How to Manage Carbon Tetrachloride Risks in Manufacturing Enterprises?
JUL 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CCl4 Risk Management Background and Objectives
Carbon tetrachloride (CCl4) has been a significant concern in manufacturing enterprises due to its potential health and environmental risks. The background of CCl4 risk management dates back to the mid-20th century when its widespread use in various industrial applications became apparent. Initially utilized as a solvent, cleaning agent, and refrigerant, CCl4's harmful effects on human health and the ozone layer gradually came to light, prompting global efforts to regulate and phase out its use.
The evolution of CCl4 risk management has been shaped by international agreements such as the Montreal Protocol, which aimed to protect the ozone layer by phasing out the production of ozone-depleting substances. This agreement, along with subsequent amendments, has played a crucial role in reducing CCl4 emissions and driving the development of alternative technologies and substances.
In recent years, the focus of CCl4 risk management has shifted towards addressing residual emissions and ensuring proper handling and disposal of existing stocks. The objectives of modern CCl4 risk management in manufacturing enterprises are multifaceted and aim to protect both human health and the environment while maintaining operational efficiency.
One primary objective is to minimize worker exposure to CCl4 through the implementation of robust safety protocols, engineering controls, and personal protective equipment. This includes regular monitoring of air quality in work areas and providing comprehensive training to employees on the proper handling and storage of CCl4.
Another key objective is to prevent environmental contamination by implementing stringent containment measures and waste management practices. This involves proper storage, handling, and disposal of CCl4 and CCl4-containing materials to prevent releases into the air, soil, or water.
Compliance with regulatory requirements is a critical objective for manufacturing enterprises. This includes adhering to emission limits, reporting requirements, and phase-out schedules set by national and international regulations. Enterprises must stay informed about evolving regulations and adapt their practices accordingly.
Developing and implementing alternatives to CCl4 in manufacturing processes is another important objective. This involves investing in research and development to find safer and more environmentally friendly substitutes that can maintain or improve production efficiency.
Continuous improvement in risk assessment and management strategies is also a key objective. This includes regularly updating risk assessments, implementing new technologies for monitoring and control, and fostering a culture of safety and environmental responsibility within the organization.
By addressing these objectives, manufacturing enterprises can effectively manage CCl4 risks, protect their workforce and the environment, and ensure long-term sustainability in their operations.
The evolution of CCl4 risk management has been shaped by international agreements such as the Montreal Protocol, which aimed to protect the ozone layer by phasing out the production of ozone-depleting substances. This agreement, along with subsequent amendments, has played a crucial role in reducing CCl4 emissions and driving the development of alternative technologies and substances.
In recent years, the focus of CCl4 risk management has shifted towards addressing residual emissions and ensuring proper handling and disposal of existing stocks. The objectives of modern CCl4 risk management in manufacturing enterprises are multifaceted and aim to protect both human health and the environment while maintaining operational efficiency.
One primary objective is to minimize worker exposure to CCl4 through the implementation of robust safety protocols, engineering controls, and personal protective equipment. This includes regular monitoring of air quality in work areas and providing comprehensive training to employees on the proper handling and storage of CCl4.
Another key objective is to prevent environmental contamination by implementing stringent containment measures and waste management practices. This involves proper storage, handling, and disposal of CCl4 and CCl4-containing materials to prevent releases into the air, soil, or water.
Compliance with regulatory requirements is a critical objective for manufacturing enterprises. This includes adhering to emission limits, reporting requirements, and phase-out schedules set by national and international regulations. Enterprises must stay informed about evolving regulations and adapt their practices accordingly.
Developing and implementing alternatives to CCl4 in manufacturing processes is another important objective. This involves investing in research and development to find safer and more environmentally friendly substitutes that can maintain or improve production efficiency.
Continuous improvement in risk assessment and management strategies is also a key objective. This includes regularly updating risk assessments, implementing new technologies for monitoring and control, and fostering a culture of safety and environmental responsibility within the organization.
By addressing these objectives, manufacturing enterprises can effectively manage CCl4 risks, protect their workforce and the environment, and ensure long-term sustainability in their operations.
Market Demand for CCl4 Alternatives
The market demand for Carbon Tetrachloride (CCl4) alternatives has been steadily increasing due to growing environmental concerns and stringent regulations surrounding the use of ozone-depleting substances. Manufacturing enterprises are actively seeking safer and more sustainable substitutes to mitigate the risks associated with CCl4 usage.
One of the primary drivers for CCl4 alternatives is the phase-out mandated by the Montreal Protocol, which has significantly restricted the production and consumption of ozone-depleting substances. This has created a substantial market opportunity for alternative solvents and processes that can effectively replace CCl4 in various industrial applications.
The electronics industry, particularly in the manufacturing of semiconductors and printed circuit boards, has been at the forefront of adopting CCl4 alternatives. Aqueous cleaning solutions, no-clean fluxes, and other environmentally friendly solvents have gained traction in this sector. The demand for these alternatives is expected to grow as the electronics industry continues to expand globally.
In the pharmaceutical and chemical industries, there is a rising demand for green solvents that can replace CCl4 in synthesis processes and as extraction agents. Supercritical CO2, ionic liquids, and bio-based solvents are emerging as promising alternatives, offering similar efficacy while reducing environmental impact and health risks.
The metal cleaning and degreasing sector, traditionally a significant user of CCl4, is transitioning towards water-based cleaners, hydrocarbon solvents, and modified alcohol solvents. This shift is driven by both regulatory pressures and the need for safer workplace environments, creating a robust market for CCl4 alternatives in this industry.
Agricultural chemical manufacturers are also seeking alternatives to CCl4, particularly in the production of pesticides and herbicides. The demand for bio-based and less toxic intermediates is growing, as companies aim to develop more sustainable and environmentally friendly crop protection products.
The market for CCl4 alternatives is further bolstered by the increasing adoption of green chemistry principles across industries. Companies are investing in research and development to create innovative, eco-friendly solutions that can match or exceed the performance of CCl4 while complying with environmental regulations.
As awareness of the health and environmental risks associated with CCl4 continues to grow, there is an expanding market for monitoring and detection technologies. These tools help enterprises ensure compliance with regulations and maintain safe working conditions, driving demand for advanced analytical instruments and safety equipment.
One of the primary drivers for CCl4 alternatives is the phase-out mandated by the Montreal Protocol, which has significantly restricted the production and consumption of ozone-depleting substances. This has created a substantial market opportunity for alternative solvents and processes that can effectively replace CCl4 in various industrial applications.
The electronics industry, particularly in the manufacturing of semiconductors and printed circuit boards, has been at the forefront of adopting CCl4 alternatives. Aqueous cleaning solutions, no-clean fluxes, and other environmentally friendly solvents have gained traction in this sector. The demand for these alternatives is expected to grow as the electronics industry continues to expand globally.
In the pharmaceutical and chemical industries, there is a rising demand for green solvents that can replace CCl4 in synthesis processes and as extraction agents. Supercritical CO2, ionic liquids, and bio-based solvents are emerging as promising alternatives, offering similar efficacy while reducing environmental impact and health risks.
The metal cleaning and degreasing sector, traditionally a significant user of CCl4, is transitioning towards water-based cleaners, hydrocarbon solvents, and modified alcohol solvents. This shift is driven by both regulatory pressures and the need for safer workplace environments, creating a robust market for CCl4 alternatives in this industry.
Agricultural chemical manufacturers are also seeking alternatives to CCl4, particularly in the production of pesticides and herbicides. The demand for bio-based and less toxic intermediates is growing, as companies aim to develop more sustainable and environmentally friendly crop protection products.
The market for CCl4 alternatives is further bolstered by the increasing adoption of green chemistry principles across industries. Companies are investing in research and development to create innovative, eco-friendly solutions that can match or exceed the performance of CCl4 while complying with environmental regulations.
As awareness of the health and environmental risks associated with CCl4 continues to grow, there is an expanding market for monitoring and detection technologies. These tools help enterprises ensure compliance with regulations and maintain safe working conditions, driving demand for advanced analytical instruments and safety equipment.
Current CCl4 Usage and Challenges
Carbon tetrachloride (CCl4) remains a significant concern in manufacturing enterprises due to its potential environmental and health risks. Despite global efforts to phase out its use, CCl4 continues to be utilized in various industrial processes, particularly as a feedstock for the production of other chemicals and as a process agent in certain manufacturing operations.
The current usage of CCl4 in manufacturing is primarily concentrated in the production of chlorofluorocarbons (CFCs) and their alternatives, such as hydrofluorocarbons (HFCs) and hydrochlorofluorocarbons (HCFCs). These substances are widely used in refrigeration, air conditioning, and foam blowing applications. Additionally, CCl4 serves as a solvent in some specialized industrial cleaning processes and as a raw material in the synthesis of pharmaceutical compounds.
One of the major challenges facing manufacturing enterprises is the strict regulatory environment surrounding CCl4 use. The Montreal Protocol and subsequent amendments have mandated the gradual elimination of CCl4 production and consumption, with exceptions for certain essential uses. This has led to increased scrutiny and compliance requirements for companies that continue to utilize CCl4 in their operations.
Another significant challenge is the management of CCl4 emissions and potential environmental contamination. Despite improved handling practices, accidental releases and fugitive emissions remain a concern. These releases can contribute to ozone depletion and pose risks to local ecosystems and human health. Enterprises must invest in sophisticated monitoring systems and implement rigorous containment measures to minimize the risk of CCl4 escaping into the environment.
The safe handling and storage of CCl4 present ongoing challenges for manufacturing facilities. The substance's high volatility and toxicity require specialized equipment and stringent safety protocols. Workers must be properly trained in handling procedures and provided with appropriate personal protective equipment to mitigate exposure risks.
Furthermore, the disposal of CCl4-containing waste and the decontamination of equipment pose significant technical and logistical challenges. Proper disposal methods, such as high-temperature incineration, are costly and require specialized facilities. The cleaning and decommissioning of CCl4-contaminated equipment also demand careful planning and execution to prevent environmental release and worker exposure.
As global regulations continue to tighten, manufacturing enterprises face the challenge of finding suitable alternatives to CCl4 in their processes. This often requires substantial research and development efforts, as well as potential modifications to existing manufacturing infrastructure. The transition to alternative substances or processes can be costly and time-consuming, impacting production efficiency and product quality.
In conclusion, while the use of CCl4 in manufacturing has decreased significantly over the past decades, its continued presence in certain industrial processes presents ongoing challenges. Enterprises must navigate a complex landscape of regulatory compliance, environmental protection, worker safety, and technological adaptation to effectively manage the risks associated with CCl4 usage.
The current usage of CCl4 in manufacturing is primarily concentrated in the production of chlorofluorocarbons (CFCs) and their alternatives, such as hydrofluorocarbons (HFCs) and hydrochlorofluorocarbons (HCFCs). These substances are widely used in refrigeration, air conditioning, and foam blowing applications. Additionally, CCl4 serves as a solvent in some specialized industrial cleaning processes and as a raw material in the synthesis of pharmaceutical compounds.
One of the major challenges facing manufacturing enterprises is the strict regulatory environment surrounding CCl4 use. The Montreal Protocol and subsequent amendments have mandated the gradual elimination of CCl4 production and consumption, with exceptions for certain essential uses. This has led to increased scrutiny and compliance requirements for companies that continue to utilize CCl4 in their operations.
Another significant challenge is the management of CCl4 emissions and potential environmental contamination. Despite improved handling practices, accidental releases and fugitive emissions remain a concern. These releases can contribute to ozone depletion and pose risks to local ecosystems and human health. Enterprises must invest in sophisticated monitoring systems and implement rigorous containment measures to minimize the risk of CCl4 escaping into the environment.
The safe handling and storage of CCl4 present ongoing challenges for manufacturing facilities. The substance's high volatility and toxicity require specialized equipment and stringent safety protocols. Workers must be properly trained in handling procedures and provided with appropriate personal protective equipment to mitigate exposure risks.
Furthermore, the disposal of CCl4-containing waste and the decontamination of equipment pose significant technical and logistical challenges. Proper disposal methods, such as high-temperature incineration, are costly and require specialized facilities. The cleaning and decommissioning of CCl4-contaminated equipment also demand careful planning and execution to prevent environmental release and worker exposure.
As global regulations continue to tighten, manufacturing enterprises face the challenge of finding suitable alternatives to CCl4 in their processes. This often requires substantial research and development efforts, as well as potential modifications to existing manufacturing infrastructure. The transition to alternative substances or processes can be costly and time-consuming, impacting production efficiency and product quality.
In conclusion, while the use of CCl4 in manufacturing has decreased significantly over the past decades, its continued presence in certain industrial processes presents ongoing challenges. Enterprises must navigate a complex landscape of regulatory compliance, environmental protection, worker safety, and technological adaptation to effectively manage the risks associated with CCl4 usage.
Existing CCl4 Risk Control Measures
01 Health and environmental hazards
Carbon tetrachloride poses significant risks to human health and the environment. It is known to be toxic, potentially carcinogenic, and can cause damage to the liver, kidneys, and central nervous system. Exposure can occur through inhalation, skin contact, or ingestion. It also contributes to ozone depletion and has been phased out in many applications due to its harmful effects.- Health and environmental hazards: Carbon tetrachloride poses significant risks to human health and the environment. It is known to be toxic, potentially carcinogenic, and can cause liver and kidney damage. Its use has been restricted due to its ozone-depleting properties and contribution to global warming.
- Industrial applications and alternatives: Despite its risks, carbon tetrachloride has been used in various industrial applications, including as a solvent, cleaning agent, and in the production of refrigerants. Efforts have been made to develop safer alternatives and phase out its use in many industries.
- Detection and monitoring methods: Various techniques have been developed to detect and monitor carbon tetrachloride in air, water, and soil. These methods are crucial for assessing contamination levels and ensuring compliance with environmental regulations.
- Remediation and treatment technologies: Research has focused on developing effective methods for treating carbon tetrachloride contamination in soil and groundwater. These technologies aim to reduce environmental impact and protect human health.
- Regulatory measures and safety protocols: Governments and organizations have implemented strict regulations and safety protocols regarding the use, handling, and disposal of carbon tetrachloride. These measures aim to minimize risks associated with its production and use in various industries.
02 Industrial applications and alternatives
Despite its risks, carbon tetrachloride has been used in various industrial applications, including as a solvent, cleaning agent, and in the production of refrigerants. However, due to its hazardous nature, efforts have been made to find safer alternatives and phase out its use in many industries. Safer substitutes and new technologies have been developed to replace carbon tetrachloride in various processes.Expand Specific Solutions03 Detection and monitoring methods
To mitigate the risks associated with carbon tetrachloride, various detection and monitoring methods have been developed. These include advanced analytical techniques for measuring carbon tetrachloride levels in air, water, and soil. Continuous monitoring systems and portable detection devices have been invented to ensure safety in industrial settings and environmental assessments.Expand Specific Solutions04 Remediation and treatment technologies
Given the persistent nature of carbon tetrachloride contamination, various remediation and treatment technologies have been developed. These include advanced oxidation processes, bioremediation techniques, and specialized filtration systems. Such technologies aim to remove or neutralize carbon tetrachloride from contaminated sites, water sources, and industrial waste streams.Expand Specific Solutions05 Regulatory measures and safety protocols
Due to the known risks of carbon tetrachloride, numerous regulatory measures and safety protocols have been implemented globally. These include strict guidelines for handling, storage, and disposal of the substance, as well as occupational exposure limits. Many countries have banned or severely restricted its use, leading to the development of new safety standards and protective equipment for scenarios where its use is still permitted.Expand Specific Solutions
Key Players in CCl4 Management Solutions
The management of carbon tetrachloride risks in manufacturing enterprises is a critical issue in the chemical industry, currently in a mature stage of development. The global market for carbon tetrachloride alternatives and risk management solutions is substantial, driven by stringent environmental regulations and increasing awareness of health hazards. Companies like DuPont de Nemours, Bayer AG, and BASF Corp. are at the forefront of developing safer alternatives and advanced risk mitigation technologies. The industry has seen significant advancements in containment, recycling, and substitution strategies, with a high level of technical maturity. However, ongoing research by institutions like the Research Institute of Innovative Technology for the Earth continues to push for more sustainable and cost-effective solutions.
DuPont de Nemours, Inc.
Technical Solution: DuPont has developed a comprehensive Carbon Tetrachloride (CCl4) risk management strategy focusing on three key areas: emission reduction, process optimization, and alternative technologies. The company has implemented advanced scrubbing systems that can capture up to 99% of CCl4 emissions[1]. They have also redesigned their manufacturing processes to minimize CCl4 use and potential exposure. DuPont's research teams are actively working on developing alternative chemicals and processes that can replace CCl4 in various applications. The company has invested in real-time monitoring systems to detect leaks and spills promptly, reducing the risk of accidental releases[2]. Additionally, DuPont has implemented strict handling and storage protocols, including the use of double-walled containers and advanced sealing technologies to prevent fugitive emissions.
Strengths: Comprehensive approach addressing multiple aspects of CCl4 risk; high-efficiency emission control systems; ongoing research into alternatives. Weaknesses: Potential high costs associated with technology upgrades and process changes; some applications may still require CCl4 use due to lack of suitable alternatives.
Bayer AG
Technical Solution: Bayer AG has implemented a multi-faceted approach to manage Carbon Tetrachloride risks in its manufacturing processes. The company has developed a closed-loop system that significantly reduces CCl4 emissions by recycling and reusing the chemical within the production cycle[3]. Bayer has also invested in advanced filtration and absorption technologies that can remove up to 98% of CCl4 from waste streams. The company's risk management strategy includes regular employee training programs on safe handling procedures and emergency response protocols. Bayer has partnered with academic institutions to research and develop green chemistry alternatives to CCl4, focusing on environmentally friendly solvents and catalysts[4]. Additionally, the company has implemented a comprehensive monitoring system using IoT sensors to detect and respond to potential CCl4 leaks in real-time, significantly reducing the risk of exposure.
Strengths: Closed-loop system minimizes emissions; strong focus on research and development of alternatives; comprehensive employee training and safety protocols. Weaknesses: High initial investment costs for technology implementation; transition to alternatives may be challenging for certain established processes.
Innovative CCl4 Substitution Technologies
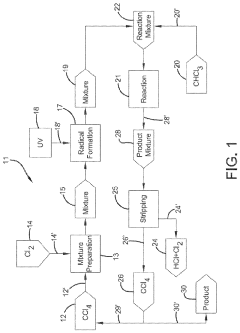
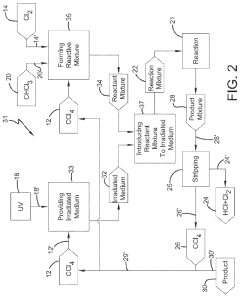
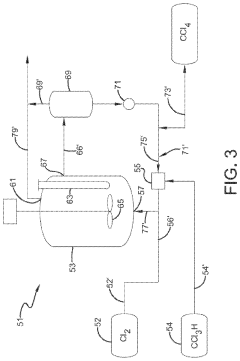
Photochlorination of partially-chlorinated chloromethanes to carbon tetrachloride
PatentActiveUS20240025823A1
Innovation
- A method involving the photochlorination of a chloromethanes stream containing chloroform, methyl chloride, and methylene chloride, combined with chlorine and additional carbon tetrachloride, and subjected to electromagnetic radiation to form carbon tetrachloride, achieving high conversion rates with reduced levels of unwanted chlorinated hydrocarbons.
Phosgene with poor carbon tetrachloride content
PatentInactiveEP1135329A1
Innovation
- Reacting carbon monoxide with chlorine in the presence of elemental carbon at controlled temperatures and pressures in conventional tubular reactors, using activated carbon as a catalyst without special preparation, to produce phosgene with a carbon tetrachloride content of less than 150 ppm.
Regulatory Framework for CCl4 in Manufacturing
The regulatory framework for carbon tetrachloride (CCl4) in manufacturing is complex and multifaceted, reflecting the substance's high toxicity and environmental impact. At the international level, the Montreal Protocol on Substances that Deplete the Ozone Layer has been instrumental in phasing out CCl4 production and consumption for non-feedstock uses. This treaty, ratified by 197 countries, has led to a significant reduction in global CCl4 emissions since its implementation in 1989.
In the United States, the Environmental Protection Agency (EPA) regulates CCl4 under various statutes. The Toxic Substances Control Act (TSCA) lists CCl4 as a priority chemical, subjecting it to risk evaluations and potential restrictions. The Clean Air Act classifies CCl4 as a hazardous air pollutant, requiring strict emission controls in manufacturing processes. Additionally, the Safe Drinking Water Act sets maximum contaminant levels for CCl4 in public water systems.
The Occupational Safety and Health Administration (OSHA) has established permissible exposure limits for CCl4 in the workplace. These standards mandate that employee exposure must not exceed 10 parts per million (ppm) as an 8-hour time-weighted average. OSHA also requires employers to implement engineering controls, work practices, and personal protective equipment to minimize worker exposure.
In the European Union, the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) regulation governs the use of CCl4. Under REACH, CCl4 is classified as a substance of very high concern (SVHC) due to its carcinogenic properties. This classification imposes strict requirements on manufacturers, importers, and downstream users, including the need for authorization for specific uses.
Many countries have implemented their own regulations to control CCl4 use and emissions. For instance, China has included CCl4 in its list of toxic chemicals subject to import and export controls. Japan regulates CCl4 under its Chemical Substances Control Law, which requires manufacturers to report production and import volumes annually.
To comply with these diverse regulations, manufacturing enterprises must implement comprehensive management systems. This includes regular monitoring of CCl4 emissions, proper handling and storage protocols, and detailed record-keeping of CCl4 use throughout the production process. Companies are also required to provide thorough training to employees on the safe handling of CCl4 and emergency response procedures in case of accidental release.
As regulations continue to evolve, manufacturers must stay informed about changes in legal requirements across different jurisdictions. This often necessitates the establishment of dedicated compliance teams or the engagement of regulatory consultants to ensure ongoing adherence to the complex and dynamic regulatory landscape surrounding CCl4 in manufacturing.
In the United States, the Environmental Protection Agency (EPA) regulates CCl4 under various statutes. The Toxic Substances Control Act (TSCA) lists CCl4 as a priority chemical, subjecting it to risk evaluations and potential restrictions. The Clean Air Act classifies CCl4 as a hazardous air pollutant, requiring strict emission controls in manufacturing processes. Additionally, the Safe Drinking Water Act sets maximum contaminant levels for CCl4 in public water systems.
The Occupational Safety and Health Administration (OSHA) has established permissible exposure limits for CCl4 in the workplace. These standards mandate that employee exposure must not exceed 10 parts per million (ppm) as an 8-hour time-weighted average. OSHA also requires employers to implement engineering controls, work practices, and personal protective equipment to minimize worker exposure.
In the European Union, the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) regulation governs the use of CCl4. Under REACH, CCl4 is classified as a substance of very high concern (SVHC) due to its carcinogenic properties. This classification imposes strict requirements on manufacturers, importers, and downstream users, including the need for authorization for specific uses.
Many countries have implemented their own regulations to control CCl4 use and emissions. For instance, China has included CCl4 in its list of toxic chemicals subject to import and export controls. Japan regulates CCl4 under its Chemical Substances Control Law, which requires manufacturers to report production and import volumes annually.
To comply with these diverse regulations, manufacturing enterprises must implement comprehensive management systems. This includes regular monitoring of CCl4 emissions, proper handling and storage protocols, and detailed record-keeping of CCl4 use throughout the production process. Companies are also required to provide thorough training to employees on the safe handling of CCl4 and emergency response procedures in case of accidental release.
As regulations continue to evolve, manufacturers must stay informed about changes in legal requirements across different jurisdictions. This often necessitates the establishment of dedicated compliance teams or the engagement of regulatory consultants to ensure ongoing adherence to the complex and dynamic regulatory landscape surrounding CCl4 in manufacturing.
Environmental Impact Assessment of CCl4 Use
The environmental impact assessment of carbon tetrachloride (CCl4) use in manufacturing enterprises is crucial for understanding and mitigating potential risks. CCl4 is a potent ozone-depleting substance and a known carcinogen, making its environmental impact a significant concern. When released into the atmosphere, CCl4 contributes to the depletion of the ozone layer, which can lead to increased ultraviolet radiation reaching the Earth's surface. This, in turn, can have detrimental effects on human health, ecosystems, and agricultural productivity.
In aquatic environments, CCl4 can persist for extended periods due to its low biodegradability. It can accumulate in sediments and bioaccumulate in aquatic organisms, potentially entering the food chain. This poses risks to aquatic ecosystems and may lead to long-term ecological impacts. Furthermore, CCl4 can contaminate groundwater resources, potentially affecting drinking water supplies and posing health risks to communities relying on these water sources.
Soil contamination is another significant concern associated with CCl4 use in manufacturing. Spills or improper disposal can lead to soil pollution, affecting soil microorganisms and plant growth. The persistence of CCl4 in soil can result in long-term contamination issues, requiring extensive remediation efforts.
Air quality is also impacted by CCl4 emissions from manufacturing processes. Indoor air quality in manufacturing facilities can be particularly affected, posing occupational health risks to workers. Outdoor air quality in surrounding areas may also be compromised, potentially affecting local communities and ecosystems.
The global nature of CCl4's environmental impact necessitates consideration of its contribution to climate change. Although not a direct greenhouse gas, CCl4's ozone-depleting properties indirectly contribute to climate change by altering atmospheric chemistry and radiation balance.
To effectively assess and manage these environmental impacts, manufacturing enterprises must implement comprehensive monitoring and control measures. This includes regular air, water, and soil quality testing, as well as emissions monitoring. Implementing best available technologies for CCl4 containment, recovery, and destruction is essential to minimize environmental releases.
Enterprises should also consider the lifecycle environmental impact of CCl4 use, from production and transportation to disposal. This holistic approach allows for a more accurate assessment of the overall environmental footprint and helps identify opportunities for improvement throughout the supply chain.
In aquatic environments, CCl4 can persist for extended periods due to its low biodegradability. It can accumulate in sediments and bioaccumulate in aquatic organisms, potentially entering the food chain. This poses risks to aquatic ecosystems and may lead to long-term ecological impacts. Furthermore, CCl4 can contaminate groundwater resources, potentially affecting drinking water supplies and posing health risks to communities relying on these water sources.
Soil contamination is another significant concern associated with CCl4 use in manufacturing. Spills or improper disposal can lead to soil pollution, affecting soil microorganisms and plant growth. The persistence of CCl4 in soil can result in long-term contamination issues, requiring extensive remediation efforts.
Air quality is also impacted by CCl4 emissions from manufacturing processes. Indoor air quality in manufacturing facilities can be particularly affected, posing occupational health risks to workers. Outdoor air quality in surrounding areas may also be compromised, potentially affecting local communities and ecosystems.
The global nature of CCl4's environmental impact necessitates consideration of its contribution to climate change. Although not a direct greenhouse gas, CCl4's ozone-depleting properties indirectly contribute to climate change by altering atmospheric chemistry and radiation balance.
To effectively assess and manage these environmental impacts, manufacturing enterprises must implement comprehensive monitoring and control measures. This includes regular air, water, and soil quality testing, as well as emissions monitoring. Implementing best available technologies for CCl4 containment, recovery, and destruction is essential to minimize environmental releases.
Enterprises should also consider the lifecycle environmental impact of CCl4 use, from production and transportation to disposal. This holistic approach allows for a more accurate assessment of the overall environmental footprint and helps identify opportunities for improvement throughout the supply chain.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!