How to Apply Carbon Tetrachloride Knowledge in Environmental Science?
JUL 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CCl4 Background and Objectives
Carbon tetrachloride (CCl4) has been a subject of significant interest in environmental science due to its historical use and environmental impact. Initially developed in the late 19th century, CCl4 found widespread applications in various industries, including dry cleaning, fire extinguishers, and as a precursor in the production of refrigerants. However, its use has been phased out in many countries due to its harmful effects on the environment and human health.
The primary objective of applying CCl4 knowledge in environmental science is to understand its behavior in the environment, assess its impact on ecosystems, and develop effective strategies for remediation and prevention of further contamination. This involves studying its chemical properties, environmental fate, and transport mechanisms in different media such as air, water, and soil.
One of the key areas of focus is the role of CCl4 in stratospheric ozone depletion. As a potent ozone-depleting substance, CCl4 has been regulated under the Montreal Protocol since 1987. Understanding its atmospheric chemistry, including its photolysis and reactions with other atmospheric components, is crucial for predicting long-term effects on the ozone layer and global climate.
In aquatic environments, CCl4 poses significant risks due to its persistence and potential for bioaccumulation. Research aims to elucidate its distribution patterns in water bodies, sediments, and aquatic organisms. This knowledge is essential for assessing ecological risks and developing water quality standards.
Soil contamination by CCl4 is another critical area of study. Environmental scientists investigate its behavior in different soil types, focusing on factors affecting its mobility, degradation, and potential for groundwater contamination. This information is vital for designing effective soil remediation strategies and preventing the spread of contamination.
The application of CCl4 knowledge extends to human health risk assessment. Understanding its toxicological profile, exposure pathways, and long-term health effects is crucial for developing public health policies and occupational safety guidelines. This includes studying its carcinogenic potential and effects on various organ systems.
Technological advancements in environmental monitoring and analysis play a significant role in CCl4 research. Developing sensitive and accurate detection methods for trace levels of CCl4 in environmental samples is an ongoing challenge. These techniques are essential for compliance monitoring, environmental impact assessments, and long-term trend analysis.
By applying CCl4 knowledge in environmental science, researchers and policymakers aim to mitigate its environmental impact, protect human health, and contribute to the broader understanding of persistent organic pollutants. This knowledge informs global environmental policies, guides remediation efforts, and supports the development of safer alternatives in industrial applications.
The primary objective of applying CCl4 knowledge in environmental science is to understand its behavior in the environment, assess its impact on ecosystems, and develop effective strategies for remediation and prevention of further contamination. This involves studying its chemical properties, environmental fate, and transport mechanisms in different media such as air, water, and soil.
One of the key areas of focus is the role of CCl4 in stratospheric ozone depletion. As a potent ozone-depleting substance, CCl4 has been regulated under the Montreal Protocol since 1987. Understanding its atmospheric chemistry, including its photolysis and reactions with other atmospheric components, is crucial for predicting long-term effects on the ozone layer and global climate.
In aquatic environments, CCl4 poses significant risks due to its persistence and potential for bioaccumulation. Research aims to elucidate its distribution patterns in water bodies, sediments, and aquatic organisms. This knowledge is essential for assessing ecological risks and developing water quality standards.
Soil contamination by CCl4 is another critical area of study. Environmental scientists investigate its behavior in different soil types, focusing on factors affecting its mobility, degradation, and potential for groundwater contamination. This information is vital for designing effective soil remediation strategies and preventing the spread of contamination.
The application of CCl4 knowledge extends to human health risk assessment. Understanding its toxicological profile, exposure pathways, and long-term health effects is crucial for developing public health policies and occupational safety guidelines. This includes studying its carcinogenic potential and effects on various organ systems.
Technological advancements in environmental monitoring and analysis play a significant role in CCl4 research. Developing sensitive and accurate detection methods for trace levels of CCl4 in environmental samples is an ongoing challenge. These techniques are essential for compliance monitoring, environmental impact assessments, and long-term trend analysis.
By applying CCl4 knowledge in environmental science, researchers and policymakers aim to mitigate its environmental impact, protect human health, and contribute to the broader understanding of persistent organic pollutants. This knowledge informs global environmental policies, guides remediation efforts, and supports the development of safer alternatives in industrial applications.
Environmental Impact Analysis
Carbon tetrachloride (CCl4) has been a significant concern in environmental science due to its detrimental effects on the ozone layer and its persistence in the environment. The application of carbon tetrachloride knowledge in environmental science primarily focuses on understanding its impact and developing strategies for mitigation and remediation.
The environmental impact of carbon tetrachloride is multifaceted, affecting both atmospheric and terrestrial ecosystems. In the atmosphere, CCl4 acts as a potent ozone-depleting substance, contributing to the depletion of the stratospheric ozone layer. This depletion leads to increased ultraviolet radiation reaching the Earth's surface, posing risks to human health and ecosystems. The long atmospheric lifetime of CCl4, estimated at 26 years, exacerbates its impact on the ozone layer.
In terrestrial and aquatic environments, carbon tetrachloride contamination poses significant risks to soil and groundwater quality. Its high mobility in soil and resistance to biodegradation make it a persistent pollutant. CCl4 can leach into groundwater, potentially contaminating drinking water sources and aquatic ecosystems. The compound's toxicity to various organisms, including humans, further compounds its environmental impact.
The bioaccumulation potential of carbon tetrachloride in aquatic organisms is another critical aspect of its environmental impact. While not as pronounced as some other organic pollutants, CCl4 can accumulate in the tissues of fish and other aquatic life, potentially entering the food chain and affecting higher trophic levels.
Understanding the environmental fate and transport of carbon tetrachloride is crucial for assessing its impact. In soil, CCl4 can volatilize into the air or migrate through soil pores, potentially reaching groundwater. Its behavior in different soil types and under various environmental conditions influences its distribution and persistence in the environment.
The impact of carbon tetrachloride on microbial communities in soil and water is an area of ongoing research. CCl4 can inhibit certain microbial populations, potentially disrupting ecosystem functions and biodegradation processes. However, some microbial species have shown the ability to degrade CCl4 under anaerobic conditions, offering potential for bioremediation strategies.
Climate change considerations add another dimension to the environmental impact of carbon tetrachloride. While not a direct greenhouse gas, CCl4's ozone-depleting properties indirectly contribute to climate change by altering atmospheric chemistry and radiation balance. The interplay between ozone depletion, climate change, and CCl4 emissions creates complex feedback loops in the Earth's climate system.
The environmental impact of carbon tetrachloride is multifaceted, affecting both atmospheric and terrestrial ecosystems. In the atmosphere, CCl4 acts as a potent ozone-depleting substance, contributing to the depletion of the stratospheric ozone layer. This depletion leads to increased ultraviolet radiation reaching the Earth's surface, posing risks to human health and ecosystems. The long atmospheric lifetime of CCl4, estimated at 26 years, exacerbates its impact on the ozone layer.
In terrestrial and aquatic environments, carbon tetrachloride contamination poses significant risks to soil and groundwater quality. Its high mobility in soil and resistance to biodegradation make it a persistent pollutant. CCl4 can leach into groundwater, potentially contaminating drinking water sources and aquatic ecosystems. The compound's toxicity to various organisms, including humans, further compounds its environmental impact.
The bioaccumulation potential of carbon tetrachloride in aquatic organisms is another critical aspect of its environmental impact. While not as pronounced as some other organic pollutants, CCl4 can accumulate in the tissues of fish and other aquatic life, potentially entering the food chain and affecting higher trophic levels.
Understanding the environmental fate and transport of carbon tetrachloride is crucial for assessing its impact. In soil, CCl4 can volatilize into the air or migrate through soil pores, potentially reaching groundwater. Its behavior in different soil types and under various environmental conditions influences its distribution and persistence in the environment.
The impact of carbon tetrachloride on microbial communities in soil and water is an area of ongoing research. CCl4 can inhibit certain microbial populations, potentially disrupting ecosystem functions and biodegradation processes. However, some microbial species have shown the ability to degrade CCl4 under anaerobic conditions, offering potential for bioremediation strategies.
Climate change considerations add another dimension to the environmental impact of carbon tetrachloride. While not a direct greenhouse gas, CCl4's ozone-depleting properties indirectly contribute to climate change by altering atmospheric chemistry and radiation balance. The interplay between ozone depletion, climate change, and CCl4 emissions creates complex feedback loops in the Earth's climate system.
CCl4 Properties and Challenges
Carbon tetrachloride (CCl4) is a synthetic chemical compound with unique properties that have made it both useful and problematic in environmental science. As a colorless, non-flammable liquid with a sweet odor, CCl4 was once widely used in various industrial and consumer applications. However, its environmental impact and health risks have led to significant restrictions on its use in recent decades.
One of the key properties of CCl4 is its high stability and low reactivity under normal conditions. This stability made it an attractive choice for use as a solvent, cleaning agent, and refrigerant. However, this same property also contributes to its persistence in the environment, as it does not readily break down through natural processes.
CCl4 is highly volatile, with a low boiling point of 76.72°C (170.1°F). This volatility allows it to easily evaporate and disperse into the atmosphere, contributing to its widespread distribution in the environment. Once in the atmosphere, CCl4 can persist for decades, with an estimated atmospheric lifetime of 26 years.
The compound's high density (1.594 g/cm³ at 20°C) means that when released into water bodies, it tends to sink and accumulate in sediments. This property makes it challenging to remove CCl4 from contaminated water sources and increases the risk of long-term environmental exposure.
One of the most significant challenges associated with CCl4 is its ozone-depleting potential. When released into the upper atmosphere, CCl4 can break down and release chlorine atoms, which catalyze the destruction of stratospheric ozone. This process contributes to the depletion of the ozone layer, which protects Earth from harmful ultraviolet radiation.
Another major concern is the toxicity of CCl4 to humans and other organisms. Exposure to CCl4 can cause liver and kidney damage, and it is classified as a probable human carcinogen. Its ability to bioaccumulate in organisms further exacerbates its potential for long-term ecological impacts.
The environmental persistence and toxicity of CCl4 pose significant challenges for remediation efforts. Traditional cleanup methods, such as pump-and-treat systems, are often ineffective due to CCl4's low solubility in water and tendency to adsorb to soil particles. This has led to the development of innovative remediation techniques, including in-situ chemical oxidation and bioremediation approaches.
In conclusion, the unique properties of CCl4, including its stability, volatility, and toxicity, present both opportunities and challenges in environmental science. Understanding these properties is crucial for developing effective strategies to mitigate its environmental impact and protect human health.
One of the key properties of CCl4 is its high stability and low reactivity under normal conditions. This stability made it an attractive choice for use as a solvent, cleaning agent, and refrigerant. However, this same property also contributes to its persistence in the environment, as it does not readily break down through natural processes.
CCl4 is highly volatile, with a low boiling point of 76.72°C (170.1°F). This volatility allows it to easily evaporate and disperse into the atmosphere, contributing to its widespread distribution in the environment. Once in the atmosphere, CCl4 can persist for decades, with an estimated atmospheric lifetime of 26 years.
The compound's high density (1.594 g/cm³ at 20°C) means that when released into water bodies, it tends to sink and accumulate in sediments. This property makes it challenging to remove CCl4 from contaminated water sources and increases the risk of long-term environmental exposure.
One of the most significant challenges associated with CCl4 is its ozone-depleting potential. When released into the upper atmosphere, CCl4 can break down and release chlorine atoms, which catalyze the destruction of stratospheric ozone. This process contributes to the depletion of the ozone layer, which protects Earth from harmful ultraviolet radiation.
Another major concern is the toxicity of CCl4 to humans and other organisms. Exposure to CCl4 can cause liver and kidney damage, and it is classified as a probable human carcinogen. Its ability to bioaccumulate in organisms further exacerbates its potential for long-term ecological impacts.
The environmental persistence and toxicity of CCl4 pose significant challenges for remediation efforts. Traditional cleanup methods, such as pump-and-treat systems, are often ineffective due to CCl4's low solubility in water and tendency to adsorb to soil particles. This has led to the development of innovative remediation techniques, including in-situ chemical oxidation and bioremediation approaches.
In conclusion, the unique properties of CCl4, including its stability, volatility, and toxicity, present both opportunities and challenges in environmental science. Understanding these properties is crucial for developing effective strategies to mitigate its environmental impact and protect human health.
Current Remediation Techniques
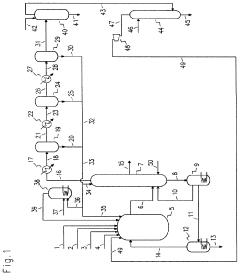
01 Production and purification of carbon tetrachloride
Various methods for producing and purifying carbon tetrachloride are described. These include chemical synthesis processes, distillation techniques, and purification methods to obtain high-quality carbon tetrachloride for industrial and laboratory use.- Production and purification of carbon tetrachloride: Various methods for producing and purifying carbon tetrachloride are described. These include chemical synthesis processes, distillation techniques, and purification methods to obtain high-quality carbon tetrachloride for industrial and laboratory use.
- Applications of carbon tetrachloride in chemical processes: Carbon tetrachloride is utilized in various chemical processes, including as a solvent, reagent, or intermediate in the production of other chemicals. Its applications span across different industries, showcasing its versatility in chemical manufacturing.
- Environmental and safety considerations: Due to its environmental impact and health hazards, research focuses on developing alternatives to carbon tetrachloride and methods for its safe handling, disposal, and remediation. This includes studies on its effects on the ozone layer and strategies for reducing its use in industrial processes.
- Detection and analysis methods: Various techniques and apparatus have been developed for detecting and analyzing carbon tetrachloride in different environments. These include spectroscopic methods, chromatography, and specialized sensors designed to measure carbon tetrachloride concentrations in air, water, or other media.
- Historical uses and patents: Early patents and historical documents reveal the diverse applications of carbon tetrachloride throughout the 20th century. These include its use in fire extinguishers, dry cleaning, and as a refrigerant, before many of these applications were phased out due to safety and environmental concerns.
02 Applications of carbon tetrachloride in chemical processes
Carbon tetrachloride is utilized in various chemical processes, including as a solvent, reagent, or intermediate in organic synthesis. It plays a role in the production of other chlorinated compounds and in specific industrial applications.Expand Specific Solutions03 Environmental and safety considerations
Due to its environmental impact and health hazards, research focuses on alternatives to carbon tetrachloride and methods for its safe handling, storage, and disposal. This includes developing eco-friendly substitutes and improving containment strategies.Expand Specific Solutions04 Detection and analysis methods
Techniques for detecting and analyzing carbon tetrachloride in various matrices are developed. These include spectroscopic methods, chromatographic techniques, and sensor-based approaches for environmental monitoring and quality control purposes.Expand Specific Solutions05 Historical uses and regulations
The historical applications of carbon tetrachloride, such as in fire extinguishers and dry cleaning, are documented. Regulatory changes and phase-out programs due to its ozone-depleting properties and toxicity are also addressed.Expand Specific Solutions
Key Stakeholders in CCl4 Management
The application of Carbon Tetrachloride knowledge in Environmental Science is at a mature stage, with a well-established understanding of its environmental impacts and regulatory frameworks. The market for related technologies and solutions is substantial, driven by global environmental concerns and stringent regulations. Companies like Occidental Chemical Corp. and Jiangsu Lee & Man Chemical Ltd. are key players in the chemical industry, likely focusing on safer alternatives and remediation technologies. Academic institutions such as Central South University and South China Agricultural University contribute to research and development in this field. The technology's maturity is evident in the involvement of diverse stakeholders, including government agencies and environmental organizations, working towards mitigating the environmental risks associated with Carbon Tetrachloride.
Occidental Chemical Corp.
Technical Solution: Occidental Chemical Corp. has developed a multi-faceted approach to address carbon tetrachloride contamination in environmental science. Their strategy includes advanced oxidation processes (AOPs) using hydrogen peroxide and UV light to break down carbon tetrachloride in water treatment systems[9]. They have also implemented thermal desorption techniques for soil remediation, effectively removing carbon tetrachloride from contaminated sites[10]. Furthermore, Occidental has invested in green chemistry initiatives to develop alternatives to carbon tetrachloride in industrial processes, reducing the overall environmental impact[11].
Strengths: Diverse remediation technologies, industrial expertise in chemical processes. Weaknesses: Potential conflict of interest as a chemical producer, high energy requirements for some remediation methods.
Panasonic Environmental Systems & Engineering Co. Ltd.
Technical Solution: Panasonic Environmental Systems & Engineering has developed an innovative air purification system that effectively removes carbon tetrachloride and other volatile organic compounds (VOCs) from indoor environments. Their technology combines advanced activated carbon filters with photocatalytic oxidation, providing a comprehensive solution for air quality management[12]. Additionally, they have created smart monitoring systems that can detect trace amounts of carbon tetrachloride in real-time, allowing for immediate response to potential contamination events[13]. Panasonic also offers integrated water treatment solutions that incorporate membrane technology and advanced oxidation processes to remove carbon tetrachloride from water sources[14].
Strengths: Integrated environmental solutions, advanced sensor technologies. Weaknesses: Focus primarily on indoor environments, limited experience in large-scale outdoor remediation.
Innovative CCl4 Detection Methods
Chlorinolysis process for producing carbon tetrachloride
PatentActiveUS20210130266A1
Innovation
- A process involving a chlorination zone with chlorine, a C1 chlorinated compound, and a carbon/second chlorine source to produce a reaction mixture that favors the formation of carbon tetrachloride over perchloroethylene, using waste products as the carbon/second chlorine source to enhance efficiency and reduce impurity formation.
Treatment of water effluent
PatentPendingUS20190092658A1
Innovation
- A method and system using a filter material comprising a porous carbon support layer and silicate wool or glass wool with distributed halogens or halides, where an electric current is passed through the filter material to adsorb and decompose hormonal contaminants from aqueous effluent streams.
Global Policy Framework
The global policy framework for carbon tetrachloride (CCl4) has evolved significantly over the past few decades, primarily driven by international efforts to protect the ozone layer and mitigate environmental pollution. The Montreal Protocol, signed in 1987, marked a crucial turning point in the regulation of ozone-depleting substances, including CCl4. This landmark agreement set the stage for a phased reduction and eventual elimination of CCl4 production and consumption worldwide.
Subsequent amendments to the Montreal Protocol, such as the London Amendment (1990) and the Copenhagen Amendment (1992), further strengthened the global commitment to addressing CCl4 and other ozone-depleting substances. These amendments accelerated the phase-out schedules and expanded the list of controlled substances, reflecting the growing scientific understanding of the environmental impacts of CCl4.
In addition to ozone layer protection, the Stockholm Convention on Persistent Organic Pollutants (POPs), adopted in 2001, has also played a role in shaping the global policy framework for CCl4. While CCl4 is not explicitly listed as a POP, the convention's principles and mechanisms have influenced the broader approach to managing persistent environmental contaminants.
The United Nations Environment Programme (UNEP) has been instrumental in coordinating international efforts and providing scientific assessments that inform policy decisions. Regular reports from UNEP's Technology and Economic Assessment Panel (TEAP) and the Scientific Assessment Panel (SAP) have provided crucial data on CCl4 emissions, atmospheric concentrations, and environmental impacts, guiding policy adjustments and enforcement strategies.
At the regional level, organizations such as the European Union have implemented stringent regulations on CCl4 use and disposal. The EU's REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) regulation, for instance, imposes strict controls on the production, import, and use of CCl4 and other hazardous substances.
Developing countries have been supported in their transition away from CCl4 through the Multilateral Fund for the Implementation of the Montreal Protocol. This financial mechanism has facilitated technology transfer and capacity building, enabling these nations to meet their phase-out commitments while addressing economic and technical challenges.
Despite the comprehensive global framework, challenges remain in addressing unexpected sources of CCl4 emissions and ensuring full compliance across all nations. Ongoing scientific research and policy reviews continue to refine the global approach to CCl4 management, emphasizing the need for continued vigilance and adaptive policymaking in environmental science and regulation.
Subsequent amendments to the Montreal Protocol, such as the London Amendment (1990) and the Copenhagen Amendment (1992), further strengthened the global commitment to addressing CCl4 and other ozone-depleting substances. These amendments accelerated the phase-out schedules and expanded the list of controlled substances, reflecting the growing scientific understanding of the environmental impacts of CCl4.
In addition to ozone layer protection, the Stockholm Convention on Persistent Organic Pollutants (POPs), adopted in 2001, has also played a role in shaping the global policy framework for CCl4. While CCl4 is not explicitly listed as a POP, the convention's principles and mechanisms have influenced the broader approach to managing persistent environmental contaminants.
The United Nations Environment Programme (UNEP) has been instrumental in coordinating international efforts and providing scientific assessments that inform policy decisions. Regular reports from UNEP's Technology and Economic Assessment Panel (TEAP) and the Scientific Assessment Panel (SAP) have provided crucial data on CCl4 emissions, atmospheric concentrations, and environmental impacts, guiding policy adjustments and enforcement strategies.
At the regional level, organizations such as the European Union have implemented stringent regulations on CCl4 use and disposal. The EU's REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) regulation, for instance, imposes strict controls on the production, import, and use of CCl4 and other hazardous substances.
Developing countries have been supported in their transition away from CCl4 through the Multilateral Fund for the Implementation of the Montreal Protocol. This financial mechanism has facilitated technology transfer and capacity building, enabling these nations to meet their phase-out commitments while addressing economic and technical challenges.
Despite the comprehensive global framework, challenges remain in addressing unexpected sources of CCl4 emissions and ensuring full compliance across all nations. Ongoing scientific research and policy reviews continue to refine the global approach to CCl4 management, emphasizing the need for continued vigilance and adaptive policymaking in environmental science and regulation.
Ecological Risk Assessment
Ecological risk assessment of carbon tetrachloride is a critical process in environmental science, focusing on evaluating the potential adverse effects of this chemical on ecosystems. The assessment typically begins with problem formulation, where the specific ecological concerns related to carbon tetrachloride exposure are identified. This includes determining the potential sources of contamination, such as industrial discharges or historical spills, and identifying the ecosystems and species at risk.
The exposure assessment phase involves quantifying the levels of carbon tetrachloride in various environmental compartments, including soil, water, and air. Advanced analytical techniques, such as gas chromatography-mass spectrometry, are employed to measure carbon tetrachloride concentrations accurately. Environmental fate models are utilized to predict the movement and persistence of the chemical in different ecosystems, considering factors like biodegradation, volatilization, and sorption to soil particles.
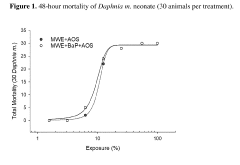
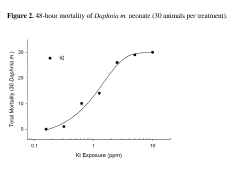
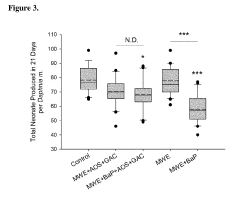
Effects assessment is a crucial component, focusing on the toxicity of carbon tetrachloride to various ecological receptors. This involves analyzing existing ecotoxicological data and conducting additional studies when necessary. Acute and chronic toxicity tests on representative species from different trophic levels, such as algae, aquatic invertebrates, and fish, provide valuable information on the chemical's ecological impacts. Bioaccumulation potential is also evaluated to assess the risk of biomagnification through food chains.
Risk characterization integrates the findings from exposure and effects assessments to estimate the likelihood and magnitude of adverse ecological effects. This often involves comparing predicted environmental concentrations with toxicity thresholds to calculate risk quotients. Probabilistic risk assessment techniques may be employed to account for uncertainties and variabilities in the data.
The ecological risk assessment process for carbon tetrachloride also considers the chemical's unique properties, such as its high volatility and potential for long-range atmospheric transport. This necessitates the evaluation of potential impacts on distant ecosystems, including polar regions where the chemical may accumulate due to global distillation effects.
Mitigation strategies based on the risk assessment findings are developed to minimize the ecological impacts of carbon tetrachloride. These may include source reduction, contaminated site remediation, and habitat restoration efforts. The assessment also informs regulatory decisions and environmental management practices to protect ecosystems from the harmful effects of this persistent organic pollutant.
The exposure assessment phase involves quantifying the levels of carbon tetrachloride in various environmental compartments, including soil, water, and air. Advanced analytical techniques, such as gas chromatography-mass spectrometry, are employed to measure carbon tetrachloride concentrations accurately. Environmental fate models are utilized to predict the movement and persistence of the chemical in different ecosystems, considering factors like biodegradation, volatilization, and sorption to soil particles.
Effects assessment is a crucial component, focusing on the toxicity of carbon tetrachloride to various ecological receptors. This involves analyzing existing ecotoxicological data and conducting additional studies when necessary. Acute and chronic toxicity tests on representative species from different trophic levels, such as algae, aquatic invertebrates, and fish, provide valuable information on the chemical's ecological impacts. Bioaccumulation potential is also evaluated to assess the risk of biomagnification through food chains.
Risk characterization integrates the findings from exposure and effects assessments to estimate the likelihood and magnitude of adverse ecological effects. This often involves comparing predicted environmental concentrations with toxicity thresholds to calculate risk quotients. Probabilistic risk assessment techniques may be employed to account for uncertainties and variabilities in the data.
The ecological risk assessment process for carbon tetrachloride also considers the chemical's unique properties, such as its high volatility and potential for long-range atmospheric transport. This necessitates the evaluation of potential impacts on distant ecosystems, including polar regions where the chemical may accumulate due to global distillation effects.
Mitigation strategies based on the risk assessment findings are developed to minimize the ecological impacts of carbon tetrachloride. These may include source reduction, contaminated site remediation, and habitat restoration efforts. The assessment also informs regulatory decisions and environmental management practices to protect ecosystems from the harmful effects of this persistent organic pollutant.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!