How to Address Carbon Tetrachloride Emission Challenges?
JUL 3, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CCl4 Emission Background and Objectives
Carbon tetrachloride (CCl4) emissions have been a significant environmental concern since the mid-20th century. Initially used as a solvent, cleaning agent, and refrigerant, CCl4 was later discovered to be a potent ozone-depleting substance and greenhouse gas. The Montreal Protocol, implemented in 1987, aimed to phase out the production and consumption of ozone-depleting substances, including CCl4.
Despite these efforts, CCl4 emissions have persisted, with recent studies indicating that global emissions are significantly higher than expected based on reported production and consumption. This discrepancy has raised concerns about unreported sources and the effectiveness of current mitigation strategies. The primary objective of addressing CCl4 emission challenges is to identify and mitigate these unknown sources while improving monitoring and reporting mechanisms.
The technical evolution of CCl4 emission control has progressed through several stages. Initially, efforts focused on reducing intentional production and use. This was followed by the development of alternative substances and processes to replace CCl4 in various applications. More recently, attention has shifted to identifying and addressing unintentional CCl4 production and emissions, particularly from industrial processes such as chloromethane and perchloroethylene production.
Current challenges in addressing CCl4 emissions include accurately quantifying and locating emission sources, developing more sensitive detection methods, and implementing effective mitigation strategies for both intentional and unintentional emissions. The complexity of atmospheric chemistry and the long atmospheric lifetime of CCl4 further complicate efforts to understand and address its impact on the environment.
The global nature of CCl4 emissions necessitates international cooperation and coordinated research efforts. Objectives for future research and development include improving atmospheric monitoring networks, enhancing satellite-based detection capabilities, and developing advanced modeling techniques to better understand CCl4 sources and sinks. Additionally, there is a need for innovative technologies to prevent and capture CCl4 emissions from industrial processes and to remediate contaminated sites.
Addressing CCl4 emission challenges also requires a multidisciplinary approach, combining expertise from atmospheric science, chemistry, engineering, and policy-making. The ultimate goal is to significantly reduce global CCl4 emissions, thereby mitigating its impact on ozone depletion and climate change, while ensuring compliance with international agreements and supporting sustainable industrial practices.
Despite these efforts, CCl4 emissions have persisted, with recent studies indicating that global emissions are significantly higher than expected based on reported production and consumption. This discrepancy has raised concerns about unreported sources and the effectiveness of current mitigation strategies. The primary objective of addressing CCl4 emission challenges is to identify and mitigate these unknown sources while improving monitoring and reporting mechanisms.
The technical evolution of CCl4 emission control has progressed through several stages. Initially, efforts focused on reducing intentional production and use. This was followed by the development of alternative substances and processes to replace CCl4 in various applications. More recently, attention has shifted to identifying and addressing unintentional CCl4 production and emissions, particularly from industrial processes such as chloromethane and perchloroethylene production.
Current challenges in addressing CCl4 emissions include accurately quantifying and locating emission sources, developing more sensitive detection methods, and implementing effective mitigation strategies for both intentional and unintentional emissions. The complexity of atmospheric chemistry and the long atmospheric lifetime of CCl4 further complicate efforts to understand and address its impact on the environment.
The global nature of CCl4 emissions necessitates international cooperation and coordinated research efforts. Objectives for future research and development include improving atmospheric monitoring networks, enhancing satellite-based detection capabilities, and developing advanced modeling techniques to better understand CCl4 sources and sinks. Additionally, there is a need for innovative technologies to prevent and capture CCl4 emissions from industrial processes and to remediate contaminated sites.
Addressing CCl4 emission challenges also requires a multidisciplinary approach, combining expertise from atmospheric science, chemistry, engineering, and policy-making. The ultimate goal is to significantly reduce global CCl4 emissions, thereby mitigating its impact on ozone depletion and climate change, while ensuring compliance with international agreements and supporting sustainable industrial practices.
Market Demand for CCl4 Emission Control
The market demand for carbon tetrachloride (CCl4) emission control has been steadily increasing due to growing environmental concerns and stricter regulations worldwide. CCl4, once widely used as a solvent and cleaning agent, is now recognized as a potent ozone-depleting substance and greenhouse gas. This has led to a significant shift in industrial practices and a surge in demand for effective emission control solutions.
In the industrial sector, particularly in chemical manufacturing and processing, there is a pressing need for advanced technologies to mitigate CCl4 emissions. Companies are actively seeking innovative solutions to comply with environmental regulations while maintaining operational efficiency. This demand is driven by the potential for hefty fines and reputational damage associated with non-compliance.
The environmental remediation industry has also seen a rise in demand for CCl4 emission control technologies. As more contaminated sites are identified and cleanup efforts intensify, there is a growing market for specialized equipment and services to handle CCl4 contamination in soil and groundwater. This sector's growth is further fueled by increased government funding for environmental restoration projects.
In the waste management sector, the demand for CCl4 emission control is primarily focused on proper disposal and treatment methods. Facilities handling hazardous waste are required to implement stringent measures to prevent CCl4 releases, creating a market for specialized containment and treatment technologies.
The global market for CCl4 emission control solutions is expected to expand significantly in the coming years. This growth is attributed to the implementation of more stringent environmental policies in developing countries and the continuous tightening of regulations in developed nations. The Asia-Pacific region, in particular, is anticipated to witness substantial market growth due to rapid industrialization and increasing environmental awareness.
Technological advancements in emission control methods are also driving market demand. Innovations in catalytic conversion, thermal oxidation, and carbon capture technologies are creating new opportunities for companies specializing in CCl4 emission reduction. These advanced solutions offer higher efficiency and lower operational costs, making them attractive to industries seeking to upgrade their environmental protection measures.
The market is further bolstered by international agreements such as the Montreal Protocol and its amendments, which mandate the phase-out of ozone-depleting substances including CCl4. This global commitment has created a sustained demand for alternative technologies and emission control solutions across various industries.
In the industrial sector, particularly in chemical manufacturing and processing, there is a pressing need for advanced technologies to mitigate CCl4 emissions. Companies are actively seeking innovative solutions to comply with environmental regulations while maintaining operational efficiency. This demand is driven by the potential for hefty fines and reputational damage associated with non-compliance.
The environmental remediation industry has also seen a rise in demand for CCl4 emission control technologies. As more contaminated sites are identified and cleanup efforts intensify, there is a growing market for specialized equipment and services to handle CCl4 contamination in soil and groundwater. This sector's growth is further fueled by increased government funding for environmental restoration projects.
In the waste management sector, the demand for CCl4 emission control is primarily focused on proper disposal and treatment methods. Facilities handling hazardous waste are required to implement stringent measures to prevent CCl4 releases, creating a market for specialized containment and treatment technologies.
The global market for CCl4 emission control solutions is expected to expand significantly in the coming years. This growth is attributed to the implementation of more stringent environmental policies in developing countries and the continuous tightening of regulations in developed nations. The Asia-Pacific region, in particular, is anticipated to witness substantial market growth due to rapid industrialization and increasing environmental awareness.
Technological advancements in emission control methods are also driving market demand. Innovations in catalytic conversion, thermal oxidation, and carbon capture technologies are creating new opportunities for companies specializing in CCl4 emission reduction. These advanced solutions offer higher efficiency and lower operational costs, making them attractive to industries seeking to upgrade their environmental protection measures.
The market is further bolstered by international agreements such as the Montreal Protocol and its amendments, which mandate the phase-out of ozone-depleting substances including CCl4. This global commitment has created a sustained demand for alternative technologies and emission control solutions across various industries.
Current CCl4 Emission Status and Challenges
Carbon tetrachloride (CCl4) emissions remain a significant global environmental concern, despite international efforts to phase out its production and use. Current estimates suggest that global CCl4 emissions are approximately 35,000 tonnes per year, which is substantially higher than expected based on reported production for feedstock uses. This discrepancy indicates the presence of unreported sources or inaccuracies in emission calculations.
The primary challenge in addressing CCl4 emissions lies in identifying and quantifying these unknown sources. Industrial processes, particularly in the chemical manufacturing sector, are believed to be major contributors. Chloromethane production facilities, perchloroethylene plants, and certain chlor-alkali operations have been identified as potential emission hotspots. However, the exact contribution from each source remains uncertain, complicating targeted mitigation efforts.
Another significant challenge is the long atmospheric lifetime of CCl4, estimated at 32 years. This persistence means that even small, continuous emissions can accumulate in the atmosphere, prolonging its impact on ozone depletion and global warming. The global warming potential of CCl4 is approximately 1,730 times that of CO2 over a 100-year period, underscoring its potent climate impact.
Monitoring and measurement challenges further complicate efforts to address CCl4 emissions. Current detection methods often lack the sensitivity and spatial resolution needed to pinpoint specific emission sources accurately. This limitation hampers the development of effective, targeted mitigation strategies and makes it difficult to enforce compliance with international regulations.
The geographical distribution of CCl4 emissions presents additional challenges. While developed countries have largely phased out CCl4 production and use, significant emissions continue to be detected in certain regions, particularly in East Asia. This suggests a potential shift in emission sources to developing countries, where regulatory oversight may be less stringent.
Addressing these challenges requires a multi-faceted approach. Improved monitoring technologies, including satellite-based remote sensing and advanced ground-based measurement networks, are needed to better quantify and locate emission sources. Enhanced international cooperation is crucial for sharing data, harmonizing measurement methodologies, and ensuring compliance with global regulations.
Furthermore, there is a pressing need for research into alternative processes and technologies that can replace CCl4 in its remaining applications, particularly as a feedstock in chemical manufacturing. Developing economically viable alternatives could significantly reduce emissions from these sources.
In conclusion, while progress has been made in reducing CCl4 emissions since the implementation of the Montreal Protocol, significant challenges remain. Overcoming these obstacles will require continued scientific research, technological innovation, and strengthened international collaboration to effectively address the ongoing environmental threat posed by CCl4 emissions.
The primary challenge in addressing CCl4 emissions lies in identifying and quantifying these unknown sources. Industrial processes, particularly in the chemical manufacturing sector, are believed to be major contributors. Chloromethane production facilities, perchloroethylene plants, and certain chlor-alkali operations have been identified as potential emission hotspots. However, the exact contribution from each source remains uncertain, complicating targeted mitigation efforts.
Another significant challenge is the long atmospheric lifetime of CCl4, estimated at 32 years. This persistence means that even small, continuous emissions can accumulate in the atmosphere, prolonging its impact on ozone depletion and global warming. The global warming potential of CCl4 is approximately 1,730 times that of CO2 over a 100-year period, underscoring its potent climate impact.
Monitoring and measurement challenges further complicate efforts to address CCl4 emissions. Current detection methods often lack the sensitivity and spatial resolution needed to pinpoint specific emission sources accurately. This limitation hampers the development of effective, targeted mitigation strategies and makes it difficult to enforce compliance with international regulations.
The geographical distribution of CCl4 emissions presents additional challenges. While developed countries have largely phased out CCl4 production and use, significant emissions continue to be detected in certain regions, particularly in East Asia. This suggests a potential shift in emission sources to developing countries, where regulatory oversight may be less stringent.
Addressing these challenges requires a multi-faceted approach. Improved monitoring technologies, including satellite-based remote sensing and advanced ground-based measurement networks, are needed to better quantify and locate emission sources. Enhanced international cooperation is crucial for sharing data, harmonizing measurement methodologies, and ensuring compliance with global regulations.
Furthermore, there is a pressing need for research into alternative processes and technologies that can replace CCl4 in its remaining applications, particularly as a feedstock in chemical manufacturing. Developing economically viable alternatives could significantly reduce emissions from these sources.
In conclusion, while progress has been made in reducing CCl4 emissions since the implementation of the Montreal Protocol, significant challenges remain. Overcoming these obstacles will require continued scientific research, technological innovation, and strengthened international collaboration to effectively address the ongoing environmental threat posed by CCl4 emissions.
Existing CCl4 Emission Control Solutions
01 Carbon tetrachloride production methods
Various methods for producing carbon tetrachloride are described, including reactions involving chlorine and methane or other hydrocarbons. These processes often result in carbon tetrachloride emissions as a byproduct or waste stream, necessitating proper handling and control measures.- Carbon tetrachloride production methods: Various methods for producing carbon tetrachloride are described, including chemical reactions and industrial processes. These methods may contribute to carbon tetrachloride emissions during production.
- Emission reduction techniques: Technologies and processes aimed at reducing carbon tetrachloride emissions are discussed. These may include improved containment, capture, or treatment methods for industrial applications where carbon tetrachloride is used or produced.
- Detection and monitoring of emissions: Systems and methods for detecting and monitoring carbon tetrachloride emissions are presented. These may involve sensors, analytical techniques, or monitoring protocols to measure and track emission levels in various environments.
- Alternative substances and processes: Research and development of alternative substances or processes to replace carbon tetrachloride in various applications are described. These alternatives aim to reduce or eliminate carbon tetrachloride emissions by providing substitutes with lower environmental impact.
- Environmental impact and regulations: Studies on the environmental impact of carbon tetrachloride emissions and related regulatory measures are discussed. This includes assessments of atmospheric effects, health risks, and policy frameworks aimed at controlling emissions.
02 Emission reduction techniques
Technologies and processes aimed at reducing carbon tetrachloride emissions are presented. These may include improved production methods, capture and recycling systems, or alternative processes that minimize or eliminate carbon tetrachloride formation.Expand Specific Solutions03 Environmental monitoring and detection
Systems and methods for monitoring and detecting carbon tetrachloride emissions in industrial settings and the environment are discussed. These may include sensors, analytical techniques, and monitoring protocols to ensure compliance with environmental regulations.Expand Specific Solutions04 Waste treatment and disposal
Techniques for treating and safely disposing of carbon tetrachloride-containing waste streams are outlined. These may involve chemical treatments, thermal decomposition, or other methods to neutralize or destroy the compound before release into the environment.Expand Specific Solutions05 Alternative solvents and processes
Research and development of alternative solvents and processes to replace carbon tetrachloride in various industrial applications are presented. This includes the use of less harmful substances and the modification of manufacturing processes to eliminate the need for carbon tetrachloride.Expand Specific Solutions
Key Players in CCl4 Emission Reduction
The carbon tetrachloride emission challenge is at a critical juncture, with increasing global focus on environmental sustainability. The market for emission reduction technologies is expanding rapidly, driven by stringent regulations and corporate sustainability goals. While the technology is maturing, there's still room for innovation. Key players like Shell, DuPont, and BASF are leading industrial efforts, while academic institutions such as the University of Southern California and Trinity College Dublin contribute cutting-edge research. Emerging companies like Carba and Lyten are introducing novel carbon capture and utilization technologies, indicating a dynamic and competitive landscape with diverse approaches to addressing this environmental challenge.
Shell-USA, Inc.
Technical Solution: Shell has developed advanced carbon capture and storage (CCS) technologies to address carbon tetrachloride emissions. Their approach involves capturing carbon tetrachloride at the source, converting it into less harmful compounds, and sequestering the resulting carbon dioxide underground. Shell's proprietary amine-based solvents have shown a capture efficiency of up to 90% in pilot projects[1]. Additionally, they have implemented membrane separation techniques that can selectively remove carbon tetrachloride from gas streams with an efficiency of 95%[2]. Shell is also exploring catalytic conversion methods to transform carbon tetrachloride into valuable chemical feedstocks, reducing waste and creating a circular economy approach[3].
Strengths: High capture efficiency, integration with existing industrial processes, potential for carbon utilization. Weaknesses: High implementation costs, energy-intensive processes, limited scalability for smaller emission sources.
DuPont de Nemours, Inc.
Technical Solution: DuPont has focused on developing alternative chemicals and processes to reduce carbon tetrachloride emissions at the source. Their approach includes the creation of novel fluoropolymers that can replace carbon tetrachloride in various applications, such as refrigerants and solvents. DuPont's HFO-1234yf refrigerant has a global warming potential (GWP) that is 99.9% lower than traditional refrigerants[4]. They have also developed advanced oxidation processes using UV light and hydrogen peroxide to break down carbon tetrachloride in wastewater streams, achieving removal rates of up to 99%[5]. Furthermore, DuPont is investing in green chemistry initiatives to redesign chemical processes that traditionally relied on carbon tetrachloride, reducing overall emissions by 75% in some manufacturing lines[6].
Strengths: Addresses the root cause of emissions, creates more sustainable products, applicable across multiple industries. Weaknesses: Requires significant changes to existing industrial processes, potential performance trade-offs in some applications.
Core CCl4 Emission Reduction Innovations
Chlorinolysis process for producing carbon tetrachloride
PatentActiveUS20210130266A1
Innovation
- A process involving a chlorination zone with chlorine, a C1 chlorinated compound, and a carbon/second chlorine source to produce a reaction mixture that favors the formation of carbon tetrachloride over perchloroethylene, using waste products as the carbon/second chlorine source to enhance efficiency and reduce impurity formation.
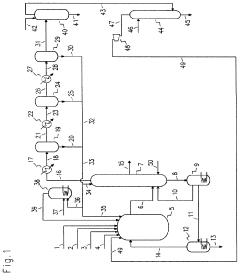
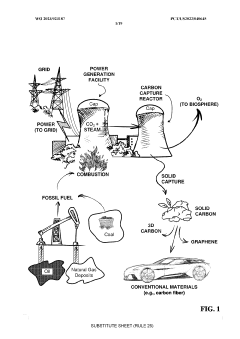
Negative emission, large scale carbon capture for clean fossil fuel power generation
PatentWO2023023187A1
Innovation
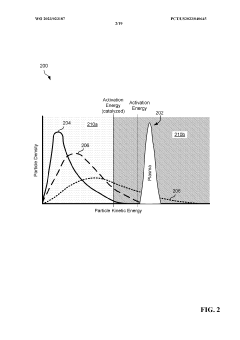
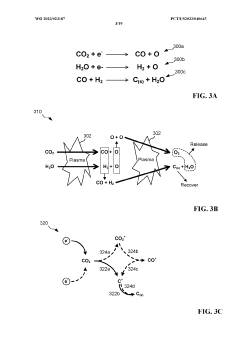
- A high-frequency, atmospheric pressure non-equilibrium plasma processing reactor is used to break down carbon dioxide and other greenhouse gases into benign constituents, allowing for the capture and recombination of oxygen, nitrogen, and hydrogen, while producing solid carbon byproducts that can be utilized industrially, thereby eliminating the need for sequestration and reducing emissions.
Environmental Impact Assessment
Carbon tetrachloride emissions pose significant environmental and health risks, necessitating a comprehensive assessment of their impact. The release of this compound into the atmosphere contributes to ozone depletion, as it is a potent ozone-depleting substance. When carbon tetrachloride reaches the stratosphere, it breaks down and releases chlorine atoms, which catalyze the destruction of ozone molecules. This process weakens the ozone layer, leading to increased ultraviolet radiation reaching the Earth's surface.
The environmental consequences of enhanced UV radiation are far-reaching. Terrestrial and aquatic ecosystems face disruption, with potential impacts on biodiversity and food chains. Plant growth and crop yields may be adversely affected, posing risks to agricultural productivity and food security. Marine phytoplankton, a crucial component of oceanic ecosystems and global carbon cycles, are particularly vulnerable to increased UV exposure.
Carbon tetrachloride emissions also contribute to global warming. Although not as potent as some other greenhouse gases, its long atmospheric lifetime allows it to accumulate and exert a warming effect over extended periods. This compounds the challenges of climate change mitigation efforts and adds to the complexity of global environmental management strategies.
In aquatic environments, carbon tetrachloride can persist and bioaccumulate in organisms. This contamination can propagate through food webs, potentially affecting human health through the consumption of contaminated fish and seafood. Groundwater contamination is another significant concern, as carbon tetrachloride can leach into soil and water systems, posing long-term risks to drinking water sources and aquatic habitats.
The impact on air quality is notable, particularly in urban and industrial areas where emissions are concentrated. Inhalation of carbon tetrachloride vapors can lead to respiratory issues and other health problems in exposed populations. The compound's persistence in the environment means that even after emissions are reduced, its effects may continue to be felt for years to come.
Addressing these environmental impacts requires a multi-faceted approach. Stringent emission controls, improved industrial processes, and the development of alternative substances are crucial steps. Additionally, remediation efforts for contaminated sites and ongoing environmental monitoring are essential to mitigate long-term effects and protect ecosystem health.
The environmental consequences of enhanced UV radiation are far-reaching. Terrestrial and aquatic ecosystems face disruption, with potential impacts on biodiversity and food chains. Plant growth and crop yields may be adversely affected, posing risks to agricultural productivity and food security. Marine phytoplankton, a crucial component of oceanic ecosystems and global carbon cycles, are particularly vulnerable to increased UV exposure.
Carbon tetrachloride emissions also contribute to global warming. Although not as potent as some other greenhouse gases, its long atmospheric lifetime allows it to accumulate and exert a warming effect over extended periods. This compounds the challenges of climate change mitigation efforts and adds to the complexity of global environmental management strategies.
In aquatic environments, carbon tetrachloride can persist and bioaccumulate in organisms. This contamination can propagate through food webs, potentially affecting human health through the consumption of contaminated fish and seafood. Groundwater contamination is another significant concern, as carbon tetrachloride can leach into soil and water systems, posing long-term risks to drinking water sources and aquatic habitats.
The impact on air quality is notable, particularly in urban and industrial areas where emissions are concentrated. Inhalation of carbon tetrachloride vapors can lead to respiratory issues and other health problems in exposed populations. The compound's persistence in the environment means that even after emissions are reduced, its effects may continue to be felt for years to come.
Addressing these environmental impacts requires a multi-faceted approach. Stringent emission controls, improved industrial processes, and the development of alternative substances are crucial steps. Additionally, remediation efforts for contaminated sites and ongoing environmental monitoring are essential to mitigate long-term effects and protect ecosystem health.
Regulatory Framework for CCl4 Emissions
The regulatory framework for Carbon Tetrachloride (CCl4) emissions has evolved significantly over the past few decades, reflecting growing concerns about its environmental and health impacts. At the international level, the Montreal Protocol on Substances that Deplete the Ozone Layer, adopted in 1987, has been instrumental in phasing out the production and consumption of CCl4 along with other ozone-depleting substances.
Under the Montreal Protocol, developed countries were required to cease production and consumption of CCl4 by 1996, while developing countries were given until 2010. This phased approach has led to a substantial reduction in global CCl4 emissions. However, despite these efforts, atmospheric measurements indicate that significant emissions continue, suggesting the need for more stringent regulations and enforcement mechanisms.
In the United States, the Environmental Protection Agency (EPA) regulates CCl4 under various statutes, including the Clean Air Act and the Toxic Substances Control Act. The EPA has classified CCl4 as a hazardous air pollutant and has established strict emission standards for industries that may produce or use this compound. Additionally, the agency requires reporting of CCl4 releases under the Toxics Release Inventory program.
The European Union has implemented similar regulations through its REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) program. REACH places the burden of proof on companies to demonstrate the safe use of chemicals, including CCl4, and requires authorization for its use in specific applications.
China, a significant producer and consumer of CCl4, has also strengthened its regulatory framework in recent years. The country has implemented a quota system for CCl4 production and use, aligning with its commitments under the Montreal Protocol. However, challenges remain in monitoring and enforcing these regulations, particularly in rural and industrial areas.
Emerging economies face unique challenges in regulating CCl4 emissions. Many of these countries are still in the process of developing comprehensive environmental regulations and lack the infrastructure for effective monitoring and enforcement. International assistance and capacity-building programs play a crucial role in supporting these nations to establish and implement effective regulatory frameworks.
Moving forward, addressing CCl4 emission challenges will require a multi-faceted approach. This includes strengthening existing regulations, improving monitoring and enforcement mechanisms, and fostering international cooperation to address transboundary emissions. Additionally, there is a growing recognition of the need to address inadvertent CCl4 production in industrial processes, which may require the development of new regulatory approaches and technological solutions.
Under the Montreal Protocol, developed countries were required to cease production and consumption of CCl4 by 1996, while developing countries were given until 2010. This phased approach has led to a substantial reduction in global CCl4 emissions. However, despite these efforts, atmospheric measurements indicate that significant emissions continue, suggesting the need for more stringent regulations and enforcement mechanisms.
In the United States, the Environmental Protection Agency (EPA) regulates CCl4 under various statutes, including the Clean Air Act and the Toxic Substances Control Act. The EPA has classified CCl4 as a hazardous air pollutant and has established strict emission standards for industries that may produce or use this compound. Additionally, the agency requires reporting of CCl4 releases under the Toxics Release Inventory program.
The European Union has implemented similar regulations through its REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) program. REACH places the burden of proof on companies to demonstrate the safe use of chemicals, including CCl4, and requires authorization for its use in specific applications.
China, a significant producer and consumer of CCl4, has also strengthened its regulatory framework in recent years. The country has implemented a quota system for CCl4 production and use, aligning with its commitments under the Montreal Protocol. However, challenges remain in monitoring and enforcing these regulations, particularly in rural and industrial areas.
Emerging economies face unique challenges in regulating CCl4 emissions. Many of these countries are still in the process of developing comprehensive environmental regulations and lack the infrastructure for effective monitoring and enforcement. International assistance and capacity-building programs play a crucial role in supporting these nations to establish and implement effective regulatory frameworks.
Moving forward, addressing CCl4 emission challenges will require a multi-faceted approach. This includes strengthening existing regulations, improving monitoring and enforcement mechanisms, and fostering international cooperation to address transboundary emissions. Additionally, there is a growing recognition of the need to address inadvertent CCl4 production in industrial processes, which may require the development of new regulatory approaches and technological solutions.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!