How to Conduct Research on Carbon Tetrachloride's Chemical Properties?
JUL 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CCl4 Research Background and Objectives
Carbon tetrachloride (CCl4) has been a subject of scientific interest for over a century due to its unique chemical properties and diverse applications. The research on CCl4 has evolved significantly, driven by both industrial needs and environmental concerns. Initially valued for its non-flammability and solvent properties, CCl4 found widespread use in fire extinguishers, cleaning agents, and as a precursor in various chemical processes.
The primary objective of conducting research on carbon tetrachloride's chemical properties is to gain a comprehensive understanding of its behavior under different conditions, its interactions with other substances, and its potential impacts on the environment and human health. This research aims to explore the molecular structure, physical characteristics, reactivity, and thermodynamic properties of CCl4, which are crucial for developing safer alternatives and mitigating its environmental effects.
One of the key areas of focus in CCl4 research is its role in stratospheric ozone depletion. As a potent ozone-depleting substance, understanding its atmospheric chemistry and lifetime has become paramount. Researchers seek to elucidate the mechanisms by which CCl4 breaks down in the atmosphere and its contribution to the formation of chlorine radicals that catalyze ozone destruction.
Another important aspect of CCl4 research is its behavior in various solvents and its interactions with other chemicals. This knowledge is essential for predicting its fate in the environment and developing effective remediation strategies for contaminated sites. Additionally, studying CCl4's chemical properties can lead to the discovery of novel applications in controlled settings where its unique characteristics can be beneficial.
The evolution of analytical techniques has significantly enhanced our ability to study CCl4. Advanced spectroscopic methods, computational chemistry, and high-resolution chromatography have enabled researchers to probe deeper into the molecular behavior of CCl4. These technological advancements have opened new avenues for investigating its reactivity, bond strengths, and electronic structure with unprecedented precision.
As global efforts to phase out ozone-depleting substances continue, research on CCl4 has shifted towards understanding its long-term environmental persistence and developing strategies for its safe disposal. This includes exploring chemical transformation processes that can convert CCl4 into less harmful substances and investigating potential biological degradation pathways.
In conclusion, the research on carbon tetrachloride's chemical properties is driven by a multifaceted approach, combining fundamental chemical studies with environmental science and toxicology. The ultimate goal is to develop a comprehensive knowledge base that can inform policy decisions, guide environmental protection efforts, and contribute to the development of safer alternatives in industrial applications.
The primary objective of conducting research on carbon tetrachloride's chemical properties is to gain a comprehensive understanding of its behavior under different conditions, its interactions with other substances, and its potential impacts on the environment and human health. This research aims to explore the molecular structure, physical characteristics, reactivity, and thermodynamic properties of CCl4, which are crucial for developing safer alternatives and mitigating its environmental effects.
One of the key areas of focus in CCl4 research is its role in stratospheric ozone depletion. As a potent ozone-depleting substance, understanding its atmospheric chemistry and lifetime has become paramount. Researchers seek to elucidate the mechanisms by which CCl4 breaks down in the atmosphere and its contribution to the formation of chlorine radicals that catalyze ozone destruction.
Another important aspect of CCl4 research is its behavior in various solvents and its interactions with other chemicals. This knowledge is essential for predicting its fate in the environment and developing effective remediation strategies for contaminated sites. Additionally, studying CCl4's chemical properties can lead to the discovery of novel applications in controlled settings where its unique characteristics can be beneficial.
The evolution of analytical techniques has significantly enhanced our ability to study CCl4. Advanced spectroscopic methods, computational chemistry, and high-resolution chromatography have enabled researchers to probe deeper into the molecular behavior of CCl4. These technological advancements have opened new avenues for investigating its reactivity, bond strengths, and electronic structure with unprecedented precision.
As global efforts to phase out ozone-depleting substances continue, research on CCl4 has shifted towards understanding its long-term environmental persistence and developing strategies for its safe disposal. This includes exploring chemical transformation processes that can convert CCl4 into less harmful substances and investigating potential biological degradation pathways.
In conclusion, the research on carbon tetrachloride's chemical properties is driven by a multifaceted approach, combining fundamental chemical studies with environmental science and toxicology. The ultimate goal is to develop a comprehensive knowledge base that can inform policy decisions, guide environmental protection efforts, and contribute to the development of safer alternatives in industrial applications.
Industrial Applications and Demand Analysis
Carbon tetrachloride, a versatile chemical compound, has found extensive applications across various industries due to its unique properties. The demand for this substance has fluctuated over the years, influenced by regulatory changes and environmental concerns. In the manufacturing sector, carbon tetrachloride has been widely used as a solvent for oils, fats, lacquers, varnishes, rubber waxes, and resins. Its ability to dissolve a wide range of organic compounds makes it particularly valuable in these processes.
The chemical industry has historically been a significant consumer of carbon tetrachloride, utilizing it as a feedstock for the production of chlorofluorocarbons (CFCs) and other chlorinated compounds. However, the phase-out of CFCs under the Montreal Protocol has led to a substantial decrease in this particular application. Despite this decline, carbon tetrachloride continues to play a role in the synthesis of various chemicals, including chlorinated rubber and pesticides.
In the pharmaceutical sector, carbon tetrachloride has been employed in the extraction and purification of pharmaceutical products. Its effectiveness as a solvent in these processes has contributed to its ongoing demand within this industry. Additionally, the compound has found use in analytical chemistry, serving as a reagent in various laboratory procedures and as a standard in spectroscopic studies.
The fire suppression industry has also utilized carbon tetrachloride in fire extinguishers, particularly for electrical fires. However, due to its toxicity and environmental impact, this application has been largely phased out in many countries, with safer alternatives taking its place. This shift highlights the evolving nature of carbon tetrachloride's industrial applications and the impact of regulatory measures on market demand.
The agricultural sector represents another area where carbon tetrachloride has been employed, primarily as a fumigant for grain storage. While effective, concerns over its environmental persistence and potential health risks have led to restrictions on this use in many regions. This trend underscores the ongoing challenge of balancing the compound's utility with its environmental and health implications.
Despite the decline in some traditional applications, research into carbon tetrachloride's chemical properties continues to drive innovation and potential new uses. The compound's unique characteristics, such as its high density and low flammability, make it an interesting subject for exploration in emerging technologies and specialized applications. As industries seek alternatives to more harmful substances, understanding the full spectrum of carbon tetrachloride's properties becomes increasingly valuable.
The chemical industry has historically been a significant consumer of carbon tetrachloride, utilizing it as a feedstock for the production of chlorofluorocarbons (CFCs) and other chlorinated compounds. However, the phase-out of CFCs under the Montreal Protocol has led to a substantial decrease in this particular application. Despite this decline, carbon tetrachloride continues to play a role in the synthesis of various chemicals, including chlorinated rubber and pesticides.
In the pharmaceutical sector, carbon tetrachloride has been employed in the extraction and purification of pharmaceutical products. Its effectiveness as a solvent in these processes has contributed to its ongoing demand within this industry. Additionally, the compound has found use in analytical chemistry, serving as a reagent in various laboratory procedures and as a standard in spectroscopic studies.
The fire suppression industry has also utilized carbon tetrachloride in fire extinguishers, particularly for electrical fires. However, due to its toxicity and environmental impact, this application has been largely phased out in many countries, with safer alternatives taking its place. This shift highlights the evolving nature of carbon tetrachloride's industrial applications and the impact of regulatory measures on market demand.
The agricultural sector represents another area where carbon tetrachloride has been employed, primarily as a fumigant for grain storage. While effective, concerns over its environmental persistence and potential health risks have led to restrictions on this use in many regions. This trend underscores the ongoing challenge of balancing the compound's utility with its environmental and health implications.
Despite the decline in some traditional applications, research into carbon tetrachloride's chemical properties continues to drive innovation and potential new uses. The compound's unique characteristics, such as its high density and low flammability, make it an interesting subject for exploration in emerging technologies and specialized applications. As industries seek alternatives to more harmful substances, understanding the full spectrum of carbon tetrachloride's properties becomes increasingly valuable.
Current State and Challenges in CCl4 Research
Carbon tetrachloride (CCl4) research has made significant strides in recent years, yet several challenges persist in fully understanding its chemical properties. The current state of CCl4 research is characterized by a comprehensive understanding of its basic physical and chemical characteristics, including its molecular structure, boiling point, and solubility. However, deeper investigations into its reactivity, environmental impact, and potential applications are ongoing.
One of the primary challenges in CCl4 research is its environmental persistence and toxicity. Despite being phased out of many applications due to its ozone-depleting properties, CCl4 remains a subject of concern due to its long atmospheric lifetime and potential health hazards. Researchers are grappling with developing accurate models to predict its atmospheric behavior and long-term environmental effects.
Another significant challenge lies in understanding CCl4's reactivity under various conditions. While its general chemical behavior is well-documented, there are still gaps in knowledge regarding its reactions in complex environmental matrices or under extreme conditions. This includes its interactions with other atmospheric pollutants and its behavior in different soil types and aquatic environments.
The development of sensitive and reliable analytical methods for detecting and quantifying CCl4 in various matrices remains an ongoing challenge. Current techniques, while effective, often require sophisticated equipment and complex sample preparation. There is a push towards developing more accessible, field-deployable methods for real-time monitoring of CCl4 in environmental samples.
In the realm of potential applications, researchers are exploring ways to safely and efficiently degrade CCl4 for environmental remediation purposes. This includes investigating catalytic processes and advanced oxidation techniques. However, scaling these methods from laboratory to field applications presents significant technical and economic hurdles.
The global nature of CCl4 research presents both opportunities and challenges. While international collaboration has led to a more comprehensive understanding of its global distribution and effects, coordinating research efforts and standardizing methodologies across different countries and institutions remains a challenge.
Lastly, the interdisciplinary nature of CCl4 research, spanning chemistry, environmental science, toxicology, and atmospheric physics, necessitates a holistic approach. Integrating findings from these diverse fields to form a cohesive understanding of CCl4's properties and impacts is an ongoing challenge that requires sophisticated data analysis and modeling techniques.
One of the primary challenges in CCl4 research is its environmental persistence and toxicity. Despite being phased out of many applications due to its ozone-depleting properties, CCl4 remains a subject of concern due to its long atmospheric lifetime and potential health hazards. Researchers are grappling with developing accurate models to predict its atmospheric behavior and long-term environmental effects.
Another significant challenge lies in understanding CCl4's reactivity under various conditions. While its general chemical behavior is well-documented, there are still gaps in knowledge regarding its reactions in complex environmental matrices or under extreme conditions. This includes its interactions with other atmospheric pollutants and its behavior in different soil types and aquatic environments.
The development of sensitive and reliable analytical methods for detecting and quantifying CCl4 in various matrices remains an ongoing challenge. Current techniques, while effective, often require sophisticated equipment and complex sample preparation. There is a push towards developing more accessible, field-deployable methods for real-time monitoring of CCl4 in environmental samples.
In the realm of potential applications, researchers are exploring ways to safely and efficiently degrade CCl4 for environmental remediation purposes. This includes investigating catalytic processes and advanced oxidation techniques. However, scaling these methods from laboratory to field applications presents significant technical and economic hurdles.
The global nature of CCl4 research presents both opportunities and challenges. While international collaboration has led to a more comprehensive understanding of its global distribution and effects, coordinating research efforts and standardizing methodologies across different countries and institutions remains a challenge.
Lastly, the interdisciplinary nature of CCl4 research, spanning chemistry, environmental science, toxicology, and atmospheric physics, necessitates a holistic approach. Integrating findings from these diverse fields to form a cohesive understanding of CCl4's properties and impacts is an ongoing challenge that requires sophisticated data analysis and modeling techniques.
Existing Methodologies for CCl4 Property Analysis
01 Physical properties of carbon tetrachloride
Carbon tetrachloride is a colorless, volatile liquid with a sweet odor. It has a high density and low boiling point, making it useful in various industrial applications. Its physical properties contribute to its effectiveness as a solvent and cleaning agent.- Physical properties of carbon tetrachloride: Carbon tetrachloride is a colorless, volatile liquid with a sweet odor. It has a high density and low boiling point, making it useful in various industrial applications. Its non-flammability and ability to dissolve many organic compounds contribute to its historical use as a solvent and cleaning agent.
- Chemical reactivity and stability: Carbon tetrachloride is relatively stable under normal conditions but can react vigorously with certain metals and strong bases. It can undergo photochemical decomposition when exposed to light, producing phosgene and chlorine. Its reactivity with water is low, but it can form hydrochloric acid in the presence of moisture and heat.
- Environmental impact and toxicity: Carbon tetrachloride is known for its ozone-depleting properties and has been phased out of many applications due to environmental concerns. It is toxic to humans and animals, with potential to cause liver and kidney damage upon exposure. Its persistence in the environment and bioaccumulation potential contribute to its classification as a hazardous substance.
- Industrial applications and alternatives: Historically, carbon tetrachloride was widely used as a solvent, cleaning agent, and refrigerant. Due to its toxicity and environmental impact, it has been largely replaced by safer alternatives in many applications. Current uses are limited to specific industrial processes where suitable substitutes have not been found, such as in the production of certain chemicals and pharmaceuticals.
- Analytical and detection methods: Various analytical techniques are used to detect and quantify carbon tetrachloride in environmental and industrial samples. These include gas chromatography, mass spectrometry, and infrared spectroscopy. Specialized sensors and monitoring systems have been developed for real-time detection of carbon tetrachloride vapors in industrial settings and for environmental monitoring purposes.
02 Chemical reactivity and stability
Carbon tetrachloride is relatively stable under normal conditions but can react with certain metals and strong bases. It undergoes photochemical decomposition when exposed to ultraviolet light, producing phosgene and chlorine radicals. Its reactivity is important in various chemical processes and environmental considerations.Expand Specific Solutions03 Solvent properties and applications
Carbon tetrachloride is an excellent solvent for organic compounds and was widely used in dry cleaning and degreasing operations. Its ability to dissolve a wide range of substances made it valuable in industrial processes and chemical synthesis. However, its use as a solvent has been restricted due to environmental and health concerns.Expand Specific Solutions04 Environmental impact and toxicity
Carbon tetrachloride is known for its ozone-depleting properties and has been phased out under the Montreal Protocol. It is toxic to humans and animals, affecting the liver, kidneys, and central nervous system. Its persistence in the environment and potential for bioaccumulation have led to strict regulations on its use and disposal.Expand Specific Solutions05 Analytical and detection methods
Various analytical techniques are used to detect and quantify carbon tetrachloride in environmental and industrial samples. These methods include gas chromatography, mass spectrometry, and spectrophotometric analysis. Accurate detection is crucial for monitoring environmental contamination and ensuring workplace safety.Expand Specific Solutions
Key Players in CCl4 Research and Production
The research on carbon tetrachloride's chemical properties is in a mature stage, with established market players and well-defined applications. The global market for carbon tetrachloride is relatively stable, driven by its use in various industrial processes. Companies like DuPont de Nemours, Occidental Chemical Corp., and The Chemours Co. are key players in this field, leveraging their extensive experience in chemical manufacturing. The technology for studying and utilizing carbon tetrachloride is well-developed, with ongoing research focusing on improving safety measures and finding alternative compounds due to environmental concerns. The market size remains significant, although growth is limited by regulatory restrictions on its use in certain applications.
DuPont de Nemours, Inc.
Technical Solution: DuPont employs advanced spectroscopic techniques, including Nuclear Magnetic Resonance (NMR) and Fourier Transform Infrared Spectroscopy (FTIR), to analyze the molecular structure and bonding of carbon tetrachloride. They utilize high-performance liquid chromatography (HPLC) and gas chromatography-mass spectrometry (GC-MS) for precise quantification and identification of impurities[1]. DuPont's research also focuses on the environmental fate of carbon tetrachloride, employing isotope tracing methods to study its degradation pathways in various ecosystems[3]. Their approach includes computational chemistry to model the reactivity and thermodynamic properties of carbon tetrachloride under different conditions, enhancing the understanding of its behavior in industrial processes and environmental systems[5].
Strengths: Comprehensive analytical capabilities, advanced computational modeling, and expertise in environmental fate studies. Weaknesses: Potential focus on industrial applications may limit broader research scope.
The Chemours Co.
Technical Solution: Chemours conducts extensive research on carbon tetrachloride's chemical properties using state-of-the-art analytical techniques. They employ X-ray diffraction (XRD) and X-ray photoelectron spectroscopy (XPS) to study the compound's crystal structure and surface properties[2]. Chemours has developed proprietary methods for analyzing the thermal stability and decomposition kinetics of carbon tetrachloride under various industrial conditions[4]. Their research extends to the development of novel catalysts for the controlled transformation of carbon tetrachloride into less harmful compounds, aiming to mitigate its environmental impact[6]. Chemours also investigates the solvent properties of carbon tetrachloride, particularly its interactions with polymers and other organic compounds, to optimize its use in industrial processes while minimizing environmental risks[8].
Strengths: Strong focus on industrial applications and environmental mitigation strategies. Weaknesses: May have limited focus on fundamental chemical properties not directly related to industrial use.
Innovative Techniques in CCl4 Characterization
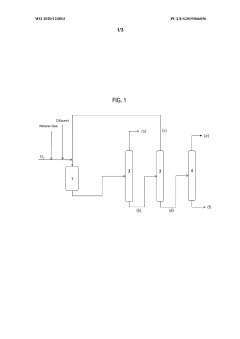
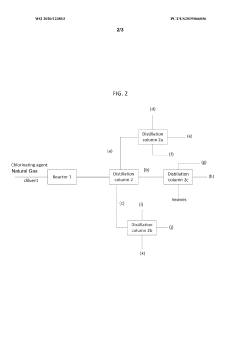
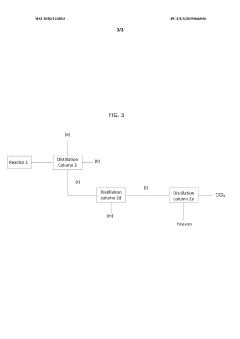
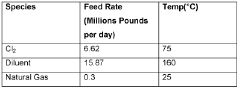
Production of carbon tetrachloride from natural gas
PatentWO2020123853A1
Innovation
- A process involving the formation of a mixture of natural gas, a chlorinating agent, and a diluent in a reactor under specific conditions to produce carbon tetrachloride with high selectivity, where the natural gas is primarily composed of methane and alkanes, and the chlorinating agent is used in excess, with the product mixture comprising anhydrous hydrogen chloride and carbon tetrachloride, which is then separated.
Chlorinolysis process for producing carbon tetrachloride
PatentActiveUS20210130266A1
Innovation
- A process involving a chlorination zone with chlorine, a C1 chlorinated compound, and a carbon/second chlorine source to produce a reaction mixture that favors the formation of carbon tetrachloride over perchloroethylene, using waste products as the carbon/second chlorine source to enhance efficiency and reduce impurity formation.
Environmental Impact and Regulations
Carbon tetrachloride, once widely used in various industrial applications, has been recognized as a significant environmental pollutant with severe impacts on both human health and ecosystems. The environmental fate of this compound is of particular concern due to its persistence and potential for long-range transport.
In aquatic environments, carbon tetrachloride can persist for extended periods, with a half-life ranging from months to years depending on conditions. It has been detected in surface waters, groundwater, and even in remote oceanic regions, indicating its widespread distribution. The compound's ability to bioaccumulate in aquatic organisms poses risks to marine life and potentially to human consumers of seafood.
Atmospheric release of carbon tetrachloride contributes to ozone depletion, as it is a potent ozone-depleting substance. Its long atmospheric lifetime allows it to reach the stratosphere, where it breaks down and releases chlorine atoms that catalyze ozone destruction. This impact on the ozone layer has led to strict regulations on its production and use under the Montreal Protocol.
Soil contamination by carbon tetrachloride is another significant concern. The compound can persist in soil for extended periods, potentially leaching into groundwater and affecting drinking water sources. Its presence in soil can also impact terrestrial ecosystems, affecting soil microorganisms and plant life.
Due to these environmental concerns, numerous regulations have been implemented globally to control the use and release of carbon tetrachloride. The Montreal Protocol, signed in 1987 and subsequently amended, has been instrumental in phasing out the production and consumption of ozone-depleting substances, including carbon tetrachloride. Many countries have banned its use in consumer products and significantly restricted its industrial applications.
In the United States, carbon tetrachloride is regulated under various environmental laws. The Clean Air Act classifies it as a hazardous air pollutant, while the Safe Drinking Water Act sets maximum contaminant levels for it in drinking water. The Toxic Substances Control Act and the Resource Conservation and Recovery Act also impose restrictions on its manufacture, use, and disposal.
Internationally, the Stockholm Convention on Persistent Organic Pollutants addresses carbon tetrachloride as a substance of concern. Many countries have implemented their own regulations to comply with these international agreements, focusing on reducing emissions, proper disposal, and remediation of contaminated sites.
Research on carbon tetrachloride's chemical properties must consider these environmental impacts and regulatory frameworks. Understanding its behavior in different environmental compartments, its transformation products, and its interactions with biota is crucial for developing effective mitigation strategies and informing policy decisions.
In aquatic environments, carbon tetrachloride can persist for extended periods, with a half-life ranging from months to years depending on conditions. It has been detected in surface waters, groundwater, and even in remote oceanic regions, indicating its widespread distribution. The compound's ability to bioaccumulate in aquatic organisms poses risks to marine life and potentially to human consumers of seafood.
Atmospheric release of carbon tetrachloride contributes to ozone depletion, as it is a potent ozone-depleting substance. Its long atmospheric lifetime allows it to reach the stratosphere, where it breaks down and releases chlorine atoms that catalyze ozone destruction. This impact on the ozone layer has led to strict regulations on its production and use under the Montreal Protocol.
Soil contamination by carbon tetrachloride is another significant concern. The compound can persist in soil for extended periods, potentially leaching into groundwater and affecting drinking water sources. Its presence in soil can also impact terrestrial ecosystems, affecting soil microorganisms and plant life.
Due to these environmental concerns, numerous regulations have been implemented globally to control the use and release of carbon tetrachloride. The Montreal Protocol, signed in 1987 and subsequently amended, has been instrumental in phasing out the production and consumption of ozone-depleting substances, including carbon tetrachloride. Many countries have banned its use in consumer products and significantly restricted its industrial applications.
In the United States, carbon tetrachloride is regulated under various environmental laws. The Clean Air Act classifies it as a hazardous air pollutant, while the Safe Drinking Water Act sets maximum contaminant levels for it in drinking water. The Toxic Substances Control Act and the Resource Conservation and Recovery Act also impose restrictions on its manufacture, use, and disposal.
Internationally, the Stockholm Convention on Persistent Organic Pollutants addresses carbon tetrachloride as a substance of concern. Many countries have implemented their own regulations to comply with these international agreements, focusing on reducing emissions, proper disposal, and remediation of contaminated sites.
Research on carbon tetrachloride's chemical properties must consider these environmental impacts and regulatory frameworks. Understanding its behavior in different environmental compartments, its transformation products, and its interactions with biota is crucial for developing effective mitigation strategies and informing policy decisions.
Safety Protocols for CCl4 Handling and Research
When conducting research on carbon tetrachloride's chemical properties, it is crucial to prioritize safety protocols for handling and research. Carbon tetrachloride (CCl4) is a highly toxic and potentially carcinogenic compound that requires strict safety measures to protect researchers and the environment.
Proper personal protective equipment (PPE) is essential when working with CCl4. Researchers must wear chemical-resistant gloves, lab coats, and safety goggles at all times. In cases where there is a risk of inhalation, a properly fitted respirator with appropriate cartridges should be used. It is important to note that CCl4 can penetrate many common glove materials, so selecting the appropriate glove type and changing them frequently is crucial.
All work involving CCl4 should be conducted in a well-ventilated area, preferably under a fume hood. This helps to minimize exposure to vapors and prevent the accumulation of potentially harmful concentrations in the air. The fume hood should be regularly inspected and certified to ensure proper functioning.
Proper storage of CCl4 is essential to prevent accidental spills or releases. The compound should be stored in tightly sealed, compatible containers in a cool, dry, and well-ventilated area. It is important to keep CCl4 away from sources of heat, sparks, or open flames, as it can decompose to form toxic phosgene gas when exposed to high temperatures or fire.
Spill response procedures must be established and communicated to all personnel working with CCl4. In the event of a spill, the area should be immediately evacuated, and proper containment and cleanup measures should be implemented by trained personnel wearing appropriate PPE. Absorbent materials specifically designed for chemical spills should be readily available in the laboratory.
Proper waste disposal is critical when working with CCl4. All waste containing the compound should be collected in designated containers and disposed of as hazardous waste according to local, state, and federal regulations. It is important to never dispose of CCl4 down the drain or in regular trash.
Regular safety training and education should be provided to all personnel working with CCl4. This should include information on the compound's hazards, proper handling techniques, emergency procedures, and the importance of following safety protocols. Maintaining up-to-date safety data sheets (SDS) and ensuring they are readily accessible is also essential.
Implementing a robust chemical hygiene plan that specifically addresses the handling of CCl4 is crucial. This plan should outline standard operating procedures, engineering controls, administrative controls, and PPE requirements. Regular safety audits and inspections should be conducted to ensure compliance with established protocols and identify areas for improvement.
Proper personal protective equipment (PPE) is essential when working with CCl4. Researchers must wear chemical-resistant gloves, lab coats, and safety goggles at all times. In cases where there is a risk of inhalation, a properly fitted respirator with appropriate cartridges should be used. It is important to note that CCl4 can penetrate many common glove materials, so selecting the appropriate glove type and changing them frequently is crucial.
All work involving CCl4 should be conducted in a well-ventilated area, preferably under a fume hood. This helps to minimize exposure to vapors and prevent the accumulation of potentially harmful concentrations in the air. The fume hood should be regularly inspected and certified to ensure proper functioning.
Proper storage of CCl4 is essential to prevent accidental spills or releases. The compound should be stored in tightly sealed, compatible containers in a cool, dry, and well-ventilated area. It is important to keep CCl4 away from sources of heat, sparks, or open flames, as it can decompose to form toxic phosgene gas when exposed to high temperatures or fire.
Spill response procedures must be established and communicated to all personnel working with CCl4. In the event of a spill, the area should be immediately evacuated, and proper containment and cleanup measures should be implemented by trained personnel wearing appropriate PPE. Absorbent materials specifically designed for chemical spills should be readily available in the laboratory.
Proper waste disposal is critical when working with CCl4. All waste containing the compound should be collected in designated containers and disposed of as hazardous waste according to local, state, and federal regulations. It is important to never dispose of CCl4 down the drain or in regular trash.
Regular safety training and education should be provided to all personnel working with CCl4. This should include information on the compound's hazards, proper handling techniques, emergency procedures, and the importance of following safety protocols. Maintaining up-to-date safety data sheets (SDS) and ensuring they are readily accessible is also essential.
Implementing a robust chemical hygiene plan that specifically addresses the handling of CCl4 is crucial. This plan should outline standard operating procedures, engineering controls, administrative controls, and PPE requirements. Regular safety audits and inspections should be conducted to ensure compliance with established protocols and identify areas for improvement.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!