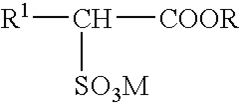How Carbon Tetrachloride is Used in Dry Cleaning Applications?
JUL 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CCl4 in Dry Cleaning: History and Objectives
Carbon tetrachloride (CCl4) has a long and complex history in the dry cleaning industry, dating back to the early 20th century. Initially introduced as a safer alternative to petroleum-based solvents, CCl4 quickly gained popularity due to its excellent cleaning properties and non-flammable nature. The compound's ability to dissolve a wide range of organic materials made it particularly effective in removing stubborn stains and dirt from various fabrics.
The use of CCl4 in dry cleaning applications reached its peak in the 1930s and 1940s. During this period, it was widely adopted by dry cleaning establishments across the United States and Europe. The compound's efficiency in cleaning delicate fabrics without causing damage or shrinkage contributed to its widespread use. Additionally, its relatively low cost and ease of handling made it an attractive option for small and large-scale dry cleaning operations alike.
However, as the understanding of environmental and health impacts grew, concerns about the use of CCl4 began to emerge. By the 1950s and 1960s, scientific studies started to reveal the potential hazards associated with prolonged exposure to CCl4 vapors. These findings led to a gradual shift away from its use in dry cleaning applications, with many countries implementing regulations to restrict or ban its use in consumer products.
The primary objective of using CCl4 in dry cleaning was to provide an effective, non-flammable cleaning agent that could safely remove stains and dirt from a variety of fabrics. Its ability to dissolve both polar and non-polar substances made it particularly versatile in tackling different types of stains. Furthermore, its low boiling point allowed for easy recovery and reuse, making it an economically viable option for dry cleaning businesses.
As environmental and health concerns grew, the objectives surrounding CCl4 use in dry cleaning evolved. The focus shifted towards finding safer alternatives that could match or exceed the cleaning efficacy of CCl4 while minimizing risks to human health and the environment. This led to the development of new dry cleaning technologies and solvents, such as perchloroethylene and hydrocarbon-based cleaners.
Today, the use of CCl4 in dry cleaning applications is largely historical, with most countries having phased out its use entirely. The evolution of dry cleaning technology serves as a prime example of how industry practices can adapt in response to scientific discoveries and changing societal priorities. The legacy of CCl4 in dry cleaning continues to influence modern cleaning methods and safety standards, driving ongoing research into more sustainable and environmentally friendly cleaning solutions.
The use of CCl4 in dry cleaning applications reached its peak in the 1930s and 1940s. During this period, it was widely adopted by dry cleaning establishments across the United States and Europe. The compound's efficiency in cleaning delicate fabrics without causing damage or shrinkage contributed to its widespread use. Additionally, its relatively low cost and ease of handling made it an attractive option for small and large-scale dry cleaning operations alike.
However, as the understanding of environmental and health impacts grew, concerns about the use of CCl4 began to emerge. By the 1950s and 1960s, scientific studies started to reveal the potential hazards associated with prolonged exposure to CCl4 vapors. These findings led to a gradual shift away from its use in dry cleaning applications, with many countries implementing regulations to restrict or ban its use in consumer products.
The primary objective of using CCl4 in dry cleaning was to provide an effective, non-flammable cleaning agent that could safely remove stains and dirt from a variety of fabrics. Its ability to dissolve both polar and non-polar substances made it particularly versatile in tackling different types of stains. Furthermore, its low boiling point allowed for easy recovery and reuse, making it an economically viable option for dry cleaning businesses.
As environmental and health concerns grew, the objectives surrounding CCl4 use in dry cleaning evolved. The focus shifted towards finding safer alternatives that could match or exceed the cleaning efficacy of CCl4 while minimizing risks to human health and the environment. This led to the development of new dry cleaning technologies and solvents, such as perchloroethylene and hydrocarbon-based cleaners.
Today, the use of CCl4 in dry cleaning applications is largely historical, with most countries having phased out its use entirely. The evolution of dry cleaning technology serves as a prime example of how industry practices can adapt in response to scientific discoveries and changing societal priorities. The legacy of CCl4 in dry cleaning continues to influence modern cleaning methods and safety standards, driving ongoing research into more sustainable and environmentally friendly cleaning solutions.
Market Analysis: Dry Cleaning Industry
The dry cleaning industry has undergone significant changes in recent years, driven by environmental concerns, technological advancements, and shifting consumer preferences. The global dry cleaning market size was valued at approximately $79 billion in 2020 and is projected to grow at a compound annual growth rate (CAGR) of 3.4% from 2021 to 2028. This growth is primarily attributed to the increasing demand for professional cleaning services in urban areas and the rising disposable income of consumers in developing countries.
Carbon tetrachloride, once a popular solvent in dry cleaning applications, has been phased out due to its harmful environmental and health effects. The industry has since shifted towards more eco-friendly alternatives, such as perchloroethylene (PERC), hydrocarbon solvents, and liquid carbon dioxide. PERC remains the most widely used solvent, accounting for about 70% of the dry cleaning market, despite growing concerns about its potential health risks.
The dry cleaning industry is highly fragmented, with small and medium-sized enterprises dominating the market. In the United States alone, there are approximately 30,000 dry cleaning establishments, with the majority being small, family-owned businesses. However, the industry is facing challenges due to the increasing popularity of casual wear and the trend towards more washable fabrics, which has led to a decline in the demand for traditional dry cleaning services.
Environmental regulations have significantly impacted the dry cleaning industry, particularly in developed countries. The European Union and several U.S. states have implemented strict regulations on the use of PERC and other potentially harmful solvents. This has led to increased adoption of alternative cleaning methods, such as wet cleaning and green dry cleaning, which use biodegradable solvents or water-based cleaning processes.
The COVID-19 pandemic has had a substantial impact on the dry cleaning industry, with many businesses experiencing a sharp decline in revenue due to lockdowns and reduced demand for professional attire. However, this has also accelerated the adoption of new technologies and services, such as contactless pickup and delivery, mobile apps for scheduling, and sanitization services for clothing and textiles.
Looking ahead, the dry cleaning industry is expected to focus on sustainability and innovation to meet changing consumer demands and regulatory requirements. This includes the development of more environmentally friendly solvents, energy-efficient equipment, and advanced cleaning technologies. Additionally, the industry is likely to see increased consolidation as larger chains and franchises expand their market share through acquisitions and technological investments.
Carbon tetrachloride, once a popular solvent in dry cleaning applications, has been phased out due to its harmful environmental and health effects. The industry has since shifted towards more eco-friendly alternatives, such as perchloroethylene (PERC), hydrocarbon solvents, and liquid carbon dioxide. PERC remains the most widely used solvent, accounting for about 70% of the dry cleaning market, despite growing concerns about its potential health risks.
The dry cleaning industry is highly fragmented, with small and medium-sized enterprises dominating the market. In the United States alone, there are approximately 30,000 dry cleaning establishments, with the majority being small, family-owned businesses. However, the industry is facing challenges due to the increasing popularity of casual wear and the trend towards more washable fabrics, which has led to a decline in the demand for traditional dry cleaning services.
Environmental regulations have significantly impacted the dry cleaning industry, particularly in developed countries. The European Union and several U.S. states have implemented strict regulations on the use of PERC and other potentially harmful solvents. This has led to increased adoption of alternative cleaning methods, such as wet cleaning and green dry cleaning, which use biodegradable solvents or water-based cleaning processes.
The COVID-19 pandemic has had a substantial impact on the dry cleaning industry, with many businesses experiencing a sharp decline in revenue due to lockdowns and reduced demand for professional attire. However, this has also accelerated the adoption of new technologies and services, such as contactless pickup and delivery, mobile apps for scheduling, and sanitization services for clothing and textiles.
Looking ahead, the dry cleaning industry is expected to focus on sustainability and innovation to meet changing consumer demands and regulatory requirements. This includes the development of more environmentally friendly solvents, energy-efficient equipment, and advanced cleaning technologies. Additionally, the industry is likely to see increased consolidation as larger chains and franchises expand their market share through acquisitions and technological investments.
CCl4 Usage: Current Status and Challenges
Carbon tetrachloride (CCl4) has been widely used in dry cleaning applications for decades due to its excellent solvent properties. However, its usage has faced significant challenges and restrictions in recent years due to environmental and health concerns.
Currently, the use of CCl4 in dry cleaning is severely limited or banned in many countries, including the United States and European Union member states. This is primarily due to its classification as an ozone-depleting substance under the Montreal Protocol and its potential carcinogenicity. Despite these restrictions, some developing countries still use CCl4 in dry cleaning processes, albeit in decreasing quantities.
The main challenge facing CCl4 usage in dry cleaning is finding suitable alternatives that offer comparable cleaning efficacy without the associated environmental and health risks. Many dry cleaning establishments have transitioned to perchloroethylene (PERC) as a replacement, but this solvent also faces scrutiny due to its own environmental concerns.
Another significant challenge is the proper disposal and management of existing CCl4 stocks. Many dry cleaning facilities still have remnant supplies of CCl4 that require careful handling and disposal to prevent environmental contamination and health hazards.
The dry cleaning industry is also grappling with the need to retrofit or replace equipment designed for CCl4 use. This transition involves substantial costs and technical challenges, particularly for small businesses operating on tight margins.
Regulatory compliance presents an ongoing challenge for businesses that continue to use CCl4 in regions where it is still permitted. Stringent safety protocols, emission controls, and worker protection measures must be implemented to mitigate risks associated with CCl4 exposure.
Research and development efforts are underway to address these challenges by developing safer, more environmentally friendly dry cleaning technologies. These include water-based cleaning systems, liquid carbon dioxide cleaning, and silicone-based solvents. However, these alternatives often face their own set of challenges, such as higher operational costs, different cleaning efficacy profiles, and the need for specialized equipment.
The dry cleaning industry is at a crossroads, balancing the need for effective cleaning solutions with environmental sustainability and public health concerns. As regulations continue to tighten globally, the future of CCl4 in dry cleaning applications appears increasingly limited, necessitating a shift towards more sustainable practices and technologies.
Currently, the use of CCl4 in dry cleaning is severely limited or banned in many countries, including the United States and European Union member states. This is primarily due to its classification as an ozone-depleting substance under the Montreal Protocol and its potential carcinogenicity. Despite these restrictions, some developing countries still use CCl4 in dry cleaning processes, albeit in decreasing quantities.
The main challenge facing CCl4 usage in dry cleaning is finding suitable alternatives that offer comparable cleaning efficacy without the associated environmental and health risks. Many dry cleaning establishments have transitioned to perchloroethylene (PERC) as a replacement, but this solvent also faces scrutiny due to its own environmental concerns.
Another significant challenge is the proper disposal and management of existing CCl4 stocks. Many dry cleaning facilities still have remnant supplies of CCl4 that require careful handling and disposal to prevent environmental contamination and health hazards.
The dry cleaning industry is also grappling with the need to retrofit or replace equipment designed for CCl4 use. This transition involves substantial costs and technical challenges, particularly for small businesses operating on tight margins.
Regulatory compliance presents an ongoing challenge for businesses that continue to use CCl4 in regions where it is still permitted. Stringent safety protocols, emission controls, and worker protection measures must be implemented to mitigate risks associated with CCl4 exposure.
Research and development efforts are underway to address these challenges by developing safer, more environmentally friendly dry cleaning technologies. These include water-based cleaning systems, liquid carbon dioxide cleaning, and silicone-based solvents. However, these alternatives often face their own set of challenges, such as higher operational costs, different cleaning efficacy profiles, and the need for specialized equipment.
The dry cleaning industry is at a crossroads, balancing the need for effective cleaning solutions with environmental sustainability and public health concerns. As regulations continue to tighten globally, the future of CCl4 in dry cleaning applications appears increasingly limited, necessitating a shift towards more sustainable practices and technologies.
CCl4-based Dry Cleaning Processes
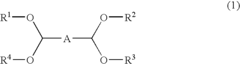
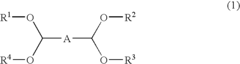
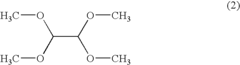
01 Production and purification of carbon tetrachloride
Various methods for producing and purifying carbon tetrachloride are described. These include chemical synthesis processes, distillation techniques, and purification methods to obtain high-quality carbon tetrachloride for industrial and laboratory use.- Production and purification of carbon tetrachloride: Various methods for producing and purifying carbon tetrachloride are described. These include chemical synthesis processes, distillation techniques, and purification methods to obtain high-quality carbon tetrachloride for industrial and laboratory use.
- Applications of carbon tetrachloride in chemical processes: Carbon tetrachloride is utilized in various chemical processes, including as a solvent, reagent, or intermediate in the production of other chemicals. Its applications span across different industries, showcasing its versatility in chemical manufacturing.
- Environmental and safety considerations: Due to its environmental impact and potential health hazards, research has been conducted on alternatives to carbon tetrachloride and methods for its safe handling, disposal, and remediation. This includes developing eco-friendly substitutes and improving safety protocols in its use.
- Detection and analysis methods: Various techniques and apparatus have been developed for detecting and analyzing carbon tetrachloride in different environments. These include spectroscopic methods, chromatography, and specialized sensors for monitoring its presence in air, water, or other media.
- Historical uses and patents: Early patents and historical documents reveal the diverse applications of carbon tetrachloride in the past, including its use in fire extinguishers, dry cleaning, and as a refrigerant. These historical patents provide insight into the evolution of carbon tetrachloride's industrial applications over time.
02 Applications of carbon tetrachloride in chemical processes
Carbon tetrachloride is utilized in various chemical processes, including as a solvent, reagent, or intermediate in organic synthesis. It plays a role in the production of other chlorinated compounds and in certain industrial applications.Expand Specific Solutions03 Environmental and safety considerations
Due to its environmental impact and health hazards, research focuses on alternatives to carbon tetrachloride and methods for its safe handling, storage, and disposal. This includes developing environmentally friendly substitutes and improving containment strategies.Expand Specific Solutions04 Detection and analysis methods
Techniques for detecting and analyzing carbon tetrachloride in various matrices are developed. These include spectroscopic methods, chromatographic techniques, and sensor-based approaches for monitoring carbon tetrachloride levels in air, water, and soil.Expand Specific Solutions05 Historical uses and regulations
The historical applications of carbon tetrachloride, such as in fire extinguishers and dry cleaning, are documented. Regulatory changes and phase-out programs due to its ozone-depleting properties and toxicity are also discussed.Expand Specific Solutions
Key Players in Dry Cleaning Chemical Industry
The competitive landscape for carbon tetrachloride in dry cleaning applications is characterized by a mature industry in a declining phase. The market size has been shrinking due to environmental and health concerns, leading to strict regulations on its use. Technologically, carbon tetrachloride is considered outdated for dry cleaning, with more eco-friendly alternatives gaining prominence. Companies like Whirlpool, LG Electronics, and Samsung Electronics have shifted focus to developing greener dry cleaning technologies and appliances. Research institutions such as the University of Leeds and Xiamen University are likely exploring safer substitutes, while chemical companies like Croda International and Clariant are adapting their product portfolios to meet evolving industry needs and regulations.
Xeros Ltd.
Technical Solution: Xeros Ltd. has developed a revolutionary polymer bead cleaning technology as an alternative to traditional dry cleaning methods using solvents like carbon tetrachloride. Their system uses specially designed polymer beads that attract and absorb dirt from fabrics, reducing water and chemical usage by up to 80%[4]. The Xeros process involves a unique drum design that optimizes the interaction between the beads, garments, and a small amount of water and detergent. This method not only cleans effectively but also helps extend the life of fabrics by reducing mechanical action[5]. While not directly using carbon tetrachloride, Xeros' technology addresses the same cleaning needs while offering a more environmentally friendly solution[6].
Strengths: Significantly reduced environmental impact, water and energy savings, and gentler on fabrics. Weaknesses: May require specialized equipment and training for adoption, potentially higher initial investment costs.
Whirlpool Corp.
Technical Solution: Whirlpool Corp. has developed advanced dry cleaning technologies that aim to replace traditional solvent-based methods, including those using carbon tetrachloride. Their approach focuses on integrating smart technologies and alternative cleaning methods into home appliances. Whirlpool's solution includes a combination of steam cleaning, air-based refreshing, and specialized detergents designed for delicate fabrics[9]. The company has also invested in developing sensor technologies that can detect fabric types and soil levels, optimizing the cleaning process for each garment. While not directly using carbon tetrachloride, Whirlpool's innovations address the same cleaning needs while offering more accessible and environmentally friendly options for consumers[10].
Strengths: Consumer-friendly solutions, integration of smart technologies, and reduced environmental impact. Weaknesses: May not be suitable for all fabric types traditionally cleaned with carbon tetrachloride, limited to home use rather than commercial applications.
Innovations in CCl4 Dry Cleaning Technology
Method for Chemically Cleaning Textile Material
PatentInactiveUS20090031504A1
Innovation
- The use of acetals with specific chemical structures as organic solvents, which offer superior cleaning capacity for fats and oils, reduced graying, and improved toxicological and ecological profiles, while meeting the requirements for dry cleaning processes, including low retention in textiles and minimal impact on fibers and dyes.
Novel cleaning method
PatentActiveUS20090217461A1
Innovation
- A solvent-free dry cleaning method using polymeric particles, such as nylon chips, with optional surfactant coating, that effectively removes stains from textile fibers with minimal water usage, avoiding the need for organic solvents and high-pressure systems.
Environmental Impact and Regulations
The use of carbon tetrachloride in dry cleaning applications has raised significant environmental concerns and prompted regulatory actions worldwide. This chemical, once widely used in the dry cleaning industry, has been identified as a potent ozone-depleting substance and a potential carcinogen. Its release into the atmosphere contributes to the depletion of the ozone layer, which protects Earth from harmful ultraviolet radiation.
The environmental impact of carbon tetrachloride extends beyond atmospheric effects. When released into soil and water, it can persist for long periods, potentially contaminating groundwater and affecting aquatic ecosystems. Its high mobility in soil and resistance to biodegradation make it a long-term environmental hazard. Studies have shown that carbon tetrachloride can accumulate in the food chain, posing risks to wildlife and human health through indirect exposure.
In response to these environmental concerns, numerous regulations have been implemented globally to restrict or ban the use of carbon tetrachloride in dry cleaning and other applications. The Montreal Protocol, an international treaty designed to protect the ozone layer, has played a crucial role in phasing out the production and consumption of ozone-depleting substances, including carbon tetrachloride.
In the United States, the Environmental Protection Agency (EPA) has classified carbon tetrachloride as a hazardous air pollutant under the Clean Air Act. Its use in consumer products, including dry cleaning, has been banned since 1970. The EPA also regulates carbon tetrachloride under the Toxic Substances Control Act and the Resource Conservation and Recovery Act, which govern its disposal and management as a hazardous waste.
The European Union has similarly implemented strict regulations on carbon tetrachloride. Under the REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) regulation, its use is severely restricted, and it is subject to authorization for specific applications. The EU has also set stringent emission limits for industrial processes that may release carbon tetrachloride.
Many countries have adopted alternatives to carbon tetrachloride in dry cleaning, such as perchloroethylene and newer, more environmentally friendly solvents. However, the legacy of carbon tetrachloride use continues to pose challenges. Contaminated sites require extensive remediation efforts, and ongoing monitoring is necessary to ensure compliance with environmental regulations.
The regulatory landscape surrounding carbon tetrachloride continues to evolve as new scientific evidence emerges about its environmental and health impacts. International cooperation remains crucial in addressing the global nature of ozone depletion and enforcing regulations across borders. As the dry cleaning industry adapts to these regulations, research into safer and more sustainable cleaning technologies continues to be a priority.
The environmental impact of carbon tetrachloride extends beyond atmospheric effects. When released into soil and water, it can persist for long periods, potentially contaminating groundwater and affecting aquatic ecosystems. Its high mobility in soil and resistance to biodegradation make it a long-term environmental hazard. Studies have shown that carbon tetrachloride can accumulate in the food chain, posing risks to wildlife and human health through indirect exposure.
In response to these environmental concerns, numerous regulations have been implemented globally to restrict or ban the use of carbon tetrachloride in dry cleaning and other applications. The Montreal Protocol, an international treaty designed to protect the ozone layer, has played a crucial role in phasing out the production and consumption of ozone-depleting substances, including carbon tetrachloride.
In the United States, the Environmental Protection Agency (EPA) has classified carbon tetrachloride as a hazardous air pollutant under the Clean Air Act. Its use in consumer products, including dry cleaning, has been banned since 1970. The EPA also regulates carbon tetrachloride under the Toxic Substances Control Act and the Resource Conservation and Recovery Act, which govern its disposal and management as a hazardous waste.
The European Union has similarly implemented strict regulations on carbon tetrachloride. Under the REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) regulation, its use is severely restricted, and it is subject to authorization for specific applications. The EU has also set stringent emission limits for industrial processes that may release carbon tetrachloride.
Many countries have adopted alternatives to carbon tetrachloride in dry cleaning, such as perchloroethylene and newer, more environmentally friendly solvents. However, the legacy of carbon tetrachloride use continues to pose challenges. Contaminated sites require extensive remediation efforts, and ongoing monitoring is necessary to ensure compliance with environmental regulations.
The regulatory landscape surrounding carbon tetrachloride continues to evolve as new scientific evidence emerges about its environmental and health impacts. International cooperation remains crucial in addressing the global nature of ozone depletion and enforcing regulations across borders. As the dry cleaning industry adapts to these regulations, research into safer and more sustainable cleaning technologies continues to be a priority.
Health and Safety Considerations
Carbon tetrachloride, once widely used in dry cleaning applications, poses significant health and safety risks that have led to its phaseout in many countries. Exposure to this chemical can occur through inhalation, skin contact, or ingestion, with potentially severe consequences for human health. Acute exposure may cause dizziness, headaches, nausea, and in severe cases, liver and kidney damage. Chronic exposure has been linked to an increased risk of cancer, particularly liver cancer.
The primary concern in dry cleaning facilities is the potential for worker exposure during handling and use of carbon tetrachloride. Proper ventilation systems are crucial to minimize inhalation risks, while personal protective equipment (PPE) such as chemical-resistant gloves, goggles, and respirators are essential for workers directly handling the substance. Strict protocols for storage, handling, and disposal of carbon tetrachloride are necessary to prevent accidental spills or releases.
Environmental considerations are also paramount, as carbon tetrachloride can persist in soil and groundwater, posing long-term contamination risks. Its ozone-depleting properties have led to its regulation under the Montreal Protocol, further restricting its use globally. Proper disposal methods, including treatment as hazardous waste, are critical to prevent environmental contamination.
Given these risks, many countries have implemented stringent regulations or outright bans on the use of carbon tetrachloride in dry cleaning. Alternative solvents and technologies, such as perchloroethylene, hydrocarbon solvents, and wet cleaning methods, have been developed to replace carbon tetrachloride. These alternatives generally offer improved safety profiles, though they may come with their own set of health and environmental considerations.
For facilities still using carbon tetrachloride, regular health monitoring of workers is essential. This includes periodic medical examinations and blood tests to detect early signs of liver or kidney damage. Emergency response plans should be in place to address potential exposures or spills, including decontamination procedures and first aid measures.
Public health concerns extend beyond the workplace, as residual carbon tetrachloride on cleaned garments could potentially expose consumers. This risk, although minimal with proper cleaning processes, underscores the importance of adhering to recommended cleaning and drying times to ensure complete solvent removal from garments before they are returned to customers.
The primary concern in dry cleaning facilities is the potential for worker exposure during handling and use of carbon tetrachloride. Proper ventilation systems are crucial to minimize inhalation risks, while personal protective equipment (PPE) such as chemical-resistant gloves, goggles, and respirators are essential for workers directly handling the substance. Strict protocols for storage, handling, and disposal of carbon tetrachloride are necessary to prevent accidental spills or releases.
Environmental considerations are also paramount, as carbon tetrachloride can persist in soil and groundwater, posing long-term contamination risks. Its ozone-depleting properties have led to its regulation under the Montreal Protocol, further restricting its use globally. Proper disposal methods, including treatment as hazardous waste, are critical to prevent environmental contamination.
Given these risks, many countries have implemented stringent regulations or outright bans on the use of carbon tetrachloride in dry cleaning. Alternative solvents and technologies, such as perchloroethylene, hydrocarbon solvents, and wet cleaning methods, have been developed to replace carbon tetrachloride. These alternatives generally offer improved safety profiles, though they may come with their own set of health and environmental considerations.
For facilities still using carbon tetrachloride, regular health monitoring of workers is essential. This includes periodic medical examinations and blood tests to detect early signs of liver or kidney damage. Emergency response plans should be in place to address potential exposures or spills, including decontamination procedures and first aid measures.
Public health concerns extend beyond the workplace, as residual carbon tetrachloride on cleaned garments could potentially expose consumers. This risk, although minimal with proper cleaning processes, underscores the importance of adhering to recommended cleaning and drying times to ensure complete solvent removal from garments before they are returned to customers.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!