How to Refine Carbon Tetrachloride Handling Guidelines?
JUL 3, 20258 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CCl4 Handling Background
Carbon tetrachloride (CCl4) has been a subject of significant concern in industrial and environmental contexts for decades. Initially used widely in various applications, including as a solvent, cleaning agent, and fire extinguisher, its usage has been dramatically curtailed due to its severe health and environmental impacts. The compound's ability to deplete the ozone layer led to its phase-out under the Montreal Protocol in 1987, marking a pivotal moment in its handling history.
The evolution of CCl4 handling guidelines reflects the growing understanding of its hazards. Early practices often underestimated the risks, leading to inadequate protection measures. As research progressed, the carcinogenic nature of CCl4 and its potential for liver and kidney damage became apparent, necessitating stricter controls. This shift in knowledge prompted regulatory bodies worldwide to implement more stringent guidelines for its use, storage, and disposal.
In recent years, the focus has shifted towards minimizing exposure and ensuring proper containment. Modern handling protocols emphasize the use of closed systems, adequate ventilation, and personal protective equipment (PPE) to mitigate risks. The Occupational Safety and Health Administration (OSHA) in the United States, for instance, has set specific exposure limits and mandated comprehensive safety measures for workplaces where CCl4 is present.
The environmental impact of CCl4 has also shaped handling practices. Its persistence in the atmosphere and potential for groundwater contamination have led to strict regulations on its release and disposal. Environmental agencies now require specialized handling and disposal methods to prevent environmental contamination, including secure storage, controlled transportation, and specialized destruction techniques.
Despite its restricted use, CCl4 remains relevant in certain industrial processes and as a chemical intermediate. This continued, albeit limited, presence in the industrial landscape underscores the ongoing need for refined handling guidelines. Current efforts focus on developing alternatives where possible and implementing best practices where its use remains necessary.
The technological advancements in detection and monitoring have also influenced CCl4 handling guidelines. More sensitive and accurate methods for detecting CCl4 in air, water, and soil have enabled better risk assessment and more targeted safety measures. These improvements have allowed for more precise control of exposure levels and quicker response to potential leaks or spills.
As global awareness of environmental and health issues continues to grow, the trajectory of CCl4 handling guidelines points towards even more stringent controls and a push for complete elimination where feasible. The ongoing refinement of these guidelines represents a critical intersection of scientific understanding, regulatory policy, and industrial practice, aimed at protecting human health and the environment while balancing necessary industrial applications.
The evolution of CCl4 handling guidelines reflects the growing understanding of its hazards. Early practices often underestimated the risks, leading to inadequate protection measures. As research progressed, the carcinogenic nature of CCl4 and its potential for liver and kidney damage became apparent, necessitating stricter controls. This shift in knowledge prompted regulatory bodies worldwide to implement more stringent guidelines for its use, storage, and disposal.
In recent years, the focus has shifted towards minimizing exposure and ensuring proper containment. Modern handling protocols emphasize the use of closed systems, adequate ventilation, and personal protective equipment (PPE) to mitigate risks. The Occupational Safety and Health Administration (OSHA) in the United States, for instance, has set specific exposure limits and mandated comprehensive safety measures for workplaces where CCl4 is present.
The environmental impact of CCl4 has also shaped handling practices. Its persistence in the atmosphere and potential for groundwater contamination have led to strict regulations on its release and disposal. Environmental agencies now require specialized handling and disposal methods to prevent environmental contamination, including secure storage, controlled transportation, and specialized destruction techniques.
Despite its restricted use, CCl4 remains relevant in certain industrial processes and as a chemical intermediate. This continued, albeit limited, presence in the industrial landscape underscores the ongoing need for refined handling guidelines. Current efforts focus on developing alternatives where possible and implementing best practices where its use remains necessary.
The technological advancements in detection and monitoring have also influenced CCl4 handling guidelines. More sensitive and accurate methods for detecting CCl4 in air, water, and soil have enabled better risk assessment and more targeted safety measures. These improvements have allowed for more precise control of exposure levels and quicker response to potential leaks or spills.
As global awareness of environmental and health issues continues to grow, the trajectory of CCl4 handling guidelines points towards even more stringent controls and a push for complete elimination where feasible. The ongoing refinement of these guidelines represents a critical intersection of scientific understanding, regulatory policy, and industrial practice, aimed at protecting human health and the environment while balancing necessary industrial applications.
Market Analysis CCl4 Use
The global market for carbon tetrachloride (CCl4) has undergone significant changes in recent decades due to environmental regulations and shifting industrial applications. Historically, CCl4 was widely used as a solvent, cleaning agent, and feedstock for various chemical processes. However, its ozone-depleting properties led to severe restrictions under the Montreal Protocol, dramatically reducing its production and consumption.
Despite these restrictions, CCl4 continues to play a role in specific industrial applications. The current market is primarily driven by its use as a feedstock for the production of hydrofluorocarbons (HFCs) and hydrofluoroolefins (HFOs), which are used as refrigerants and blowing agents. This sector accounts for a substantial portion of the global CCl4 demand, as these compounds are considered more environmentally friendly alternatives to ozone-depleting substances.
The pharmaceutical industry represents another significant market for CCl4, where it is used in the synthesis of various drugs and active pharmaceutical ingredients. Its application in this sector is tightly controlled and subject to stringent handling guidelines to minimize environmental impact and worker exposure.
In the agrochemical sector, CCl4 finds limited use in the production of certain pesticides and herbicides. However, this application has been declining due to the development of alternative synthesis routes and increasing regulatory pressure.
Geographically, the CCl4 market is concentrated in regions with strong chemical and pharmaceutical industries, particularly in Asia-Pacific, North America, and Europe. China remains a major producer and consumer of CCl4, despite international efforts to phase out its production.
The market size for CCl4 has contracted significantly since its peak usage in the late 20th century. Current global production is estimated to be a fraction of historical levels, with the majority being used as feedstock in controlled processes rather than as an end-product.
Looking forward, the CCl4 market is expected to face continued pressure from environmental regulations and the development of alternative technologies. The refinement of handling guidelines is crucial for maintaining its use in essential applications while minimizing environmental impact. This includes improving containment strategies, enhancing recycling and destruction technologies, and developing more efficient production processes that reduce emissions and waste.
Despite these restrictions, CCl4 continues to play a role in specific industrial applications. The current market is primarily driven by its use as a feedstock for the production of hydrofluorocarbons (HFCs) and hydrofluoroolefins (HFOs), which are used as refrigerants and blowing agents. This sector accounts for a substantial portion of the global CCl4 demand, as these compounds are considered more environmentally friendly alternatives to ozone-depleting substances.
The pharmaceutical industry represents another significant market for CCl4, where it is used in the synthesis of various drugs and active pharmaceutical ingredients. Its application in this sector is tightly controlled and subject to stringent handling guidelines to minimize environmental impact and worker exposure.
In the agrochemical sector, CCl4 finds limited use in the production of certain pesticides and herbicides. However, this application has been declining due to the development of alternative synthesis routes and increasing regulatory pressure.
Geographically, the CCl4 market is concentrated in regions with strong chemical and pharmaceutical industries, particularly in Asia-Pacific, North America, and Europe. China remains a major producer and consumer of CCl4, despite international efforts to phase out its production.
The market size for CCl4 has contracted significantly since its peak usage in the late 20th century. Current global production is estimated to be a fraction of historical levels, with the majority being used as feedstock in controlled processes rather than as an end-product.
Looking forward, the CCl4 market is expected to face continued pressure from environmental regulations and the development of alternative technologies. The refinement of handling guidelines is crucial for maintaining its use in essential applications while minimizing environmental impact. This includes improving containment strategies, enhancing recycling and destruction technologies, and developing more efficient production processes that reduce emissions and waste.
Current CCl4 Challenges
Carbon tetrachloride (CCl4) handling presents several significant challenges in today's industrial and research environments. The primary concern is its high toxicity, which poses severe health risks to workers and researchers. Exposure to CCl4 can cause liver and kidney damage, central nervous system depression, and in extreme cases, death. This necessitates stringent safety protocols and protective measures, often complicating routine procedures and increasing operational costs.
Another major challenge is the environmental impact of CCl4. As a potent ozone-depleting substance, its use is heavily regulated under the Montreal Protocol. This has led to restrictions on its production and use, making it increasingly difficult to obtain and utilize in various applications. The environmental concerns also extend to potential soil and groundwater contamination, requiring careful disposal and cleanup procedures.
The volatility of CCl4 presents additional handling difficulties. Its high vapor pressure means it readily evaporates at room temperature, increasing the risk of inhalation exposure and making containment more challenging. This volatility also contributes to fire hazards, as CCl4 vapors can form explosive mixtures with air under certain conditions.
Storage and transportation of CCl4 pose their own set of challenges. The compound is corrosive to certain metals and can degrade some types of plastics and rubber, limiting the materials that can be used for containers and equipment. This corrosivity also increases the risk of leaks and spills, necessitating specialized containment systems and regular integrity checks.
The analytical and experimental use of CCl4 faces obstacles due to its reactivity. While its inertness makes it useful as a solvent in some applications, it can also undergo unexpected reactions under certain conditions, potentially interfering with experimental results or producing hazardous byproducts. This reactivity requires careful consideration in experimental design and execution.
Disposal of CCl4 and CCl4-contaminated materials is another significant challenge. As a hazardous waste, it requires specialized treatment and disposal methods to prevent environmental contamination. This not only increases costs but also demands expertise in hazardous waste management, which may not be readily available in all settings.
Lastly, the regulatory landscape surrounding CCl4 is complex and evolving. Different countries and regions have varying regulations regarding its use, storage, and disposal. Keeping up with these changing regulations and ensuring compliance across different jurisdictions can be a significant burden for organizations working with CCl4, particularly those operating internationally.
Another major challenge is the environmental impact of CCl4. As a potent ozone-depleting substance, its use is heavily regulated under the Montreal Protocol. This has led to restrictions on its production and use, making it increasingly difficult to obtain and utilize in various applications. The environmental concerns also extend to potential soil and groundwater contamination, requiring careful disposal and cleanup procedures.
The volatility of CCl4 presents additional handling difficulties. Its high vapor pressure means it readily evaporates at room temperature, increasing the risk of inhalation exposure and making containment more challenging. This volatility also contributes to fire hazards, as CCl4 vapors can form explosive mixtures with air under certain conditions.
Storage and transportation of CCl4 pose their own set of challenges. The compound is corrosive to certain metals and can degrade some types of plastics and rubber, limiting the materials that can be used for containers and equipment. This corrosivity also increases the risk of leaks and spills, necessitating specialized containment systems and regular integrity checks.
The analytical and experimental use of CCl4 faces obstacles due to its reactivity. While its inertness makes it useful as a solvent in some applications, it can also undergo unexpected reactions under certain conditions, potentially interfering with experimental results or producing hazardous byproducts. This reactivity requires careful consideration in experimental design and execution.
Disposal of CCl4 and CCl4-contaminated materials is another significant challenge. As a hazardous waste, it requires specialized treatment and disposal methods to prevent environmental contamination. This not only increases costs but also demands expertise in hazardous waste management, which may not be readily available in all settings.
Lastly, the regulatory landscape surrounding CCl4 is complex and evolving. Different countries and regions have varying regulations regarding its use, storage, and disposal. Keeping up with these changing regulations and ensuring compliance across different jurisdictions can be a significant burden for organizations working with CCl4, particularly those operating internationally.
Existing CCl4 Protocols
01 Safety precautions and protective equipment
When handling carbon tetrachloride, it is essential to use appropriate personal protective equipment (PPE) such as chemical-resistant gloves, safety goggles, and respiratory protection. Work should be conducted in well-ventilated areas or under fume hoods to minimize exposure to vapors. Proper training on safe handling procedures is crucial for all personnel working with this hazardous substance.- Safety precautions and protective equipment: When handling carbon tetrachloride, it is essential to use appropriate personal protective equipment (PPE) such as chemical-resistant gloves, safety goggles, and respiratory protection. Work should be conducted in a well-ventilated area or under a fume hood to minimize exposure to vapors. Proper training on safe handling procedures is crucial for all personnel working with this hazardous substance.
- Storage and containment guidelines: Carbon tetrachloride should be stored in tightly sealed containers in a cool, dry, and well-ventilated area away from sources of heat, ignition, and direct sunlight. Containers should be properly labeled and kept separate from incompatible materials. Secondary containment measures should be implemented to prevent spills and leaks from contaminating the environment.
- Disposal and waste management: Proper disposal of carbon tetrachloride and its waste products is critical to prevent environmental contamination. It should not be released into sewers, drains, or water sources. Waste should be collected in appropriate containers and disposed of through licensed waste management facilities. Recycling and recovery methods should be considered when possible to minimize environmental impact.
- Emergency response and spill management: In case of spills or accidental releases, immediate action should be taken to contain and clean up the material. Evacuation of the affected area may be necessary. Absorbent materials should be used for small spills, while larger spills may require professional hazardous material response teams. Proper decontamination procedures must be followed, and all cleanup materials should be disposed of as hazardous waste.
- Alternatives and substitution strategies: Due to its high toxicity and environmental concerns, efforts should be made to find safer alternatives to carbon tetrachloride whenever possible. This may involve using less hazardous solvents or developing new processes that eliminate the need for carbon tetrachloride. When substitution is not feasible, minimizing its use and implementing strict control measures is essential to reduce risks associated with its handling.
02 Storage and containment guidelines
Carbon tetrachloride should be stored in tightly sealed containers in a cool, dry, and well-ventilated area away from sources of heat or ignition. Containers should be properly labeled and kept away from incompatible materials. Secondary containment measures should be implemented to prevent spills or leaks from spreading. Regular inspections of storage areas and containers are necessary to ensure integrity.Expand Specific Solutions03 Disposal and environmental considerations
Proper disposal of carbon tetrachloride and its waste products is critical to prevent environmental contamination. It should not be released into sewers, soil, or water bodies. Waste should be handled as hazardous material and disposed of through authorized waste management facilities. Recycling or recovery processes should be considered when possible to minimize environmental impact.Expand Specific Solutions04 Emergency response and spill management
In case of spills or accidental releases, immediate action is required. The area should be evacuated, and only trained personnel with proper PPE should handle the cleanup. Absorbent materials should be used to contain the spill, and contaminated materials must be disposed of as hazardous waste. Adequate ventilation should be ensured, and all sources of ignition should be removed. Emergency response plans should be in place and regularly updated.Expand Specific Solutions05 Handling and transportation procedures
When handling or transporting carbon tetrachloride, use only in closed systems or with adequate ventilation. Avoid generating vapors or mists. Use proper grounding and bonding procedures to prevent static electricity buildup. During transportation, ensure containers are securely sealed and properly labeled. Follow all applicable regulations for the transportation of hazardous materials, including proper documentation and placarding of vehicles.Expand Specific Solutions
Key CCl4 Industry Players
The carbon tetrachloride handling guidelines refinement landscape is in a mature phase, with established players and well-developed safety protocols. The market size is relatively stable, driven by industrial applications and environmental regulations. Technologically, the field is advanced, with companies like DuPont de Nemours, Occidental Chemical Corp., and BASF Corp. leading innovations in safe handling practices. These industry giants, along with specialized chemical firms like Evonik Operations GmbH and The Chemours Co., are continuously improving guidelines through research and development, focusing on worker safety, environmental protection, and process efficiency. The competitive landscape is characterized by a focus on regulatory compliance and sustainable practices.
Occidental Chemical Corp.
Technical Solution: Occidental Chemical Corp. has developed advanced handling guidelines for carbon tetrachloride, focusing on a multi-layered approach to safety and environmental protection. Their system includes state-of-the-art containment technologies, such as double-walled storage tanks with leak detection systems[1]. They have implemented a comprehensive monitoring program using real-time sensors to detect any potential releases[3]. The company has also developed specialized training programs for employees, emphasizing proper handling techniques and emergency response procedures[5]. Additionally, they have invested in research to explore alternative chemicals and processes to reduce reliance on carbon tetrachloride where possible[7].
Strengths: Comprehensive safety measures, advanced monitoring systems, and employee training. Weaknesses: Potential high implementation costs and the ongoing challenge of finding suitable alternatives to carbon tetrachloride.
DuPont de Nemours, Inc.
Technical Solution: DuPont has refined its carbon tetrachloride handling guidelines by implementing a holistic approach that combines engineering controls, administrative procedures, and personal protective equipment. They have developed proprietary closed-loop systems that minimize exposure risks during production and handling processes[2]. DuPont's guidelines emphasize the use of inert gas blanketing in storage and transfer operations to prevent moisture ingress and potential decomposition[4]. The company has also pioneered the use of advanced materials for seals and gaskets that are highly resistant to carbon tetrachloride degradation, reducing the risk of leaks[6]. Furthermore, DuPont has implemented a rigorous waste management protocol, including on-site treatment facilities to neutralize and safely dispose of carbon tetrachloride-containing waste[8].
Strengths: Innovative engineering solutions, comprehensive safety protocols, and advanced waste management. Weaknesses: High initial investment costs and the need for continuous training and updating of specialized equipment.
CCl4 Safety Innovations
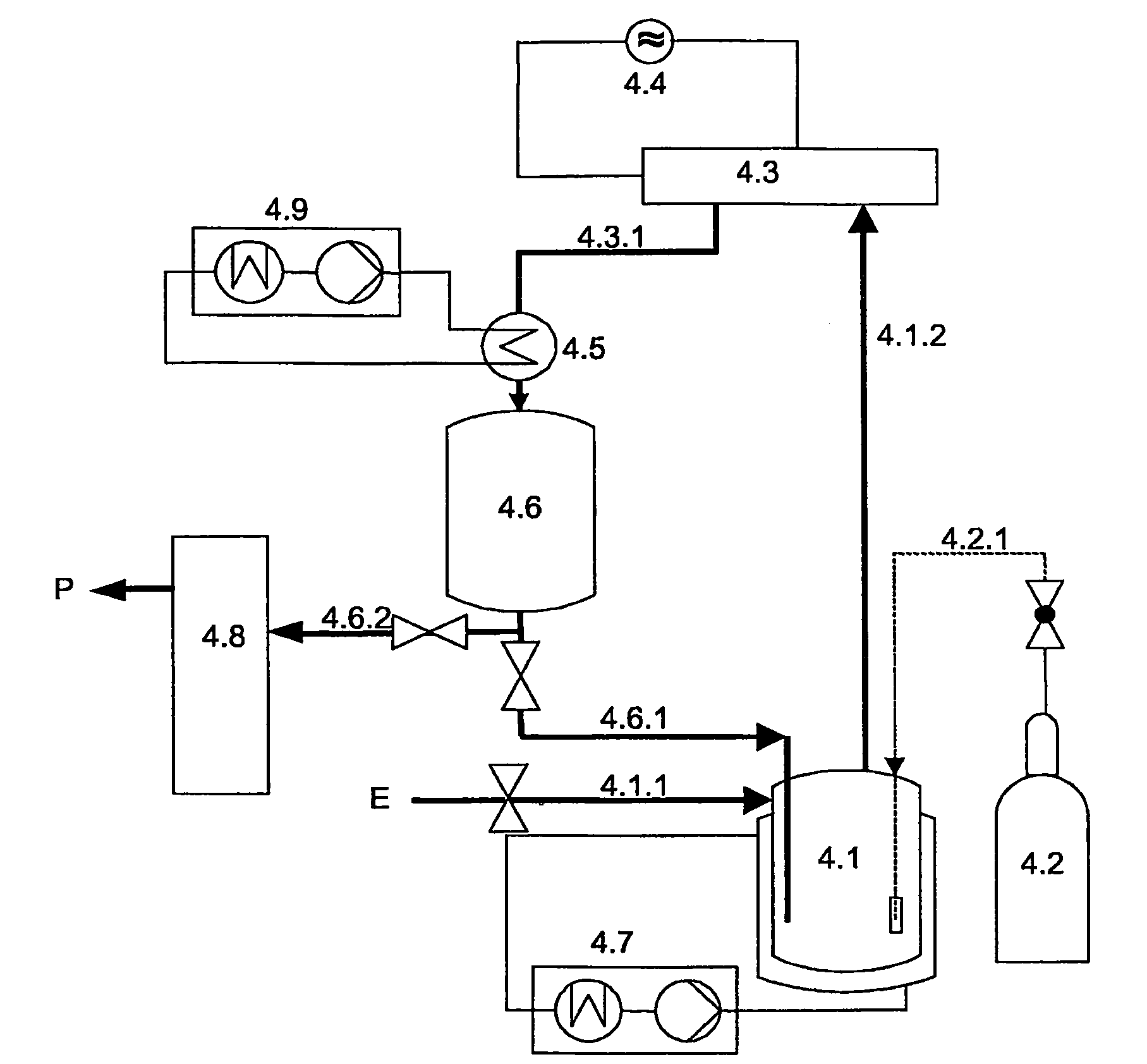
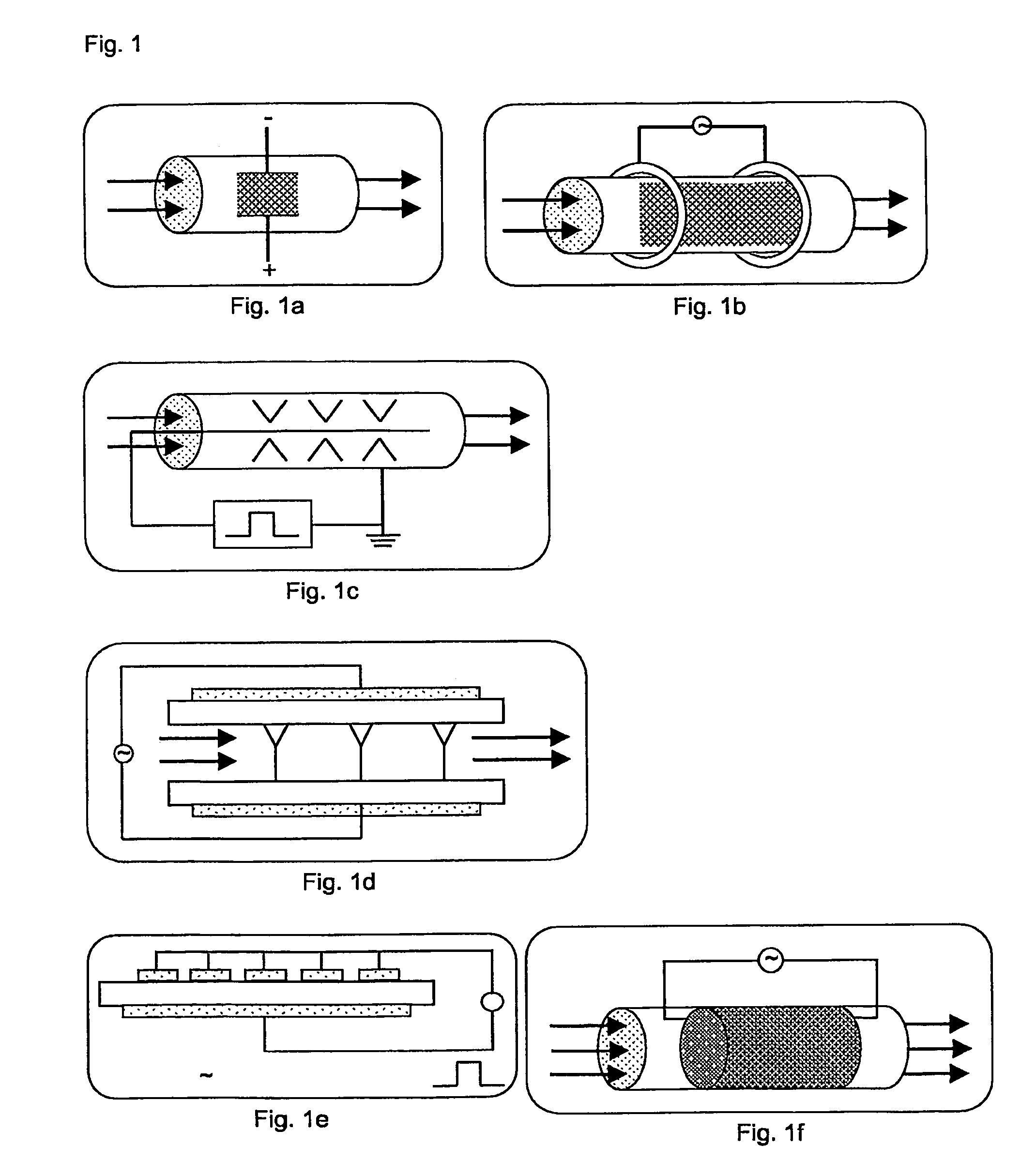
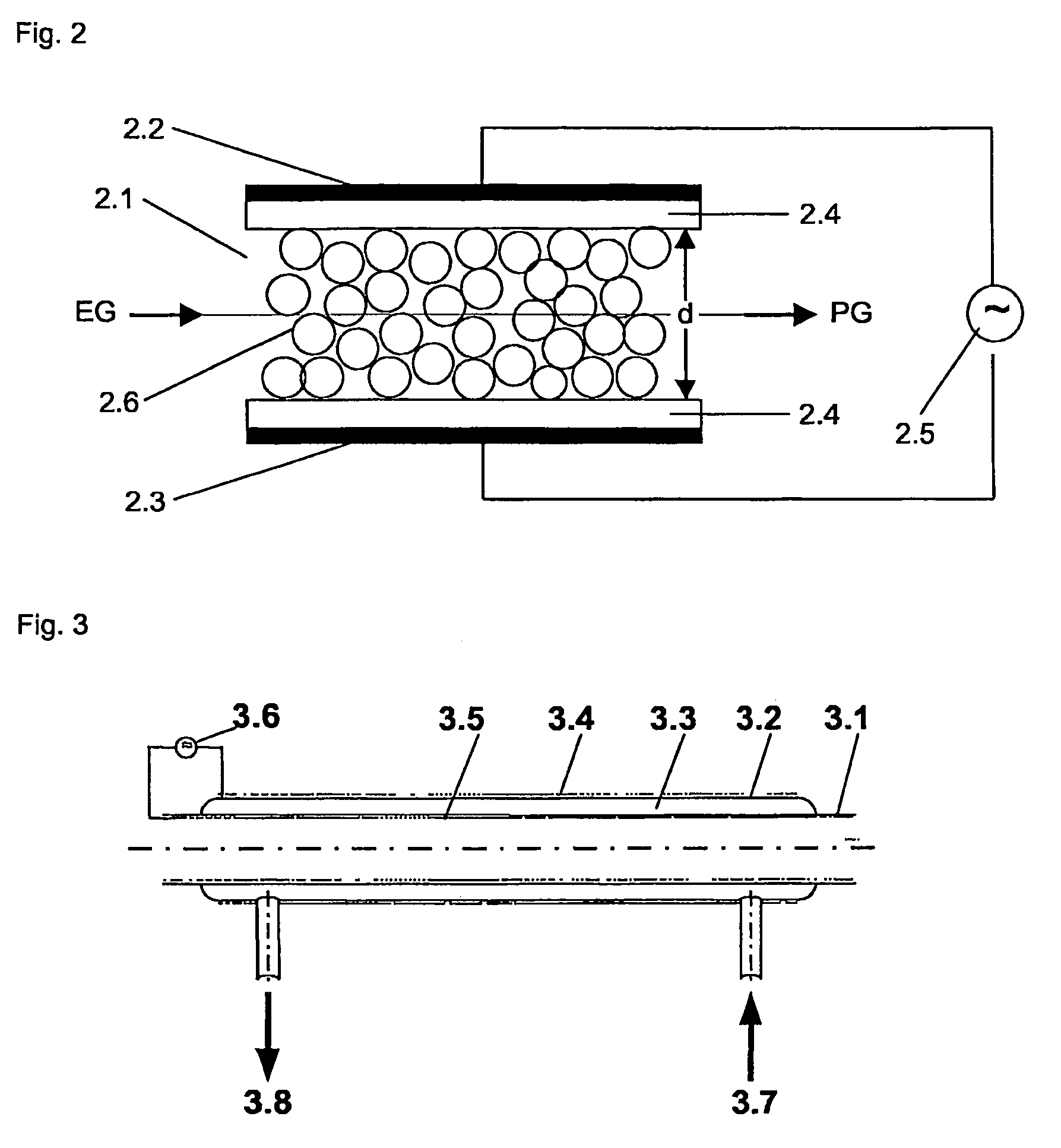
Process and apparatus for purifying silicon tetrachloride or germanium tetrachloride containing hydrogen compounds
PatentActiveUS8002954B2
Innovation
- Treatment of silicon tetrachloride or germanium tetrachloride using a cold plasma, specifically a dielectrically hindered discharge (DBD), which converts hydrogen-containing compounds into separable species without the need for a reducing agent, allowing for subsequent distillation and achieving high-purity tetrachloride.
Method for reusing heavy end by-products in the manufacture of polychlorinated alkanes
PatentInactiveEP1663920A2
Innovation
- Recovering heavy end by-products through distillation, subjecting them to exhaustive high-temperature chlorination to produce carbon tetrachloride, and recycling it back into the process to produce additional polychlorinated alkanes, thereby maximizing the utilization of starting materials and reducing waste.
Environmental Impact CCl4
Carbon tetrachloride (CCl4) is a potent environmental pollutant with significant impacts on both human health and ecosystems. Its release into the environment can have far-reaching consequences, particularly on air quality and the ozone layer. When CCl4 enters the atmosphere, it contributes to the depletion of stratospheric ozone, which protects the Earth from harmful ultraviolet radiation. This ozone depletion can lead to increased UV exposure, potentially causing skin cancer, cataracts, and damage to marine ecosystems.
In aquatic environments, CCl4 can persist for extended periods due to its low biodegradability. It can accumulate in sediments and bioaccumulate in aquatic organisms, potentially entering the food chain. This persistence poses risks to aquatic ecosystems, affecting the health and reproduction of various species. Groundwater contamination is another significant concern, as CCl4 can leach into soil and eventually reach underground water sources, potentially impacting drinking water supplies.
Terrestrial ecosystems are also at risk from CCl4 exposure. Soil contamination can lead to reduced plant growth and altered microbial communities, affecting overall ecosystem health. Animals may be exposed through contaminated soil, water, or food sources, leading to potential toxicity and bioaccumulation issues.
The global transport of CCl4 through atmospheric and oceanic currents means that its environmental impacts are not limited to the areas of release. This long-range transport capability makes CCl4 a global pollutant, affecting regions far from its point of origin. Climate change may exacerbate these impacts, as altered weather patterns and temperature changes could affect the distribution and persistence of CCl4 in various environmental compartments.
Efforts to mitigate the environmental impact of CCl4 have led to international agreements such as the Montreal Protocol, which aims to phase out the production and use of ozone-depleting substances. However, ongoing monitoring and research are crucial to understand the long-term effects of historical CCl4 releases and to address any continuing emissions. Improved handling guidelines for CCl4 must consider its potential for environmental contamination at all stages of its lifecycle, from production and use to disposal and remediation of contaminated sites.
In aquatic environments, CCl4 can persist for extended periods due to its low biodegradability. It can accumulate in sediments and bioaccumulate in aquatic organisms, potentially entering the food chain. This persistence poses risks to aquatic ecosystems, affecting the health and reproduction of various species. Groundwater contamination is another significant concern, as CCl4 can leach into soil and eventually reach underground water sources, potentially impacting drinking water supplies.
Terrestrial ecosystems are also at risk from CCl4 exposure. Soil contamination can lead to reduced plant growth and altered microbial communities, affecting overall ecosystem health. Animals may be exposed through contaminated soil, water, or food sources, leading to potential toxicity and bioaccumulation issues.
The global transport of CCl4 through atmospheric and oceanic currents means that its environmental impacts are not limited to the areas of release. This long-range transport capability makes CCl4 a global pollutant, affecting regions far from its point of origin. Climate change may exacerbate these impacts, as altered weather patterns and temperature changes could affect the distribution and persistence of CCl4 in various environmental compartments.
Efforts to mitigate the environmental impact of CCl4 have led to international agreements such as the Montreal Protocol, which aims to phase out the production and use of ozone-depleting substances. However, ongoing monitoring and research are crucial to understand the long-term effects of historical CCl4 releases and to address any continuing emissions. Improved handling guidelines for CCl4 must consider its potential for environmental contamination at all stages of its lifecycle, from production and use to disposal and remediation of contaminated sites.
Regulatory Framework CCl4
The regulatory framework for carbon tetrachloride (CCl4) handling has evolved significantly over the years, reflecting growing concerns about its environmental and health impacts. At the international level, the Montreal Protocol on Substances that Deplete the Ozone Layer, adopted in 1987, has been instrumental in phasing out the production and consumption of CCl4. This treaty has been universally ratified and has led to a dramatic reduction in the global use of ozone-depleting substances, including CCl4.
In the United States, the Environmental Protection Agency (EPA) regulates CCl4 under various statutes. The Toxic Substances Control Act (TSCA) provides the EPA with authority to require reporting, record-keeping, and testing requirements, and restrictions relating to chemical substances and/or mixtures. Under the Clean Air Act, CCl4 is listed as a hazardous air pollutant, subject to National Emission Standards for Hazardous Air Pollutants (NESHAP).
The Occupational Safety and Health Administration (OSHA) has established permissible exposure limits for CCl4 in the workplace. The current permissible exposure limit (PEL) is set at 10 ppm as an 8-hour time-weighted average, with a ceiling limit of 25 ppm. OSHA also requires employers to implement engineering controls and work practices to reduce employee exposure.
At the European level, the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) regulation governs the use of CCl4. Under REACH, CCl4 is classified as a substance of very high concern (SVHC) due to its carcinogenic properties. This classification imposes strict controls on its manufacture, import, and use within the European Union.
Many countries have implemented their own regulations for CCl4 handling, often aligning with international standards. For instance, Japan's Chemical Substances Control Law regulates the manufacture, import, and use of CCl4, while China has included CCl4 in its list of priority controlled chemicals.
Despite these regulations, challenges remain in enforcing and updating guidelines to address emerging concerns. Recent studies have highlighted the need for more stringent controls on inadvertent production and emissions of CCl4 from industrial processes. This has prompted calls for revisions to existing regulatory frameworks to incorporate new scientific findings and technological advancements in monitoring and control techniques.
In the United States, the Environmental Protection Agency (EPA) regulates CCl4 under various statutes. The Toxic Substances Control Act (TSCA) provides the EPA with authority to require reporting, record-keeping, and testing requirements, and restrictions relating to chemical substances and/or mixtures. Under the Clean Air Act, CCl4 is listed as a hazardous air pollutant, subject to National Emission Standards for Hazardous Air Pollutants (NESHAP).
The Occupational Safety and Health Administration (OSHA) has established permissible exposure limits for CCl4 in the workplace. The current permissible exposure limit (PEL) is set at 10 ppm as an 8-hour time-weighted average, with a ceiling limit of 25 ppm. OSHA also requires employers to implement engineering controls and work practices to reduce employee exposure.
At the European level, the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) regulation governs the use of CCl4. Under REACH, CCl4 is classified as a substance of very high concern (SVHC) due to its carcinogenic properties. This classification imposes strict controls on its manufacture, import, and use within the European Union.
Many countries have implemented their own regulations for CCl4 handling, often aligning with international standards. For instance, Japan's Chemical Substances Control Law regulates the manufacture, import, and use of CCl4, while China has included CCl4 in its list of priority controlled chemicals.
Despite these regulations, challenges remain in enforcing and updating guidelines to address emerging concerns. Recent studies have highlighted the need for more stringent controls on inadvertent production and emissions of CCl4 from industrial processes. This has prompted calls for revisions to existing regulatory frameworks to incorporate new scientific findings and technological advancements in monitoring and control techniques.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!