How Researchers Study Carbon Tetrachloride's Atmospheric Impact?
JUL 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Carbon Tetrachloride Research Background and Objectives
Carbon tetrachloride (CCl4) has been a subject of intense scientific scrutiny due to its significant impact on the Earth's atmosphere. This compound, once widely used in various industrial applications, has emerged as a potent ozone-depleting substance and greenhouse gas. The study of CCl4's atmospheric impact has evolved over several decades, driven by growing environmental concerns and advancements in scientific methodologies.
The primary objective of research in this field is to comprehensively understand the behavior, distribution, and effects of CCl4 in the atmosphere. Scientists aim to quantify its contribution to ozone depletion and global warming, track its sources and sinks, and predict future atmospheric concentrations. These efforts are crucial for informing policy decisions and guiding international environmental agreements.
Researchers employ a multifaceted approach to study CCl4's atmospheric impact. Atmospheric measurements form the backbone of this research, utilizing ground-based, airborne, and satellite-based instruments to monitor CCl4 concentrations at various altitudes and geographical locations. Long-term monitoring programs, such as the Advanced Global Atmospheric Gases Experiment (AGAGE) network, provide invaluable data on global CCl4 trends.
Laboratory experiments play a vital role in elucidating the chemical reactions involving CCl4 in the atmosphere. Scientists investigate its photolysis rates, reactions with atmospheric radicals, and interactions with aerosols to better understand its atmospheric lifetime and degradation pathways. These studies help refine our knowledge of CCl4's ozone-depleting potential and its role in stratospheric chemistry.
Atmospheric modeling is another crucial tool in CCl4 research. Global circulation models and chemical transport models are used to simulate the distribution and transport of CCl4 in the atmosphere. These models integrate observational data with theoretical understanding to predict future CCl4 concentrations and assess the effectiveness of mitigation strategies.
The technological evolution in analytical techniques has significantly enhanced our ability to study CCl4. Advanced mass spectrometry methods, for instance, allow for precise measurements of CCl4 and its isotopes, helping to differentiate between natural and anthropogenic sources. Remote sensing technologies have also improved, enabling more accurate global-scale observations of atmospheric CCl4.
As research progresses, emerging areas of focus include investigating discrepancies between observed atmospheric concentrations and reported emissions, understanding the role of ocean-atmosphere exchange in the global CCl4 budget, and exploring potential natural sources of CCl4. These ongoing efforts aim to resolve uncertainties in our understanding of CCl4's atmospheric behavior and improve predictions of its future impact on the Earth's atmosphere.
The primary objective of research in this field is to comprehensively understand the behavior, distribution, and effects of CCl4 in the atmosphere. Scientists aim to quantify its contribution to ozone depletion and global warming, track its sources and sinks, and predict future atmospheric concentrations. These efforts are crucial for informing policy decisions and guiding international environmental agreements.
Researchers employ a multifaceted approach to study CCl4's atmospheric impact. Atmospheric measurements form the backbone of this research, utilizing ground-based, airborne, and satellite-based instruments to monitor CCl4 concentrations at various altitudes and geographical locations. Long-term monitoring programs, such as the Advanced Global Atmospheric Gases Experiment (AGAGE) network, provide invaluable data on global CCl4 trends.
Laboratory experiments play a vital role in elucidating the chemical reactions involving CCl4 in the atmosphere. Scientists investigate its photolysis rates, reactions with atmospheric radicals, and interactions with aerosols to better understand its atmospheric lifetime and degradation pathways. These studies help refine our knowledge of CCl4's ozone-depleting potential and its role in stratospheric chemistry.
Atmospheric modeling is another crucial tool in CCl4 research. Global circulation models and chemical transport models are used to simulate the distribution and transport of CCl4 in the atmosphere. These models integrate observational data with theoretical understanding to predict future CCl4 concentrations and assess the effectiveness of mitigation strategies.
The technological evolution in analytical techniques has significantly enhanced our ability to study CCl4. Advanced mass spectrometry methods, for instance, allow for precise measurements of CCl4 and its isotopes, helping to differentiate between natural and anthropogenic sources. Remote sensing technologies have also improved, enabling more accurate global-scale observations of atmospheric CCl4.
As research progresses, emerging areas of focus include investigating discrepancies between observed atmospheric concentrations and reported emissions, understanding the role of ocean-atmosphere exchange in the global CCl4 budget, and exploring potential natural sources of CCl4. These ongoing efforts aim to resolve uncertainties in our understanding of CCl4's atmospheric behavior and improve predictions of its future impact on the Earth's atmosphere.
Atmospheric Impact Assessment Needs
The assessment of carbon tetrachloride's atmospheric impact requires a comprehensive approach that integrates various scientific disciplines and advanced monitoring technologies. Researchers employ a multi-faceted strategy to study this compound's effects on the atmosphere, focusing on its distribution, persistence, and interactions with other atmospheric components.
Atmospheric scientists utilize a network of ground-based monitoring stations equipped with high-precision instruments to measure carbon tetrachloride concentrations at different locations and altitudes. These stations provide crucial data on local and regional variations in atmospheric levels. Complementing ground-based measurements, satellite-based remote sensing technologies offer a global perspective on carbon tetrachloride distribution. Instruments such as the Atmospheric Chemistry Experiment Fourier Transform Spectrometer (ACE-FTS) aboard satellites enable researchers to track the compound's presence across vast geographical areas and at various atmospheric layers.
To understand the long-term trends and historical context of carbon tetrachloride in the atmosphere, scientists analyze air samples trapped in polar ice cores and firn (compacted snow). These natural archives provide invaluable information on atmospheric composition dating back several decades or even centuries, allowing researchers to reconstruct past concentrations and emission patterns.
Laboratory experiments play a vital role in elucidating the chemical reactions and degradation processes involving carbon tetrachloride in the atmosphere. Researchers use controlled environments to simulate various atmospheric conditions and study the compound's interactions with other gases, aerosols, and radiation. These experiments help in determining the atmospheric lifetime of carbon tetrachloride and its potential for ozone depletion.
Atmospheric transport models are essential tools for understanding how carbon tetrachloride moves through the atmosphere and identifying potential source regions. By integrating observational data with meteorological information, these models can simulate the dispersion and fate of the compound on regional and global scales. This modeling approach is crucial for assessing the impact of emissions from different geographical areas and predicting future atmospheric concentrations under various scenarios.
The study of carbon tetrachloride's atmospheric impact also necessitates interdisciplinary collaboration. Atmospheric chemists work alongside climate scientists, oceanographers, and ecologists to investigate the compound's role in global biogeochemical cycles and its potential effects on ecosystems. This holistic approach ensures a comprehensive understanding of carbon tetrachloride's environmental footprint beyond its immediate atmospheric effects.
Atmospheric scientists utilize a network of ground-based monitoring stations equipped with high-precision instruments to measure carbon tetrachloride concentrations at different locations and altitudes. These stations provide crucial data on local and regional variations in atmospheric levels. Complementing ground-based measurements, satellite-based remote sensing technologies offer a global perspective on carbon tetrachloride distribution. Instruments such as the Atmospheric Chemistry Experiment Fourier Transform Spectrometer (ACE-FTS) aboard satellites enable researchers to track the compound's presence across vast geographical areas and at various atmospheric layers.
To understand the long-term trends and historical context of carbon tetrachloride in the atmosphere, scientists analyze air samples trapped in polar ice cores and firn (compacted snow). These natural archives provide invaluable information on atmospheric composition dating back several decades or even centuries, allowing researchers to reconstruct past concentrations and emission patterns.
Laboratory experiments play a vital role in elucidating the chemical reactions and degradation processes involving carbon tetrachloride in the atmosphere. Researchers use controlled environments to simulate various atmospheric conditions and study the compound's interactions with other gases, aerosols, and radiation. These experiments help in determining the atmospheric lifetime of carbon tetrachloride and its potential for ozone depletion.
Atmospheric transport models are essential tools for understanding how carbon tetrachloride moves through the atmosphere and identifying potential source regions. By integrating observational data with meteorological information, these models can simulate the dispersion and fate of the compound on regional and global scales. This modeling approach is crucial for assessing the impact of emissions from different geographical areas and predicting future atmospheric concentrations under various scenarios.
The study of carbon tetrachloride's atmospheric impact also necessitates interdisciplinary collaboration. Atmospheric chemists work alongside climate scientists, oceanographers, and ecologists to investigate the compound's role in global biogeochemical cycles and its potential effects on ecosystems. This holistic approach ensures a comprehensive understanding of carbon tetrachloride's environmental footprint beyond its immediate atmospheric effects.
Current Challenges in CCl4 Atmospheric Studies
Despite significant progress in understanding the atmospheric impact of carbon tetrachloride (CCl4), researchers still face several challenges in their studies. One of the primary obstacles is the complexity of atmospheric chemistry involving CCl4. The compound's interactions with other atmospheric constituents and its role in various chemical reactions are not fully understood, making it difficult to accurately model its behavior and long-term effects on the ozone layer.
Another challenge lies in the measurement and detection of CCl4 in the atmosphere. While advanced technologies have improved our ability to detect trace amounts of the compound, there are still limitations in terms of spatial and temporal resolution. This affects our capacity to track CCl4 emissions and distribution patterns accurately, especially in remote or under-sampled regions of the globe.
The discrepancy between observed atmospheric concentrations and reported emissions presents a significant puzzle for researchers. Despite the global phase-out of CCl4 production for dispersive uses under the Montreal Protocol, atmospheric levels have not declined as rapidly as expected. This suggests the existence of unreported sources or previously unaccounted for natural emissions, which researchers are struggling to identify and quantify.
Climate change introduces additional complexities to CCl4 studies. As global temperatures rise and atmospheric circulation patterns shift, the behavior and distribution of CCl4 in the atmosphere may change in ways that are difficult to predict. This uncertainty complicates efforts to project future impacts and develop effective mitigation strategies.
The long atmospheric lifetime of CCl4, estimated at around 32 years, poses challenges for both measurement and modeling. It requires long-term, consistent monitoring programs to track changes accurately, which can be resource-intensive and technologically demanding. Additionally, modeling the long-term fate of CCl4 in the atmosphere requires sophisticated computational tools and a deep understanding of various atmospheric processes over extended time scales.
Lastly, the interdisciplinary nature of CCl4 research presents coordination challenges. Effective studies require collaboration among atmospheric chemists, climate scientists, remote sensing experts, and policymakers. Integrating diverse datasets, methodologies, and perspectives to form a cohesive understanding of CCl4's atmospheric impact remains an ongoing challenge in the field.
Another challenge lies in the measurement and detection of CCl4 in the atmosphere. While advanced technologies have improved our ability to detect trace amounts of the compound, there are still limitations in terms of spatial and temporal resolution. This affects our capacity to track CCl4 emissions and distribution patterns accurately, especially in remote or under-sampled regions of the globe.
The discrepancy between observed atmospheric concentrations and reported emissions presents a significant puzzle for researchers. Despite the global phase-out of CCl4 production for dispersive uses under the Montreal Protocol, atmospheric levels have not declined as rapidly as expected. This suggests the existence of unreported sources or previously unaccounted for natural emissions, which researchers are struggling to identify and quantify.
Climate change introduces additional complexities to CCl4 studies. As global temperatures rise and atmospheric circulation patterns shift, the behavior and distribution of CCl4 in the atmosphere may change in ways that are difficult to predict. This uncertainty complicates efforts to project future impacts and develop effective mitigation strategies.
The long atmospheric lifetime of CCl4, estimated at around 32 years, poses challenges for both measurement and modeling. It requires long-term, consistent monitoring programs to track changes accurately, which can be resource-intensive and technologically demanding. Additionally, modeling the long-term fate of CCl4 in the atmosphere requires sophisticated computational tools and a deep understanding of various atmospheric processes over extended time scales.
Lastly, the interdisciplinary nature of CCl4 research presents coordination challenges. Effective studies require collaboration among atmospheric chemists, climate scientists, remote sensing experts, and policymakers. Integrating diverse datasets, methodologies, and perspectives to form a cohesive understanding of CCl4's atmospheric impact remains an ongoing challenge in the field.
Methodologies for CCl4 Atmospheric Impact Analysis
01 Atmospheric ozone depletion
Carbon tetrachloride is known to contribute to the depletion of the ozone layer in the Earth's atmosphere. When released into the air, it can break down and release chlorine atoms, which catalyze ozone destruction. This impact has led to regulations and efforts to reduce its use and emissions.- Atmospheric impact and ozone depletion: Carbon tetrachloride has significant atmospheric impacts, particularly its role in ozone depletion. As a chlorinated compound, it can release chlorine atoms in the stratosphere, which catalyze the breakdown of ozone molecules. This contributes to the thinning of the ozone layer, increasing the amount of harmful UV radiation reaching the Earth's surface.
- Global warming potential: Carbon tetrachloride is a potent greenhouse gas with a high global warming potential. Its long atmospheric lifetime allows it to accumulate in the atmosphere, contributing to the enhancement of the greenhouse effect and global climate change. Efforts to reduce its emissions are crucial for mitigating its impact on global temperatures.
- Atmospheric monitoring and detection: Monitoring and detecting carbon tetrachloride in the atmosphere is essential for understanding its distribution and impact. Various techniques and instruments have been developed to measure its concentration in air samples, including gas chromatography and spectroscopic methods. These measurements help in assessing the effectiveness of emission control measures and tracking long-term trends.
- Emission sources and reduction strategies: Identifying and controlling sources of carbon tetrachloride emissions is crucial for minimizing its atmospheric impact. Major sources include industrial processes, solvent use, and inadvertent production. Strategies for reducing emissions include implementing alternative technologies, improving process efficiency, and enforcing stricter regulations on its production and use.
- Atmospheric chemistry and degradation: Understanding the atmospheric chemistry of carbon tetrachloride is important for assessing its long-term impact. While it is relatively stable in the troposphere, it can undergo photolysis in the stratosphere, leading to the formation of reactive chlorine species. Research into its degradation pathways and atmospheric lifetime helps in predicting its future concentrations and environmental effects.
02 Global warming potential
Carbon tetrachloride has a significant global warming potential, contributing to climate change. Its long atmospheric lifetime allows it to trap heat in the atmosphere, making it a potent greenhouse gas. Efforts to mitigate its impact on global warming include reducing emissions and finding alternative substances.Expand Specific Solutions03 Atmospheric monitoring and detection
Various methods and technologies have been developed to monitor and detect carbon tetrachloride in the atmosphere. These include advanced sensing techniques, satellite observations, and ground-based monitoring stations. Accurate detection is crucial for assessing its environmental impact and enforcing regulations.Expand Specific Solutions04 Atmospheric degradation and transformation
Carbon tetrachloride undergoes various degradation and transformation processes in the atmosphere. Understanding these processes is important for predicting its long-term impact and developing strategies to mitigate its effects. Research focuses on reaction mechanisms, byproducts, and environmental fate.Expand Specific Solutions05 Emission reduction and alternatives
Efforts to reduce carbon tetrachloride emissions and find suitable alternatives are ongoing. This includes developing new industrial processes, improving containment and disposal methods, and identifying environmentally friendly substitutes. These measures aim to minimize its atmospheric impact while maintaining necessary industrial applications.Expand Specific Solutions
Key Organizations in CCl4 Research
The study of carbon tetrachloride's atmospheric impact is in a mature research phase, with ongoing efforts to refine understanding of its effects. The global market for related atmospheric research and monitoring equipment is substantial, driven by environmental concerns and regulatory requirements. Technologically, the field is well-developed, with advanced analytical techniques employed by leading institutions and companies. Central South University, DuPont, and California Institute of Technology are among the key players contributing to this research area, leveraging their expertise in environmental science, chemical engineering, and atmospheric modeling to advance knowledge and develop mitigation strategies for this potent ozone-depleting substance.
The Regents of the University of California
Technical Solution: The University of California has developed advanced atmospheric modeling techniques to study carbon tetrachloride's impact. They utilize a combination of satellite observations, ground-based measurements, and sophisticated computer simulations to track the global distribution and transport of carbon tetrachloride in the atmosphere[1]. Their research involves analyzing long-term trends in atmospheric concentrations, identifying potential sources, and assessing the compound's role in ozone depletion. The university's approach integrates data from multiple platforms, including the Microwave Limb Sounder on NASA's Aura satellite, to provide a comprehensive view of carbon tetrachloride's atmospheric behavior[2].
Strengths: Comprehensive approach combining multiple data sources and advanced modeling techniques. Weaknesses: Reliance on complex models may introduce uncertainties in predictions.
California Institute of Technology
Technical Solution: Caltech researchers have developed innovative spectroscopic techniques to study carbon tetrachloride in the atmosphere. They use high-resolution Fourier transform spectrometers to measure infrared absorption spectra of carbon tetrachloride in laboratory settings, which are then applied to interpret atmospheric observations[3]. This approach allows for precise quantification of carbon tetrachloride concentrations at various altitudes. Additionally, Caltech scientists have contributed to the development of atmospheric chemistry models that incorporate carbon tetrachloride's interactions with other compounds and its impact on stratospheric ozone[4].
Strengths: High-precision spectroscopic measurements and detailed atmospheric chemistry modeling. Weaknesses: Laboratory-based measurements may not fully capture real-world atmospheric complexities.
Breakthrough Studies on CCl4 Atmospheric Effects
Device and method for measuring the concentration of elementary carbon in the atmospheric particulate
PatentWO2020225770A1
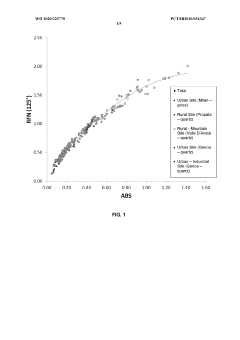
Innovation
- A non-destructive method using a single optical reflection measurement correlated with absorption values through an empirical equation, derived from a large dataset of reference samples, to determine Black Carbon concentration in atmospheric particulates, allowing for real-time, online monitoring compatible with standard sequential samplers.
Process of preparation of monobasic lead salt of 2,4 di nitro resorcinol using trichloro ethylene as an inert
PatentInactiveIN201821045441A
Innovation
- The process involves using trichloroethylene as an inert solvent media to replace carbon tetrachloride, involving nitrosation of resorcinol followed by alkaline oxidation and subsequent purification of 2,4-dinitroso resorcinol to produce monobasic lead salt of 2,4-di nitro resorcinol, which is then used in detonator compositions and fuze applications.
Environmental Regulations on CCl4 Usage
Environmental regulations on carbon tetrachloride (CCl4) usage have evolved significantly over the past few decades, reflecting growing concerns about its impact on the atmosphere and ozone layer. The Montreal Protocol, signed in 1987, marked a pivotal moment in global efforts to phase out ozone-depleting substances, including CCl4.
Under the Montreal Protocol, developed countries were required to cease production and consumption of CCl4 by 1996, while developing countries were given until 2010. This international agreement has been instrumental in reducing CCl4 emissions and atmospheric concentrations.
In the United States, the Environmental Protection Agency (EPA) has implemented stringent regulations on CCl4 under the Clean Air Act. The substance is classified as a hazardous air pollutant and is subject to strict emission controls. Industrial facilities using CCl4 must adhere to maximum achievable control technology (MACT) standards to minimize emissions.
The European Union has also imposed strict controls on CCl4 through its REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation. CCl4 is listed as a substance of very high concern (SVHC) due to its persistent, bioaccumulative, and toxic properties.
Despite these regulations, challenges remain in completely eliminating CCl4 emissions. Some countries still use CCl4 as a feedstock for the production of other chemicals, which is permitted under the Montreal Protocol but can lead to inadvertent emissions. Additionally, there are concerns about unreported or illegal production and use of CCl4 in some regions.
Researchers studying CCl4's atmospheric impact must navigate this complex regulatory landscape. They often collaborate with regulatory bodies to identify potential sources of emissions and assess the effectiveness of current regulations. This includes analyzing atmospheric measurements, tracking industrial processes, and developing models to predict future trends in CCl4 concentrations.
The ongoing research into CCl4's atmospheric impact has led to calls for further tightening of regulations. Some scientists advocate for stricter controls on feedstock uses and improved monitoring of industrial processes to prevent unintended releases. There is also a push for enhanced international cooperation to address potential illegal production and trade of CCl4.
As our understanding of CCl4's atmospheric impact continues to evolve, it is likely that environmental regulations will be further refined. This dynamic interplay between scientific research and policy-making underscores the importance of continued study in this field.
Under the Montreal Protocol, developed countries were required to cease production and consumption of CCl4 by 1996, while developing countries were given until 2010. This international agreement has been instrumental in reducing CCl4 emissions and atmospheric concentrations.
In the United States, the Environmental Protection Agency (EPA) has implemented stringent regulations on CCl4 under the Clean Air Act. The substance is classified as a hazardous air pollutant and is subject to strict emission controls. Industrial facilities using CCl4 must adhere to maximum achievable control technology (MACT) standards to minimize emissions.
The European Union has also imposed strict controls on CCl4 through its REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation. CCl4 is listed as a substance of very high concern (SVHC) due to its persistent, bioaccumulative, and toxic properties.
Despite these regulations, challenges remain in completely eliminating CCl4 emissions. Some countries still use CCl4 as a feedstock for the production of other chemicals, which is permitted under the Montreal Protocol but can lead to inadvertent emissions. Additionally, there are concerns about unreported or illegal production and use of CCl4 in some regions.
Researchers studying CCl4's atmospheric impact must navigate this complex regulatory landscape. They often collaborate with regulatory bodies to identify potential sources of emissions and assess the effectiveness of current regulations. This includes analyzing atmospheric measurements, tracking industrial processes, and developing models to predict future trends in CCl4 concentrations.
The ongoing research into CCl4's atmospheric impact has led to calls for further tightening of regulations. Some scientists advocate for stricter controls on feedstock uses and improved monitoring of industrial processes to prevent unintended releases. There is also a push for enhanced international cooperation to address potential illegal production and trade of CCl4.
As our understanding of CCl4's atmospheric impact continues to evolve, it is likely that environmental regulations will be further refined. This dynamic interplay between scientific research and policy-making underscores the importance of continued study in this field.
Global Collaboration in CCl4 Research
The study of carbon tetrachloride's (CCl4) atmospheric impact has become a global endeavor, requiring extensive collaboration among researchers, institutions, and nations. This international cooperation is crucial due to the widespread distribution of CCl4 in the atmosphere and its significant ozone-depleting potential.
One of the primary platforms for global collaboration in CCl4 research is the World Meteorological Organization's Global Atmosphere Watch (GAW) program. This initiative coordinates a network of monitoring stations worldwide, enabling continuous measurement of CCl4 concentrations in various atmospheric layers. The data collected through this network provides a comprehensive picture of CCl4 distribution and trends on a global scale.
The United Nations Environment Programme (UNEP) plays a pivotal role in facilitating international cooperation on CCl4 research. Through its Ozone Secretariat, UNEP organizes regular meetings and workshops that bring together scientists, policymakers, and industry representatives to discuss the latest findings and coordinate research efforts.
Satellite-based observations have become an essential tool in CCl4 research, necessitating collaboration between space agencies such as NASA, ESA, and JAXA. These agencies share data and resources to provide global coverage of CCl4 concentrations and their temporal variations. The Atmospheric Chemistry Experiment (ACE) satellite mission, for instance, involves scientists from multiple countries working together to analyze CCl4 data.
International scientific bodies, such as the World Climate Research Programme (WCRP) and the International Global Atmospheric Chemistry (IGAC) project, foster collaboration by organizing focused research initiatives on CCl4 and other ozone-depleting substances. These projects often involve joint field campaigns, data sharing, and collaborative modeling efforts.
The Intergovernmental Panel on Climate Change (IPCC) serves as a crucial platform for synthesizing global research on CCl4 and its atmospheric impacts. Scientists from various countries contribute to IPCC reports, providing a comprehensive assessment of the current state of knowledge on CCl4 and its role in climate change.
Collaborative efforts also extend to laboratory studies, where researchers from different institutions pool resources and expertise to investigate CCl4's chemical properties and reactions in controlled environments. These studies often involve international teams working together to develop and refine experimental techniques.
In conclusion, the global nature of CCl4's atmospheric impact necessitates a coordinated international approach to research. Through various platforms and initiatives, scientists worldwide are working together to better understand and address the challenges posed by this ozone-depleting substance.
One of the primary platforms for global collaboration in CCl4 research is the World Meteorological Organization's Global Atmosphere Watch (GAW) program. This initiative coordinates a network of monitoring stations worldwide, enabling continuous measurement of CCl4 concentrations in various atmospheric layers. The data collected through this network provides a comprehensive picture of CCl4 distribution and trends on a global scale.
The United Nations Environment Programme (UNEP) plays a pivotal role in facilitating international cooperation on CCl4 research. Through its Ozone Secretariat, UNEP organizes regular meetings and workshops that bring together scientists, policymakers, and industry representatives to discuss the latest findings and coordinate research efforts.
Satellite-based observations have become an essential tool in CCl4 research, necessitating collaboration between space agencies such as NASA, ESA, and JAXA. These agencies share data and resources to provide global coverage of CCl4 concentrations and their temporal variations. The Atmospheric Chemistry Experiment (ACE) satellite mission, for instance, involves scientists from multiple countries working together to analyze CCl4 data.
International scientific bodies, such as the World Climate Research Programme (WCRP) and the International Global Atmospheric Chemistry (IGAC) project, foster collaboration by organizing focused research initiatives on CCl4 and other ozone-depleting substances. These projects often involve joint field campaigns, data sharing, and collaborative modeling efforts.
The Intergovernmental Panel on Climate Change (IPCC) serves as a crucial platform for synthesizing global research on CCl4 and its atmospheric impacts. Scientists from various countries contribute to IPCC reports, providing a comprehensive assessment of the current state of knowledge on CCl4 and its role in climate change.
Collaborative efforts also extend to laboratory studies, where researchers from different institutions pool resources and expertise to investigate CCl4's chemical properties and reactions in controlled environments. These studies often involve international teams working together to develop and refine experimental techniques.
In conclusion, the global nature of CCl4's atmospheric impact necessitates a coordinated international approach to research. Through various platforms and initiatives, scientists worldwide are working together to better understand and address the challenges posed by this ozone-depleting substance.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!