of the environmental consequences of CCl4 use has grown, there has been a shift towards greener alternatives in industrial processes. This transition not only addresses the immediate environmental concerns but also promotes the development of more sustainable chemical technologies for the future.
Regulatory Framework for CCl4 Handling
The regulatory framework for handling Carbon Tetrachloride (CCl4) in industrial chemical processes has evolved significantly over the years, reflecting growing concerns about its environmental and health impacts. At the international level, the Montreal Protocol on Substances that Deplete the Ozone Layer, which came into force in 1989, has been instrumental in phasing out the production and consumption of CCl4 along with other ozone-depleting substances.
In the United States, the Environmental Protection Agency (EPA) regulates CCl4 under various statutes. The Toxic Substances Control Act (TSCA) requires reporting, record-keeping, and testing requirements for CCl4. The Clean Air Act classifies CCl4 as a hazardous air pollutant, subjecting it to stringent emission controls. The Safe Drinking Water Act sets maximum contaminant levels for CCl4 in public water systems.
The Occupational Safety and Health Administration (OSHA) has established permissible exposure limits for CCl4 in the workplace. The current standard is 10 parts per million (ppm) as an 8-hour time-weighted average, with a ceiling limit of 25 ppm. OSHA also mandates specific safety measures, including personal protective equipment and engineering controls, for workers handling CCl4.
In the European Union, the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation governs the use of CCl4. Under REACH, CCl4 is classified as a substance of very high concern due to its carcinogenic properties and environmental persistence. Its use is severely restricted, requiring special authorization for specific applications.
Many countries have implemented their own regulations for CCl4 handling, often aligning with international agreements. For instance, China has banned the use of CCl4 as a solvent and cleaning agent in most industries, allowing its use only in limited, controlled applications.
The regulatory landscape for CCl4 continues to evolve, with a trend towards stricter controls and phase-out schedules. This has led to increased focus on developing alternative substances and processes in various industrial applications. Companies working with CCl4 must stay abreast of these regulatory changes and invest in compliance measures to ensure safe handling and minimize environmental impact.
In the United States, the Environmental Protection Agency (EPA) regulates CCl4 under various statutes. The Toxic Substances Control Act (TSCA) requires reporting, record-keeping, and testing requirements for CCl4. The Clean Air Act classifies CCl4 as a hazardous air pollutant, subjecting it to stringent emission controls. The Safe Drinking Water Act sets maximum contaminant levels for CCl4 in public water systems.
The Occupational Safety and Health Administration (OSHA) has established permissible exposure limits for CCl4 in the workplace. The current standard is 10 parts per million (ppm) as an 8-hour time-weighted average, with a ceiling limit of 25 ppm. OSHA also mandates specific safety measures, including personal protective equipment and engineering controls, for workers handling CCl4.
In the European Union, the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation governs the use of CCl4. Under REACH, CCl4 is classified as a substance of very high concern due to its carcinogenic properties and environmental persistence. Its use is severely restricted, requiring special authorization for specific applications.
Many countries have implemented their own regulations for CCl4 handling, often aligning with international agreements. For instance, China has banned the use of CCl4 as a solvent and cleaning agent in most industries, allowing its use only in limited, controlled applications.
The regulatory landscape for CCl4 continues to evolve, with a trend towards stricter controls and phase-out schedules. This has led to increased focus on developing alternative substances and processes in various industrial applications. Companies working with CCl4 must stay abreast of these regulatory changes and invest in compliance measures to ensure safe handling and minimize environmental impact.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!