Carbon Tetrachloride's Historical Usage and Evolution in Industry
JUL 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CCl4 Industrial History
Carbon tetrachloride (CCl4) has a rich and complex history in industrial applications, spanning over a century of use across various sectors. Initially synthesized in the mid-19th century, CCl4 gained prominence in the early 1900s due to its unique chemical properties and versatility.
The industrial journey of CCl4 began with its widespread adoption as a cleaning solvent and degreasing agent. Its non-flammability and excellent solvency made it particularly valuable in metal cleaning and textile processing. During World War I, CCl4 found extensive use in the production of smoke screens and as a fire extinguishing agent, owing to its ability to displace oxygen and suppress combustion.
In the 1920s and 1930s, CCl4 became a key component in the refrigeration industry. It served as both a refrigerant and a working fluid in air conditioning systems, contributing significantly to the rapid growth of climate control technologies. This period also saw its increased use in the dry cleaning industry, where it replaced more flammable solvents like gasoline and benzene.
The chemical industry further expanded CCl4's applications during the mid-20th century. It became an important intermediate in the production of chlorofluorocarbons (CFCs), which were widely used as refrigerants, propellants, and blowing agents. CCl4 also played a crucial role in the synthesis of various pesticides and pharmaceuticals.
However, the 1970s marked a turning point in CCl4's industrial history. Growing awareness of its environmental impact, particularly its role in ozone depletion and its toxicity to human health, led to increased scrutiny and regulation. The Montreal Protocol of 1987 initiated the phase-out of ozone-depleting substances, including CCl4, in many applications.
Despite these restrictions, CCl4 continued to find use in certain niche applications. It remained valuable in analytical chemistry as a solvent and reagent. In the semiconductor industry, high-purity CCl4 was utilized in the production of silicon wafers and in plasma etching processes.
The late 20th and early 21st centuries saw a significant decline in CCl4 production and use globally. Alternative substances and technologies were developed to replace CCl4 in most of its traditional applications. However, its legacy in industrial history remains significant, having played a pivotal role in the development of numerous technologies and products that shaped modern industry.
The industrial journey of CCl4 began with its widespread adoption as a cleaning solvent and degreasing agent. Its non-flammability and excellent solvency made it particularly valuable in metal cleaning and textile processing. During World War I, CCl4 found extensive use in the production of smoke screens and as a fire extinguishing agent, owing to its ability to displace oxygen and suppress combustion.
In the 1920s and 1930s, CCl4 became a key component in the refrigeration industry. It served as both a refrigerant and a working fluid in air conditioning systems, contributing significantly to the rapid growth of climate control technologies. This period also saw its increased use in the dry cleaning industry, where it replaced more flammable solvents like gasoline and benzene.
The chemical industry further expanded CCl4's applications during the mid-20th century. It became an important intermediate in the production of chlorofluorocarbons (CFCs), which were widely used as refrigerants, propellants, and blowing agents. CCl4 also played a crucial role in the synthesis of various pesticides and pharmaceuticals.
However, the 1970s marked a turning point in CCl4's industrial history. Growing awareness of its environmental impact, particularly its role in ozone depletion and its toxicity to human health, led to increased scrutiny and regulation. The Montreal Protocol of 1987 initiated the phase-out of ozone-depleting substances, including CCl4, in many applications.
Despite these restrictions, CCl4 continued to find use in certain niche applications. It remained valuable in analytical chemistry as a solvent and reagent. In the semiconductor industry, high-purity CCl4 was utilized in the production of silicon wafers and in plasma etching processes.
The late 20th and early 21st centuries saw a significant decline in CCl4 production and use globally. Alternative substances and technologies were developed to replace CCl4 in most of its traditional applications. However, its legacy in industrial history remains significant, having played a pivotal role in the development of numerous technologies and products that shaped modern industry.
Market Demand Analysis
Carbon tetrachloride, once a widely used industrial chemical, has experienced significant shifts in market demand over the years. Initially, its versatile properties made it a popular choice across various industries. In the early to mid-20th century, the compound found extensive use as a cleaning agent, solvent, and fire extinguishing agent. Its non-flammability and excellent solvency properties drove high demand in the dry cleaning industry, metal degreasing processes, and as a precursor in the production of refrigerants.
However, the market landscape for carbon tetrachloride began to change dramatically in the latter half of the 20th century. The discovery of its ozone-depleting properties and potential health hazards led to a sharp decline in demand. The Montreal Protocol, implemented in 1987, significantly restricted the production and consumption of ozone-depleting substances, including carbon tetrachloride. This global agreement had a profound impact on the market, effectively eliminating its use in many applications.
Despite these restrictions, a niche market for carbon tetrachloride persisted in certain industrial processes. It continued to be used as a feedstock for the production of other chemicals, particularly in the manufacture of hydrofluorocarbons (HFCs) and hydrofluoroolefins (HFOs). These substances were developed as alternatives to ozone-depleting chlorofluorocarbons (CFCs) and hydrochlorofluorocarbons (HCFCs).
The pharmaceutical industry also maintained a limited demand for carbon tetrachloride, primarily as a solvent in the synthesis of certain drugs. Additionally, some specialized laboratory applications continued to require small quantities of the compound for analytical and research purposes.
In recent years, the global push towards more environmentally friendly and sustainable practices has further impacted the market demand for carbon tetrachloride. Industries have been actively seeking alternatives that offer similar performance characteristics without the associated environmental and health risks. This trend has led to the development of new, safer solvents and cleaning agents, further reducing the reliance on carbon tetrachloride.
The evolving regulatory landscape has also played a crucial role in shaping market demand. Stricter environmental regulations and increased awareness of the compound's hazards have prompted industries to invest in alternative technologies and processes. This shift has not only affected direct users of carbon tetrachloride but also industries involved in its production and distribution.
Looking forward, the market demand for carbon tetrachloride is expected to continue its downward trend. The focus on sustainability, coupled with ongoing research into safer alternatives, suggests that its use will become increasingly limited to highly specialized applications where no viable substitutes exist. The chemical industry's efforts to develop greener processes and products are likely to further diminish the need for carbon tetrachloride in the coming years.
However, the market landscape for carbon tetrachloride began to change dramatically in the latter half of the 20th century. The discovery of its ozone-depleting properties and potential health hazards led to a sharp decline in demand. The Montreal Protocol, implemented in 1987, significantly restricted the production and consumption of ozone-depleting substances, including carbon tetrachloride. This global agreement had a profound impact on the market, effectively eliminating its use in many applications.
Despite these restrictions, a niche market for carbon tetrachloride persisted in certain industrial processes. It continued to be used as a feedstock for the production of other chemicals, particularly in the manufacture of hydrofluorocarbons (HFCs) and hydrofluoroolefins (HFOs). These substances were developed as alternatives to ozone-depleting chlorofluorocarbons (CFCs) and hydrochlorofluorocarbons (HCFCs).
The pharmaceutical industry also maintained a limited demand for carbon tetrachloride, primarily as a solvent in the synthesis of certain drugs. Additionally, some specialized laboratory applications continued to require small quantities of the compound for analytical and research purposes.
In recent years, the global push towards more environmentally friendly and sustainable practices has further impacted the market demand for carbon tetrachloride. Industries have been actively seeking alternatives that offer similar performance characteristics without the associated environmental and health risks. This trend has led to the development of new, safer solvents and cleaning agents, further reducing the reliance on carbon tetrachloride.
The evolving regulatory landscape has also played a crucial role in shaping market demand. Stricter environmental regulations and increased awareness of the compound's hazards have prompted industries to invest in alternative technologies and processes. This shift has not only affected direct users of carbon tetrachloride but also industries involved in its production and distribution.
Looking forward, the market demand for carbon tetrachloride is expected to continue its downward trend. The focus on sustainability, coupled with ongoing research into safer alternatives, suggests that its use will become increasingly limited to highly specialized applications where no viable substitutes exist. The chemical industry's efforts to develop greener processes and products are likely to further diminish the need for carbon tetrachloride in the coming years.
Technical Challenges
Carbon tetrachloride's historical usage in industry has faced significant technical challenges over the years, primarily due to its environmental and health impacts. One of the main obstacles has been finding suitable alternatives that can match its effectiveness in various applications while being less harmful.
In the realm of fire extinguishing, carbon tetrachloride was widely used due to its excellent fire-suppressing properties. However, the toxic fumes produced during its use posed severe health risks to both firefighters and victims. This led to a major technical challenge in developing equally effective but safer fire extinguishing agents.
The dry cleaning industry heavily relied on carbon tetrachloride for its excellent solvent properties. The challenge here was to find a replacement that could effectively remove stains and dirt from fabrics without causing damage or leaving residues. This required extensive research into alternative solvents and cleaning technologies.
In the field of refrigeration, carbon tetrachloride was used as a refrigerant due to its thermodynamic properties. The technical challenge lay in developing new refrigerants that could provide similar cooling efficiency while being ozone-friendly and having a lower global warming potential.
The semiconductor industry utilized carbon tetrachloride in the production of silicon chips. Finding a substitute that could effectively etch silicon wafers without compromising the quality or efficiency of the manufacturing process presented a significant technical hurdle.
Another major challenge has been the remediation of sites contaminated with carbon tetrachloride. Its persistence in the environment and tendency to form dense non-aqueous phase liquids (DNAPLs) make it difficult to remove from soil and groundwater. Developing effective and economically viable cleanup technologies has been an ongoing technical challenge.
The analytical chemistry sector faced the challenge of replacing carbon tetrachloride in various laboratory procedures, particularly as a solvent in spectroscopic analyses. This required the development of new analytical methods and the adaptation of existing ones to work with alternative solvents.
Lastly, the phase-out of carbon tetrachloride production itself presented technical challenges. Industries had to redesign their processes and equipment to accommodate new substances, often requiring significant investments in research, development, and infrastructure modifications.
These technical challenges have driven innovation across multiple industries, leading to the development of safer alternatives and more environmentally friendly practices. However, some applications still lack perfect substitutes, highlighting the ongoing need for research and development in this area.
In the realm of fire extinguishing, carbon tetrachloride was widely used due to its excellent fire-suppressing properties. However, the toxic fumes produced during its use posed severe health risks to both firefighters and victims. This led to a major technical challenge in developing equally effective but safer fire extinguishing agents.
The dry cleaning industry heavily relied on carbon tetrachloride for its excellent solvent properties. The challenge here was to find a replacement that could effectively remove stains and dirt from fabrics without causing damage or leaving residues. This required extensive research into alternative solvents and cleaning technologies.
In the field of refrigeration, carbon tetrachloride was used as a refrigerant due to its thermodynamic properties. The technical challenge lay in developing new refrigerants that could provide similar cooling efficiency while being ozone-friendly and having a lower global warming potential.
The semiconductor industry utilized carbon tetrachloride in the production of silicon chips. Finding a substitute that could effectively etch silicon wafers without compromising the quality or efficiency of the manufacturing process presented a significant technical hurdle.
Another major challenge has been the remediation of sites contaminated with carbon tetrachloride. Its persistence in the environment and tendency to form dense non-aqueous phase liquids (DNAPLs) make it difficult to remove from soil and groundwater. Developing effective and economically viable cleanup technologies has been an ongoing technical challenge.
The analytical chemistry sector faced the challenge of replacing carbon tetrachloride in various laboratory procedures, particularly as a solvent in spectroscopic analyses. This required the development of new analytical methods and the adaptation of existing ones to work with alternative solvents.
Lastly, the phase-out of carbon tetrachloride production itself presented technical challenges. Industries had to redesign their processes and equipment to accommodate new substances, often requiring significant investments in research, development, and infrastructure modifications.
These technical challenges have driven innovation across multiple industries, leading to the development of safer alternatives and more environmentally friendly practices. However, some applications still lack perfect substitutes, highlighting the ongoing need for research and development in this area.
Current Applications
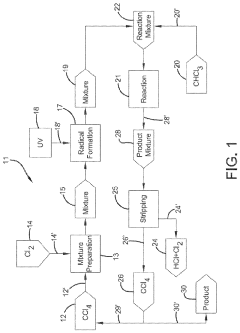
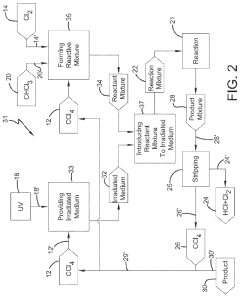
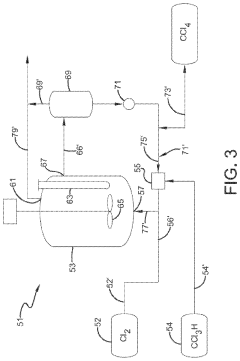
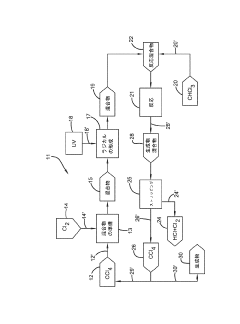
01 Production and purification of carbon tetrachloride
Various methods for producing and purifying carbon tetrachloride are described. These include chemical reactions, distillation processes, and other purification techniques to obtain high-quality carbon tetrachloride for industrial and laboratory use.- Production and purification of carbon tetrachloride: Various methods for producing and purifying carbon tetrachloride are described. These include chemical synthesis processes, distillation techniques, and purification methods to obtain high-quality carbon tetrachloride for industrial applications.
- Applications of carbon tetrachloride in chemical processes: Carbon tetrachloride is utilized in various chemical processes, including as a solvent, reagent, or intermediate in the production of other chemicals. Its properties make it suitable for specific industrial applications and chemical reactions.
- Environmental and safety considerations: Due to its environmental impact and health hazards, research focuses on alternatives to carbon tetrachloride and methods for its safe handling, storage, and disposal. This includes developing eco-friendly substitutes and improving safety measures in industrial settings.
- Detection and analysis methods: Various techniques and devices are developed for detecting and analyzing carbon tetrachloride in different environments. These include spectroscopic methods, chromatography, and specialized sensors for monitoring its presence in air, water, or other media.
- Historical uses and patents: Early patents and historical documents describe various applications of carbon tetrachloride, including its use in fire extinguishers, dry cleaning, and as a degreasing agent. These historical uses provide context for the compound's industrial significance over time.
02 Applications of carbon tetrachloride in chemical processes
Carbon tetrachloride is utilized in various chemical processes, including as a solvent, reagent, or intermediate in the production of other chemicals. Its unique properties make it valuable in specific industrial applications and synthetic reactions.Expand Specific Solutions03 Environmental and safety considerations
Due to its environmental impact and potential health hazards, there are concerns and regulations surrounding the use of carbon tetrachloride. Alternative substances and methods are being developed to replace its use in various applications, and proper handling and disposal procedures are emphasized.Expand Specific Solutions04 Detection and analysis methods for carbon tetrachloride
Various analytical techniques and methods have been developed for detecting and quantifying carbon tetrachloride in different matrices. These include spectroscopic methods, chromatography, and specialized sensors for environmental monitoring and quality control purposes.Expand Specific Solutions05 Historical uses and patents related to carbon tetrachloride
Carbon tetrachloride has a long history of industrial and commercial use. Early patents and historical documents describe various applications, including its use as a fire extinguishing agent, cleaning solvent, and in the production of refrigerants. Many of these uses have since been phased out due to safety and environmental concerns.Expand Specific Solutions
Key Industry Players
The carbon tetrachloride industry has evolved significantly over the years, transitioning from widespread industrial use to a highly regulated and limited market. Currently, the industry is in a mature phase, with a relatively small global market size due to environmental concerns and regulatory restrictions. The technology for carbon tetrachloride production and handling is well-established, with key players like Occidental Chemical Corp., Eastman Chemical Co., and Wacker Chemie AG possessing advanced capabilities. These companies, along with others such as Tronox LLC and CITIC Dicastal Co., Ltd., have adapted their operations to focus on specialized applications and alternative products, reflecting the industry's shift towards more sustainable practices and stringent environmental standards.
Occidental Chemical Corp.
Technical Solution: Occidental Chemical Corp. has been a significant player in the carbon tetrachloride industry, focusing on its production and application in various chemical processes. The company developed advanced manufacturing techniques to produce high-purity CCl4, which was crucial for its use as a solvent and reagent in industrial applications[4]. Occidental also implemented stringent safety measures and containment systems to minimize environmental impact and worker exposure during CCl4 production and handling[5]. As regulations tightened, the company shifted its focus to developing alternative chlorinated solvents and exploring new applications for CCl4 by-products, such as in the production of perchloroethylene and other chlorinated hydrocarbons[6].
Strengths: Expertise in chlorinated solvent production, established safety protocols for handling hazardous materials. Weaknesses: Dependence on chlorine-based chemistry, potential environmental liabilities from historical CCl4 production.
Eastman Chemical Co.
Technical Solution: Eastman Chemical Co. has been involved in the historical usage and evolution of carbon tetrachloride in industry, particularly in the development of alternative solvents and chemical intermediates. The company focused on creating safer and more environmentally friendly substitutes for CCl4 in various applications, including as a solvent in the production of polymers and pharmaceuticals[10]. Eastman developed proprietary processes for the synthesis of chlorinated hydrocarbons that reduced reliance on CCl4 as a feedstock[11]. Additionally, the company invested in research to repurpose existing CCl4 production facilities for the manufacture of more sustainable chemical products, demonstrating adaptability in the face of changing regulations and market demands[12].
Strengths: Diversified product portfolio, strong capabilities in process innovation and optimization. Weaknesses: Historical involvement in chlorinated solvent production, potential remediation costs for legacy sites.
CCl4 Alternatives
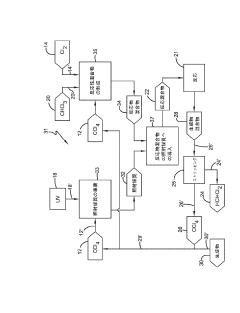
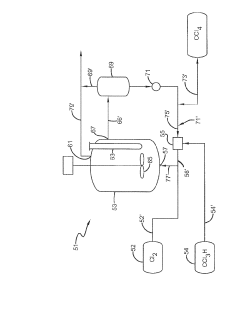
Photochlorination of partially-chlorinated chloromethanes to carbon tetrachloride
PatentActiveUS20240025823A1
Innovation
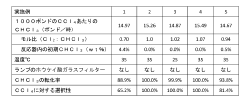
- A method involving the photochlorination of a chloromethanes stream containing chloroform, methyl chloride, and methylene chloride, combined with chlorine and additional carbon tetrachloride, and subjected to electromagnetic radiation to form carbon tetrachloride, achieving high conversion rates with reduced levels of unwanted chlorinated hydrocarbons.
Producing carbon tetrachloride by photochlorination of chloroform
PatentActiveJP2024069268A
Innovation
- A method involving the photochlorination of chloroform with chlorine in the presence of electromagnetic radiation, maintaining a low concentration of chloroform and a stoichiometric concentration of chlorine, to produce carbon tetrachloride with high selectivity and minimize the formation of hexachloroethane.
Environmental Impact
Carbon tetrachloride's historical usage in industry has had significant environmental impacts, particularly on air and water quality, as well as ecosystem health. The widespread use of this chemical compound in various industrial applications led to its release into the environment through emissions, spills, and improper disposal practices.
In the atmosphere, carbon tetrachloride contributes to ozone depletion. As a chlorinated compound, it releases chlorine atoms when exposed to ultraviolet radiation in the stratosphere. These chlorine atoms catalyze the breakdown of ozone molecules, leading to the thinning of the ozone layer. This depletion increases the amount of harmful UV radiation reaching the Earth's surface, posing risks to human health and ecosystems.
Water contamination has been another major environmental concern associated with carbon tetrachloride. Its high solubility and persistence in water have resulted in the contamination of groundwater and surface water sources. This pollution has affected drinking water quality in many areas, potentially exposing populations to health risks associated with long-term exposure to the chemical.
Soil contamination from carbon tetrachloride has also been observed in areas with historical industrial activity. The compound's ability to persist in soil and migrate through subsurface layers has led to the creation of contaminated sites that require extensive remediation efforts. This contamination not only affects soil quality but also poses risks to plants, soil organisms, and animals in the affected ecosystems.
The bioaccumulation of carbon tetrachloride in aquatic organisms has been documented, leading to potential impacts on food chains and ecosystem balance. Fish and other aquatic life exposed to contaminated water bodies may accumulate the chemical in their tissues, potentially transferring it to higher trophic levels, including humans who consume these organisms.
Recognizing these environmental impacts, regulatory bodies worldwide have implemented strict controls on the production, use, and disposal of carbon tetrachloride. The Montreal Protocol, an international treaty designed to protect the ozone layer, phased out the production of carbon tetrachloride for dispersive uses. This action has significantly reduced atmospheric emissions and their associated environmental impacts.
Efforts to address the legacy of carbon tetrachloride contamination continue through environmental remediation projects. These initiatives aim to clean up contaminated sites, restore affected ecosystems, and mitigate long-term environmental risks. Advanced treatment technologies, such as pump-and-treat systems and in-situ chemical oxidation, are being employed to remove carbon tetrachloride from contaminated soil and groundwater.
In the atmosphere, carbon tetrachloride contributes to ozone depletion. As a chlorinated compound, it releases chlorine atoms when exposed to ultraviolet radiation in the stratosphere. These chlorine atoms catalyze the breakdown of ozone molecules, leading to the thinning of the ozone layer. This depletion increases the amount of harmful UV radiation reaching the Earth's surface, posing risks to human health and ecosystems.
Water contamination has been another major environmental concern associated with carbon tetrachloride. Its high solubility and persistence in water have resulted in the contamination of groundwater and surface water sources. This pollution has affected drinking water quality in many areas, potentially exposing populations to health risks associated with long-term exposure to the chemical.
Soil contamination from carbon tetrachloride has also been observed in areas with historical industrial activity. The compound's ability to persist in soil and migrate through subsurface layers has led to the creation of contaminated sites that require extensive remediation efforts. This contamination not only affects soil quality but also poses risks to plants, soil organisms, and animals in the affected ecosystems.
The bioaccumulation of carbon tetrachloride in aquatic organisms has been documented, leading to potential impacts on food chains and ecosystem balance. Fish and other aquatic life exposed to contaminated water bodies may accumulate the chemical in their tissues, potentially transferring it to higher trophic levels, including humans who consume these organisms.
Recognizing these environmental impacts, regulatory bodies worldwide have implemented strict controls on the production, use, and disposal of carbon tetrachloride. The Montreal Protocol, an international treaty designed to protect the ozone layer, phased out the production of carbon tetrachloride for dispersive uses. This action has significantly reduced atmospheric emissions and their associated environmental impacts.
Efforts to address the legacy of carbon tetrachloride contamination continue through environmental remediation projects. These initiatives aim to clean up contaminated sites, restore affected ecosystems, and mitigate long-term environmental risks. Advanced treatment technologies, such as pump-and-treat systems and in-situ chemical oxidation, are being employed to remove carbon tetrachloride from contaminated soil and groundwater.
Regulatory Framework
The regulatory framework surrounding Carbon Tetrachloride (CCl4) has evolved significantly over the years, reflecting growing awareness of its environmental and health impacts. In the early 20th century, CCl4 was widely used in various industrial applications with minimal restrictions. However, as scientific evidence emerged about its ozone-depleting properties and potential health hazards, governments worldwide began implementing stringent regulations.
The Montreal Protocol, signed in 1987, marked a turning point in CCl4 regulation. This international treaty aimed to phase out the production of ozone-depleting substances, including CCl4. Signatories committed to gradually reducing and eventually eliminating the production and consumption of CCl4 for non-feedstock uses. The protocol set specific timelines for developed and developing countries to achieve these goals.
In the United States, the Environmental Protection Agency (EPA) has played a crucial role in regulating CCl4. The Clean Air Act Amendments of 1990 incorporated the Montreal Protocol's objectives into domestic law. The EPA subsequently implemented regulations to control CCl4 production, import, and use. These regulations included a ban on the manufacture of CCl4 for dispersive uses and strict reporting requirements for its production and handling.
The European Union has also implemented comprehensive regulations on CCl4. The REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation, enacted in 2007, placed additional restrictions on the use of CCl4 in industrial processes. Under REACH, CCl4 is classified as a substance of very high concern, requiring special authorization for its use.
Internationally, the Stockholm Convention on Persistent Organic Pollutants, which came into force in 2004, further reinforced global efforts to restrict CCl4 use. While CCl4 is not directly listed in the convention, many countries have included it in their national implementation plans due to its persistent nature and long-range transport potential.
Despite these regulations, CCl4 continues to be produced and used in certain controlled applications, primarily as a feedstock for the production of other chemicals. However, strict monitoring and reporting requirements are in place to ensure compliance with international and national regulations. The regulatory framework also includes provisions for the safe disposal of CCl4 and remediation of contaminated sites.
As scientific understanding of CCl4's environmental and health impacts continues to evolve, regulatory frameworks are periodically updated. Recent focus has been on addressing unexpected emissions and improving monitoring techniques to ensure compliance with existing regulations. The ongoing regulatory efforts reflect a global commitment to minimizing the environmental and health risks associated with CCl4 while balancing industrial needs.
The Montreal Protocol, signed in 1987, marked a turning point in CCl4 regulation. This international treaty aimed to phase out the production of ozone-depleting substances, including CCl4. Signatories committed to gradually reducing and eventually eliminating the production and consumption of CCl4 for non-feedstock uses. The protocol set specific timelines for developed and developing countries to achieve these goals.
In the United States, the Environmental Protection Agency (EPA) has played a crucial role in regulating CCl4. The Clean Air Act Amendments of 1990 incorporated the Montreal Protocol's objectives into domestic law. The EPA subsequently implemented regulations to control CCl4 production, import, and use. These regulations included a ban on the manufacture of CCl4 for dispersive uses and strict reporting requirements for its production and handling.
The European Union has also implemented comprehensive regulations on CCl4. The REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation, enacted in 2007, placed additional restrictions on the use of CCl4 in industrial processes. Under REACH, CCl4 is classified as a substance of very high concern, requiring special authorization for its use.
Internationally, the Stockholm Convention on Persistent Organic Pollutants, which came into force in 2004, further reinforced global efforts to restrict CCl4 use. While CCl4 is not directly listed in the convention, many countries have included it in their national implementation plans due to its persistent nature and long-range transport potential.
Despite these regulations, CCl4 continues to be produced and used in certain controlled applications, primarily as a feedstock for the production of other chemicals. However, strict monitoring and reporting requirements are in place to ensure compliance with international and national regulations. The regulatory framework also includes provisions for the safe disposal of CCl4 and remediation of contaminated sites.
As scientific understanding of CCl4's environmental and health impacts continues to evolve, regulatory frameworks are periodically updated. Recent focus has been on addressing unexpected emissions and improving monitoring techniques to ensure compliance with existing regulations. The ongoing regulatory efforts reflect a global commitment to minimizing the environmental and health risks associated with CCl4 while balancing industrial needs.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!