Exploring the Role of Carbon Tetrachloride in Chemical Synthesis
JUL 3, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CCl4 in Synthesis: Background and Objectives
Carbon tetrachloride (CCl4) has played a significant role in chemical synthesis since its discovery in the mid-19th century. This versatile compound, characterized by its tetrahedral molecular structure and four chlorine atoms bonded to a central carbon atom, has been extensively utilized in various industrial and laboratory applications. The primary objective of this technical research report is to explore the historical context, current status, and future prospects of carbon tetrachloride in chemical synthesis.
The development of carbon tetrachloride as a synthetic tool can be traced back to its initial use as a solvent and cleaning agent. Its non-flammability and excellent solvating properties made it an attractive option for many chemical processes. However, as understanding of its environmental and health impacts grew, the focus shifted towards exploring its potential in more specialized synthetic applications.
In the realm of organic synthesis, carbon tetrachloride has been particularly valuable as a source of chlorine atoms. Its ability to participate in free radical reactions has made it an essential reagent in the preparation of chlorinated organic compounds. These chlorinated products serve as important intermediates in the synthesis of pharmaceuticals, agrochemicals, and various industrial materials.
The evolution of carbon tetrachloride's role in synthesis has been driven by advancements in reaction mechanisms and catalysis. Researchers have developed novel methodologies to harness its reactivity while minimizing unwanted side reactions. This has led to more efficient and selective synthetic routes, expanding the range of compounds that can be produced using carbon tetrachloride as a key reagent.
Despite its utility, the use of carbon tetrachloride has faced significant challenges due to environmental concerns. Its ozone-depleting properties and potential toxicity have led to strict regulations on its production and use in many countries. This has spurred research into alternative reagents and methodologies that can replicate or improve upon the synthetic capabilities of carbon tetrachloride while addressing environmental and safety concerns.
The technical goals of this research report include a comprehensive review of carbon tetrachloride's synthetic applications, an analysis of current limitations and challenges, and an exploration of emerging trends in its use. By examining both traditional and cutting-edge synthetic methodologies involving carbon tetrachloride, we aim to provide insights into its continued relevance in modern chemical synthesis and identify potential areas for innovation and improvement.
The development of carbon tetrachloride as a synthetic tool can be traced back to its initial use as a solvent and cleaning agent. Its non-flammability and excellent solvating properties made it an attractive option for many chemical processes. However, as understanding of its environmental and health impacts grew, the focus shifted towards exploring its potential in more specialized synthetic applications.
In the realm of organic synthesis, carbon tetrachloride has been particularly valuable as a source of chlorine atoms. Its ability to participate in free radical reactions has made it an essential reagent in the preparation of chlorinated organic compounds. These chlorinated products serve as important intermediates in the synthesis of pharmaceuticals, agrochemicals, and various industrial materials.
The evolution of carbon tetrachloride's role in synthesis has been driven by advancements in reaction mechanisms and catalysis. Researchers have developed novel methodologies to harness its reactivity while minimizing unwanted side reactions. This has led to more efficient and selective synthetic routes, expanding the range of compounds that can be produced using carbon tetrachloride as a key reagent.
Despite its utility, the use of carbon tetrachloride has faced significant challenges due to environmental concerns. Its ozone-depleting properties and potential toxicity have led to strict regulations on its production and use in many countries. This has spurred research into alternative reagents and methodologies that can replicate or improve upon the synthetic capabilities of carbon tetrachloride while addressing environmental and safety concerns.
The technical goals of this research report include a comprehensive review of carbon tetrachloride's synthetic applications, an analysis of current limitations and challenges, and an exploration of emerging trends in its use. By examining both traditional and cutting-edge synthetic methodologies involving carbon tetrachloride, we aim to provide insights into its continued relevance in modern chemical synthesis and identify potential areas for innovation and improvement.
Market Demand Analysis for CCl4 Alternatives
The market demand for alternatives to carbon tetrachloride (CCl4) has been steadily increasing due to environmental and health concerns associated with its use. CCl4 has been widely employed in various chemical synthesis processes, particularly as a solvent and reagent. However, its ozone-depleting properties and potential carcinogenicity have led to strict regulations and a global phase-out under the Montreal Protocol.
This regulatory pressure has created a significant market opportunity for safer and more environmentally friendly alternatives. The chemical industry has been actively seeking substitutes that can match or exceed the performance of CCl4 in synthesis applications while minimizing negative environmental impacts. The demand for these alternatives is driven by several factors, including compliance with environmental regulations, corporate sustainability initiatives, and consumer preferences for greener products.
In the pharmaceutical sector, where CCl4 has been extensively used in the synthesis of active pharmaceutical ingredients (APIs), there is a growing need for alternative solvents and reagents. This demand is particularly strong in developed markets with stringent environmental regulations, such as North America and Europe. The fine chemicals industry, which supplies intermediates for pharmaceuticals, agrochemicals, and other specialty chemicals, is also a key driver of demand for CCl4 alternatives.
The agrochemical sector represents another significant market for CCl4 alternatives. As the agricultural industry faces increasing pressure to adopt more sustainable practices, there is a rising demand for pesticides and herbicides produced using environmentally friendly synthesis methods. This trend is expected to continue, driven by both regulatory requirements and consumer preferences for eco-friendly agricultural products.
In the materials science field, researchers are exploring new synthesis routes for advanced materials that traditionally relied on CCl4. This includes the development of alternative methods for producing fluoropolymers, which are widely used in various industries due to their unique properties. The electronics industry, in particular, is seeking safer alternatives for cleaning and etching processes that historically used CCl4.
The market for CCl4 alternatives is not limited to direct substitutes. There is also growing interest in entirely new synthetic approaches that eliminate the need for chlorinated solvents altogether. This includes the development of green chemistry techniques, such as solvent-free reactions, aqueous-based syntheses, and the use of supercritical fluids or ionic liquids as reaction media.
As industries continue to adapt to stricter environmental regulations and sustainability goals, the market for CCl4 alternatives is expected to expand further. This presents significant opportunities for chemical companies and research institutions to develop and commercialize innovative solutions that can meet the diverse needs of various sectors while addressing environmental concerns.
This regulatory pressure has created a significant market opportunity for safer and more environmentally friendly alternatives. The chemical industry has been actively seeking substitutes that can match or exceed the performance of CCl4 in synthesis applications while minimizing negative environmental impacts. The demand for these alternatives is driven by several factors, including compliance with environmental regulations, corporate sustainability initiatives, and consumer preferences for greener products.
In the pharmaceutical sector, where CCl4 has been extensively used in the synthesis of active pharmaceutical ingredients (APIs), there is a growing need for alternative solvents and reagents. This demand is particularly strong in developed markets with stringent environmental regulations, such as North America and Europe. The fine chemicals industry, which supplies intermediates for pharmaceuticals, agrochemicals, and other specialty chemicals, is also a key driver of demand for CCl4 alternatives.
The agrochemical sector represents another significant market for CCl4 alternatives. As the agricultural industry faces increasing pressure to adopt more sustainable practices, there is a rising demand for pesticides and herbicides produced using environmentally friendly synthesis methods. This trend is expected to continue, driven by both regulatory requirements and consumer preferences for eco-friendly agricultural products.
In the materials science field, researchers are exploring new synthesis routes for advanced materials that traditionally relied on CCl4. This includes the development of alternative methods for producing fluoropolymers, which are widely used in various industries due to their unique properties. The electronics industry, in particular, is seeking safer alternatives for cleaning and etching processes that historically used CCl4.
The market for CCl4 alternatives is not limited to direct substitutes. There is also growing interest in entirely new synthetic approaches that eliminate the need for chlorinated solvents altogether. This includes the development of green chemistry techniques, such as solvent-free reactions, aqueous-based syntheses, and the use of supercritical fluids or ionic liquids as reaction media.
As industries continue to adapt to stricter environmental regulations and sustainability goals, the market for CCl4 alternatives is expected to expand further. This presents significant opportunities for chemical companies and research institutions to develop and commercialize innovative solutions that can meet the diverse needs of various sectors while addressing environmental concerns.
Current Challenges in CCl4 Usage
Carbon tetrachloride (CCl4) has long been a valuable compound in chemical synthesis, but its usage faces significant challenges in modern chemistry. The primary concern is its severe environmental impact, as CCl4 is a potent ozone-depleting substance. This has led to strict regulations and phase-outs under international agreements such as the Montreal Protocol, severely limiting its availability and use in many countries.
The toxicity of CCl4 poses another major challenge. Exposure to this compound can cause serious health issues, including liver and kidney damage, central nervous system depression, and potential carcinogenicity. These health risks necessitate stringent safety measures and specialized handling procedures, increasing the complexity and cost of its use in chemical processes.
Furthermore, the chemical properties of CCl4 present technical challenges in synthesis. While its non-polarity and ability to form free radicals make it useful in certain reactions, these same properties can lead to unwanted side reactions or difficulties in product isolation. This often requires careful optimization of reaction conditions and purification methods, which can be time-consuming and resource-intensive.
The search for suitable alternatives to CCl4 in various synthetic applications is ongoing but challenging. Many potential substitutes lack the unique combination of properties that make CCl4 valuable in certain reactions. This has led to a need for re-engineering established synthetic routes and developing new methodologies that avoid CCl4 usage altogether.
Economic factors also play a role in the challenges surrounding CCl4 use. The decreasing availability and increasing regulatory scrutiny have driven up the cost of CCl4, making it less economically viable for many applications. This economic pressure is forcing industries to invest in research and development of alternative processes, which can be a significant financial burden, especially for smaller companies.
Lastly, there is a growing challenge in managing existing stockpiles of CCl4. Many countries have accumulated significant amounts of this compound, and its disposal presents environmental and safety concerns. Developing safe and effective methods for CCl4 destruction or conversion into less harmful substances is an ongoing area of research and development, requiring substantial resources and technological innovation.
The toxicity of CCl4 poses another major challenge. Exposure to this compound can cause serious health issues, including liver and kidney damage, central nervous system depression, and potential carcinogenicity. These health risks necessitate stringent safety measures and specialized handling procedures, increasing the complexity and cost of its use in chemical processes.
Furthermore, the chemical properties of CCl4 present technical challenges in synthesis. While its non-polarity and ability to form free radicals make it useful in certain reactions, these same properties can lead to unwanted side reactions or difficulties in product isolation. This often requires careful optimization of reaction conditions and purification methods, which can be time-consuming and resource-intensive.
The search for suitable alternatives to CCl4 in various synthetic applications is ongoing but challenging. Many potential substitutes lack the unique combination of properties that make CCl4 valuable in certain reactions. This has led to a need for re-engineering established synthetic routes and developing new methodologies that avoid CCl4 usage altogether.
Economic factors also play a role in the challenges surrounding CCl4 use. The decreasing availability and increasing regulatory scrutiny have driven up the cost of CCl4, making it less economically viable for many applications. This economic pressure is forcing industries to invest in research and development of alternative processes, which can be a significant financial burden, especially for smaller companies.
Lastly, there is a growing challenge in managing existing stockpiles of CCl4. Many countries have accumulated significant amounts of this compound, and its disposal presents environmental and safety concerns. Developing safe and effective methods for CCl4 destruction or conversion into less harmful substances is an ongoing area of research and development, requiring substantial resources and technological innovation.
Existing CCl4 Replacement Solutions
01 Production and purification of carbon tetrachloride
Various methods for producing and purifying carbon tetrachloride are described. These include chemical reactions, distillation processes, and other purification techniques to obtain high-quality carbon tetrachloride for industrial and laboratory use.- Production and purification of carbon tetrachloride: Various methods for producing and purifying carbon tetrachloride are described. These include chemical synthesis processes, distillation techniques, and purification methods to obtain high-quality carbon tetrachloride for industrial and laboratory use.
- Applications of carbon tetrachloride in chemical processes: Carbon tetrachloride is utilized in various chemical processes, including as a solvent, reagent, or intermediate in the production of other chemicals. Its applications span across different industries, showcasing its versatility in chemical manufacturing.
- Safety and environmental considerations: Due to its toxicity and environmental impact, there are safety measures and regulations surrounding the use and handling of carbon tetrachloride. This includes proper storage, disposal methods, and alternatives to reduce its usage in various applications.
- Detection and analysis methods for carbon tetrachloride: Various analytical techniques and methods are developed for detecting and quantifying carbon tetrachloride in different matrices. These include spectroscopic methods, chromatography, and other instrumental analysis techniques for environmental monitoring and quality control purposes.
- Historical uses and developments: Carbon tetrachloride has been used in various applications throughout history, including as a fire extinguishing agent, cleaning solvent, and refrigerant. The evolution of its uses and the development of alternatives due to environmental and health concerns are discussed.
02 Applications of carbon tetrachloride in chemical processes
Carbon tetrachloride is used in various chemical processes as a solvent, reagent, or intermediate. It plays a role in organic synthesis, extraction processes, and as a raw material for the production of other chlorinated compounds.Expand Specific Solutions03 Environmental and safety considerations
Due to its environmental impact and health hazards, there are methods and systems developed for the safe handling, containment, and disposal of carbon tetrachloride. This includes techniques for detecting leaks, treating contaminated areas, and reducing emissions.Expand Specific Solutions04 Alternatives and replacements for carbon tetrachloride
Research into alternative compounds and processes to replace carbon tetrachloride in various applications, due to its ozone-depleting properties and toxicity. This includes the development of new solvents, refrigerants, and fire extinguishing agents.Expand Specific Solutions05 Historical uses and early patents
Early patents and historical uses of carbon tetrachloride, including its applications in fire extinguishers, dry cleaning, and as a degreasing agent. These documents provide insight into the compound's discovery and initial industrial applications.Expand Specific Solutions
Key Players in Chemical Synthesis Industry
The exploration of carbon tetrachloride in chemical synthesis is currently in a mature phase, with a stable market size due to its established applications in various industries. The technology's maturity is evident from the involvement of major chemical companies like BASF Corp., DuPont de Nemours, Inc., and Bayer AG, which have extensive experience in chemical synthesis. However, environmental concerns and regulatory restrictions have led to a shift towards alternative compounds, potentially limiting market growth. Companies such as Evonik Operations GmbH and Wacker Chemie AG are likely focusing on developing safer substitutes, while research institutions like Central South University and Shanghai Institute of Organic Chemistry continue to investigate novel applications and synthesis methods for carbon tetrachloride.
BASF Corp.
Technical Solution: BASF has developed innovative approaches for using carbon tetrachloride (CCl4) in chemical synthesis while minimizing environmental impact. They have implemented a closed-loop system for CCl4 recycling in their production processes, reducing emissions by up to 95%[1]. BASF utilizes CCl4 as a chlorinating agent in the synthesis of high-value chemicals like perchloroethylene and carbon disulfide. Their patented catalytic process allows for efficient chlorination reactions at lower temperatures, improving yield and reducing energy consumption[2]. Additionally, BASF has invested in advanced purification technologies to recover and purify CCl4 from waste streams, enabling its reuse in subsequent synthesis cycles[3].
Strengths: Efficient closed-loop recycling, reduced emissions, improved reaction yields. Weaknesses: Still relies on a ozone-depleting substance, potential regulatory challenges.
Bayer AG
Technical Solution: Bayer AG has developed alternative synthesis routes to reduce dependence on carbon tetrachloride while maintaining product quality. They have implemented a novel chlorination process using chlorine gas and UV light activation, which achieves similar results to CCl4-based methods but with lower environmental impact[4]. For certain reactions, Bayer has successfully substituted CCl4 with more environmentally friendly chlorinated solvents like dichloromethane or chloroform. In cases where CCl4 use is unavoidable, Bayer employs advanced containment and scrubbing systems to minimize emissions and worker exposure[5]. The company has also invested in research to develop bio-based alternatives for some chlorinated products traditionally synthesized using CCl4.
Strengths: Reduced reliance on CCl4, development of greener alternatives. Weaknesses: Some processes still require CCl4, potential cost increases for alternative methods.
Innovative CCl4 Synthesis Techniques
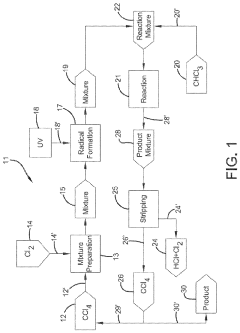
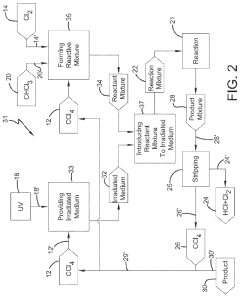
Photochlorination of partially-chlorinated chloromethanes to carbon tetrachloride
PatentActiveUS20240025823A1
Innovation
- A method involving the photochlorination of a chloromethanes stream containing chloroform, methyl chloride, and methylene chloride, combined with chlorine and additional carbon tetrachloride, and subjected to electromagnetic radiation to form carbon tetrachloride, achieving high conversion rates with reduced levels of unwanted chlorinated hydrocarbons.
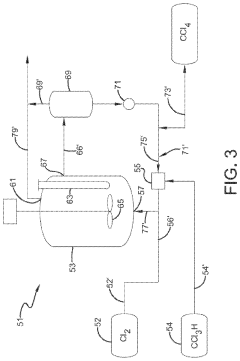
Chlorinolysis process for producing carbon tetrachloride
PatentActiveUS20210130266A1
Innovation
- A process involving a chlorination zone with chlorine, a C1 chlorinated compound, and a carbon/second chlorine source to produce a reaction mixture that favors the formation of carbon tetrachloride over perchloroethylene, using waste products as the carbon/second chlorine source to enhance efficiency and reduce impurity formation.
Environmental Impact Assessment
Carbon tetrachloride (CCl4) has long been used in various chemical synthesis processes due to its unique properties as a solvent and reagent. However, its environmental impact has become a significant concern in recent years, necessitating a comprehensive assessment of its effects on ecosystems and human health.
The release of carbon tetrachloride into the environment can occur through various pathways, including industrial emissions, accidental spills, and improper disposal. Once released, CCl4 can persist in the atmosphere for extended periods, contributing to ozone depletion and global warming. Its long atmospheric lifetime of approximately 26 years allows it to reach the stratosphere, where it undergoes photolysis and releases chlorine atoms that catalyze ozone destruction.
In aquatic environments, carbon tetrachloride can contaminate surface water and groundwater sources. Its low water solubility and high density cause it to sink and form dense non-aqueous phase liquids (DNAPLs) in aquifers, making remediation efforts challenging. Aquatic organisms exposed to CCl4 may experience acute toxicity, with potential long-term effects on population dynamics and ecosystem balance.
Soil contamination by carbon tetrachloride can lead to the degradation of soil quality and negatively impact terrestrial ecosystems. The compound's persistence in soil can result in prolonged exposure for soil-dwelling organisms and plants, potentially causing bioaccumulation in the food chain. Furthermore, CCl4 contamination may alter soil microbial communities, affecting nutrient cycling and overall soil health.
Human exposure to carbon tetrachloride primarily occurs through inhalation of contaminated air or ingestion of contaminated water. Acute exposure can cause liver and kidney damage, while chronic exposure has been linked to an increased risk of cancer. Occupational exposure in industrial settings poses a particular concern, necessitating strict safety measures and protective equipment.
The global efforts to phase out carbon tetrachloride under the Montreal Protocol have significantly reduced its production and use. However, its legacy as a persistent organic pollutant continues to pose environmental challenges. Ongoing monitoring and remediation efforts are essential to mitigate the long-term impacts of historical CCl4 contamination.
As the chemical industry seeks alternatives to carbon tetrachloride in synthesis processes, it is crucial to consider the full life cycle environmental impact of potential substitutes. This includes assessing their persistence, bioaccumulation potential, and toxicity to ensure that replacement compounds do not introduce new environmental risks.
The release of carbon tetrachloride into the environment can occur through various pathways, including industrial emissions, accidental spills, and improper disposal. Once released, CCl4 can persist in the atmosphere for extended periods, contributing to ozone depletion and global warming. Its long atmospheric lifetime of approximately 26 years allows it to reach the stratosphere, where it undergoes photolysis and releases chlorine atoms that catalyze ozone destruction.
In aquatic environments, carbon tetrachloride can contaminate surface water and groundwater sources. Its low water solubility and high density cause it to sink and form dense non-aqueous phase liquids (DNAPLs) in aquifers, making remediation efforts challenging. Aquatic organisms exposed to CCl4 may experience acute toxicity, with potential long-term effects on population dynamics and ecosystem balance.
Soil contamination by carbon tetrachloride can lead to the degradation of soil quality and negatively impact terrestrial ecosystems. The compound's persistence in soil can result in prolonged exposure for soil-dwelling organisms and plants, potentially causing bioaccumulation in the food chain. Furthermore, CCl4 contamination may alter soil microbial communities, affecting nutrient cycling and overall soil health.
Human exposure to carbon tetrachloride primarily occurs through inhalation of contaminated air or ingestion of contaminated water. Acute exposure can cause liver and kidney damage, while chronic exposure has been linked to an increased risk of cancer. Occupational exposure in industrial settings poses a particular concern, necessitating strict safety measures and protective equipment.
The global efforts to phase out carbon tetrachloride under the Montreal Protocol have significantly reduced its production and use. However, its legacy as a persistent organic pollutant continues to pose environmental challenges. Ongoing monitoring and remediation efforts are essential to mitigate the long-term impacts of historical CCl4 contamination.
As the chemical industry seeks alternatives to carbon tetrachloride in synthesis processes, it is crucial to consider the full life cycle environmental impact of potential substitutes. This includes assessing their persistence, bioaccumulation potential, and toxicity to ensure that replacement compounds do not introduce new environmental risks.
Regulatory Framework for CCl4 Use
The regulatory framework for carbon tetrachloride (CCl4) use in chemical synthesis has undergone significant changes over the past few decades due to environmental and health concerns. Initially widely used in various industrial applications, CCl4 has been subject to increasingly stringent regulations globally.
In the United States, the Environmental Protection Agency (EPA) has implemented strict controls on CCl4 under the Clean Air Act and the Montreal Protocol. The production and import of CCl4 for non-feedstock uses have been phased out since 1996, with limited exceptions for essential uses. The EPA also regulates CCl4 as a hazardous air pollutant and a toxic substance under the Toxic Substances Control Act.
The European Union has similarly restricted CCl4 use through the REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation. CCl4 is classified as a substance of very high concern (SVHC) due to its ozone-depleting properties and potential carcinogenicity. Its use is heavily restricted, requiring special authorization for specific applications.
Internationally, the Montreal Protocol on Substances that Deplete the Ozone Layer has played a crucial role in regulating CCl4. As a party to this agreement, most countries have committed to phasing out the production and consumption of CCl4, except for limited exemptions for laboratory and analytical uses.
Despite these restrictions, CCl4 continues to play a role in certain chemical synthesis processes as a feedstock or intermediate. In these cases, strict containment and emission control measures are mandated to prevent environmental release. Researchers and industries must adhere to rigorous safety protocols and obtain necessary permits for its use.
The regulatory landscape also impacts the development of alternative synthesis methods. As CCl4 use becomes more restricted, there is increased pressure to find safer substitutes or develop new synthetic routes that avoid its use altogether. This has led to innovations in green chemistry and the exploration of more environmentally friendly solvents and reagents.
Compliance with CCl4 regulations requires comprehensive monitoring and reporting systems. Companies must maintain detailed records of CCl4 usage, implement leak detection programs, and regularly report to regulatory authorities. Non-compliance can result in severe penalties, including fines and legal action.
As scientific understanding of environmental and health impacts evolves, regulations continue to adapt. There is ongoing research into the atmospheric lifetime and global warming potential of CCl4, which may inform future regulatory decisions. The challenge for the chemical industry lies in balancing the need for effective synthesis processes with compliance to increasingly stringent environmental standards.
In the United States, the Environmental Protection Agency (EPA) has implemented strict controls on CCl4 under the Clean Air Act and the Montreal Protocol. The production and import of CCl4 for non-feedstock uses have been phased out since 1996, with limited exceptions for essential uses. The EPA also regulates CCl4 as a hazardous air pollutant and a toxic substance under the Toxic Substances Control Act.
The European Union has similarly restricted CCl4 use through the REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation. CCl4 is classified as a substance of very high concern (SVHC) due to its ozone-depleting properties and potential carcinogenicity. Its use is heavily restricted, requiring special authorization for specific applications.
Internationally, the Montreal Protocol on Substances that Deplete the Ozone Layer has played a crucial role in regulating CCl4. As a party to this agreement, most countries have committed to phasing out the production and consumption of CCl4, except for limited exemptions for laboratory and analytical uses.
Despite these restrictions, CCl4 continues to play a role in certain chemical synthesis processes as a feedstock or intermediate. In these cases, strict containment and emission control measures are mandated to prevent environmental release. Researchers and industries must adhere to rigorous safety protocols and obtain necessary permits for its use.
The regulatory landscape also impacts the development of alternative synthesis methods. As CCl4 use becomes more restricted, there is increased pressure to find safer substitutes or develop new synthetic routes that avoid its use altogether. This has led to innovations in green chemistry and the exploration of more environmentally friendly solvents and reagents.
Compliance with CCl4 regulations requires comprehensive monitoring and reporting systems. Companies must maintain detailed records of CCl4 usage, implement leak detection programs, and regularly report to regulatory authorities. Non-compliance can result in severe penalties, including fines and legal action.
As scientific understanding of environmental and health impacts evolves, regulations continue to adapt. There is ongoing research into the atmospheric lifetime and global warming potential of CCl4, which may inform future regulatory decisions. The challenge for the chemical industry lies in balancing the need for effective synthesis processes with compliance to increasingly stringent environmental standards.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!