How to Leverage Carbon Tetrachloride Properties in Product Development?
JUL 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Carbon Tetrachloride Overview and Development Goals
Carbon tetrachloride, a colorless liquid with a sweet odor, has been a subject of interest in various industries due to its unique properties. This compound, with the chemical formula CCl4, has a rich history dating back to its discovery in the 19th century. Initially used as a solvent and cleaning agent, carbon tetrachloride's applications have evolved significantly over time.
The development of carbon tetrachloride has been marked by both technological advancements and environmental concerns. Its high solvency power and non-flammability made it an attractive choice for many industrial processes. However, the recognition of its ozone-depleting properties and potential health hazards led to a shift in its usage and regulation.
In recent years, the focus has been on finding alternative applications that can leverage carbon tetrachloride's unique properties while minimizing environmental impact. This has sparked renewed interest in research and development efforts to explore novel uses in controlled environments.
The current technological landscape presents both challenges and opportunities for carbon tetrachloride utilization. While its use as a general-purpose solvent has been largely phased out, there are emerging applications in specialized fields such as analytical chemistry and materials science.
One of the primary goals in carbon tetrachloride development is to harness its properties for high-value, low-volume applications. This includes exploring its potential in advanced manufacturing processes, where its chemical stability and solvent properties can be advantageous.
Another key objective is to investigate carbon tetrachloride's role in the synthesis of new materials. Its ability to form stable compounds with certain elements opens up possibilities for creating innovative substances with unique characteristics.
Researchers are also focusing on developing safer handling and containment methods for carbon tetrachloride. This is crucial for enabling its use in controlled laboratory settings and specialized industrial processes while ensuring environmental and worker safety.
The future of carbon tetrachloride in product development lies in identifying niche applications where its properties offer significant advantages over alternative substances. This requires a careful balance between leveraging its beneficial characteristics and addressing the associated risks and regulatory constraints.
As we move forward, the goal is to integrate carbon tetrachloride into sustainable product development strategies. This involves not only finding new applications but also developing closed-loop systems that minimize environmental exposure and maximize resource efficiency.
The development of carbon tetrachloride has been marked by both technological advancements and environmental concerns. Its high solvency power and non-flammability made it an attractive choice for many industrial processes. However, the recognition of its ozone-depleting properties and potential health hazards led to a shift in its usage and regulation.
In recent years, the focus has been on finding alternative applications that can leverage carbon tetrachloride's unique properties while minimizing environmental impact. This has sparked renewed interest in research and development efforts to explore novel uses in controlled environments.
The current technological landscape presents both challenges and opportunities for carbon tetrachloride utilization. While its use as a general-purpose solvent has been largely phased out, there are emerging applications in specialized fields such as analytical chemistry and materials science.
One of the primary goals in carbon tetrachloride development is to harness its properties for high-value, low-volume applications. This includes exploring its potential in advanced manufacturing processes, where its chemical stability and solvent properties can be advantageous.
Another key objective is to investigate carbon tetrachloride's role in the synthesis of new materials. Its ability to form stable compounds with certain elements opens up possibilities for creating innovative substances with unique characteristics.
Researchers are also focusing on developing safer handling and containment methods for carbon tetrachloride. This is crucial for enabling its use in controlled laboratory settings and specialized industrial processes while ensuring environmental and worker safety.
The future of carbon tetrachloride in product development lies in identifying niche applications where its properties offer significant advantages over alternative substances. This requires a careful balance between leveraging its beneficial characteristics and addressing the associated risks and regulatory constraints.
As we move forward, the goal is to integrate carbon tetrachloride into sustainable product development strategies. This involves not only finding new applications but also developing closed-loop systems that minimize environmental exposure and maximize resource efficiency.
Market Analysis for Carbon Tetrachloride Applications
The global market for carbon tetrachloride applications has undergone significant changes in recent years due to environmental regulations and shifting industrial demands. Despite restrictions on its use in many consumer products, carbon tetrachloride continues to find niche applications in various industrial sectors. The chemical industry remains the primary consumer, utilizing carbon tetrachloride as a feedstock for the production of chlorofluorocarbons (CFCs) and their alternatives. While the phase-out of CFCs under the Montreal Protocol has reduced demand, the transition to hydrochlorofluorocarbons (HCFCs) and hydrofluorocarbons (HFCs) has maintained a steady market for carbon tetrachloride in this sector.
In the pharmaceutical industry, carbon tetrachloride serves as a solvent and reagent in the synthesis of certain drugs and intermediates. Its unique properties make it valuable in specific chemical processes, although manufacturers are actively seeking safer alternatives. The agrochemical sector also utilizes carbon tetrachloride in the production of pesticides and herbicides, albeit in limited quantities due to environmental concerns.
The electronics industry represents a growing market for high-purity carbon tetrachloride, which is used in the manufacture of semiconductors and optical fibers. Its excellent solvency and non-flammability make it suitable for cleaning and etching processes in these high-tech applications. However, stringent regulations and the push for greener technologies are driving research into substitutes.
Geographically, the Asia-Pacific region dominates the carbon tetrachloride market, with China being the largest producer and consumer. The region's robust chemical and electronics manufacturing sectors contribute significantly to this demand. North America and Europe have seen a decline in carbon tetrachloride usage due to strict environmental regulations, but specialized industrial applications persist.
Market trends indicate a gradual shift towards more sustainable alternatives across all sectors. This transition is driven by both regulatory pressures and corporate sustainability initiatives. Manufacturers are investing in research and development to find environmentally friendly substitutes that can match the performance characteristics of carbon tetrachloride in various applications.
The future market for carbon tetrachloride is expected to contract further as industries continue to phase out its use. However, its unique properties ensure that it will retain a place in certain specialized applications where suitable alternatives are not yet available. The challenge for product developers lies in leveraging these properties while addressing environmental concerns, potentially through the development of closed-loop systems or recovery processes that minimize emissions and environmental impact.
In the pharmaceutical industry, carbon tetrachloride serves as a solvent and reagent in the synthesis of certain drugs and intermediates. Its unique properties make it valuable in specific chemical processes, although manufacturers are actively seeking safer alternatives. The agrochemical sector also utilizes carbon tetrachloride in the production of pesticides and herbicides, albeit in limited quantities due to environmental concerns.
The electronics industry represents a growing market for high-purity carbon tetrachloride, which is used in the manufacture of semiconductors and optical fibers. Its excellent solvency and non-flammability make it suitable for cleaning and etching processes in these high-tech applications. However, stringent regulations and the push for greener technologies are driving research into substitutes.
Geographically, the Asia-Pacific region dominates the carbon tetrachloride market, with China being the largest producer and consumer. The region's robust chemical and electronics manufacturing sectors contribute significantly to this demand. North America and Europe have seen a decline in carbon tetrachloride usage due to strict environmental regulations, but specialized industrial applications persist.
Market trends indicate a gradual shift towards more sustainable alternatives across all sectors. This transition is driven by both regulatory pressures and corporate sustainability initiatives. Manufacturers are investing in research and development to find environmentally friendly substitutes that can match the performance characteristics of carbon tetrachloride in various applications.
The future market for carbon tetrachloride is expected to contract further as industries continue to phase out its use. However, its unique properties ensure that it will retain a place in certain specialized applications where suitable alternatives are not yet available. The challenge for product developers lies in leveraging these properties while addressing environmental concerns, potentially through the development of closed-loop systems or recovery processes that minimize emissions and environmental impact.
Current Challenges in Carbon Tetrachloride Utilization
Despite its historical significance in various industrial applications, carbon tetrachloride (CCl4) faces numerous challenges in modern product development due to environmental and health concerns. The primary obstacle is its classification as an ozone-depleting substance, which has led to strict regulations and phase-outs under the Montreal Protocol. This has significantly limited its use in many countries, forcing industries to seek alternatives.
Another major challenge is the toxicity of carbon tetrachloride. Exposure to this compound can cause severe liver and kidney damage, as well as potential carcinogenic effects. This toxicity profile necessitates stringent safety measures in any product development process, increasing costs and complexity of manufacturing.
The environmental persistence of carbon tetrachloride poses additional challenges. Its long atmospheric lifetime and potential for bioaccumulation raise concerns about long-term ecological impacts. This has led to increased scrutiny from regulatory bodies and environmental organizations, making it difficult to justify its use in new product development.
From a technical standpoint, finding suitable replacements that match carbon tetrachloride's unique properties has proven challenging. Its excellent solvency for non-polar compounds and its non-flammability made it ideal for certain applications, such as dry cleaning and fire extinguishers. Developing alternatives that offer similar performance without the associated risks has been an ongoing challenge for researchers and product developers.
The legacy contamination from historical use of carbon tetrachloride presents another hurdle. Many industrial sites and groundwater sources are still dealing with CCl4 pollution, necessitating costly remediation efforts. This ongoing environmental liability makes companies hesitant to incorporate the compound into new products, even in applications where it might offer significant benefits.
Furthermore, the negative public perception surrounding carbon tetrachloride creates marketing challenges for any product utilizing this compound. Consumers are increasingly environmentally conscious, and the association with ozone depletion and toxicity could lead to product rejection in the marketplace.
Lastly, the global shift towards sustainable and green chemistry principles in product development is at odds with the use of carbon tetrachloride. As industries strive to reduce their environmental footprint and adopt more eco-friendly practices, the incorporation of CCl4 in new products becomes increasingly difficult to justify, both from an ethical and a regulatory compliance perspective.
Another major challenge is the toxicity of carbon tetrachloride. Exposure to this compound can cause severe liver and kidney damage, as well as potential carcinogenic effects. This toxicity profile necessitates stringent safety measures in any product development process, increasing costs and complexity of manufacturing.
The environmental persistence of carbon tetrachloride poses additional challenges. Its long atmospheric lifetime and potential for bioaccumulation raise concerns about long-term ecological impacts. This has led to increased scrutiny from regulatory bodies and environmental organizations, making it difficult to justify its use in new product development.
From a technical standpoint, finding suitable replacements that match carbon tetrachloride's unique properties has proven challenging. Its excellent solvency for non-polar compounds and its non-flammability made it ideal for certain applications, such as dry cleaning and fire extinguishers. Developing alternatives that offer similar performance without the associated risks has been an ongoing challenge for researchers and product developers.
The legacy contamination from historical use of carbon tetrachloride presents another hurdle. Many industrial sites and groundwater sources are still dealing with CCl4 pollution, necessitating costly remediation efforts. This ongoing environmental liability makes companies hesitant to incorporate the compound into new products, even in applications where it might offer significant benefits.
Furthermore, the negative public perception surrounding carbon tetrachloride creates marketing challenges for any product utilizing this compound. Consumers are increasingly environmentally conscious, and the association with ozone depletion and toxicity could lead to product rejection in the marketplace.
Lastly, the global shift towards sustainable and green chemistry principles in product development is at odds with the use of carbon tetrachloride. As industries strive to reduce their environmental footprint and adopt more eco-friendly practices, the incorporation of CCl4 in new products becomes increasingly difficult to justify, both from an ethical and a regulatory compliance perspective.
Existing Carbon Tetrachloride-Based Solutions
01 Production and purification of carbon tetrachloride
Various methods for producing and purifying carbon tetrachloride are described. These include chemical synthesis processes, distillation techniques, and purification methods to obtain high-quality carbon tetrachloride for industrial and laboratory use.- Production and purification of carbon tetrachloride: Various methods for producing and purifying carbon tetrachloride are described. These include chemical synthesis processes, distillation techniques, and purification methods to obtain high-quality carbon tetrachloride for industrial and laboratory use.
- Applications of carbon tetrachloride in chemical processes: Carbon tetrachloride is utilized in various chemical processes, including as a solvent, reagent, or intermediate in the production of other chemicals. Its applications span across different industries, showcasing its versatility in chemical manufacturing.
- Environmental and safety considerations: Due to its environmental impact and health hazards, research focuses on developing alternatives to carbon tetrachloride and methods for its safe handling, disposal, and remediation. This includes studies on its effects on the ozone layer and strategies for reducing its use in industrial processes.
- Detection and analysis methods: Various techniques and apparatus have been developed for detecting and analyzing carbon tetrachloride in different environments. These include spectroscopic methods, chromatography, and specialized sensors designed to measure carbon tetrachloride concentrations in air, water, or other media.
- Historical uses and patents: Early patents and historical documents reveal the diverse applications of carbon tetrachloride in the past, including its use in fire extinguishers, dry cleaning, and as a refrigerant. These historical patents provide insight into the evolution of carbon tetrachloride's industrial applications over time.
02 Applications of carbon tetrachloride in chemical processes
Carbon tetrachloride is utilized in various chemical processes, including as a solvent, reagent, or intermediate in the production of other chemicals. Its applications span across different industries, such as pharmaceuticals, plastics, and agrochemicals.Expand Specific Solutions03 Environmental and safety considerations
Due to its environmental impact and health hazards, research focuses on developing alternatives to carbon tetrachloride and methods for its safe handling, storage, and disposal. This includes techniques for detecting and monitoring carbon tetrachloride in various environments.Expand Specific Solutions04 Historical uses and regulations
Carbon tetrachloride has been used historically in various applications, such as fire extinguishers and cleaning agents. However, due to its toxicity and environmental concerns, many countries have implemented regulations restricting its use and production.Expand Specific Solutions05 Analytical methods involving carbon tetrachloride
Carbon tetrachloride is used in various analytical methods and laboratory techniques. This includes its use as a solvent in spectroscopy, chromatography, and other analytical procedures for chemical analysis and material characterization.Expand Specific Solutions
Key Industry Players in Carbon Tetrachloride Production
The carbon tetrachloride market is in a mature phase, with limited growth potential due to environmental regulations restricting its use. The global market size is relatively small, estimated at around $30-40 million annually. Technologically, carbon tetrachloride production is well-established, with key players like Occidental Chemical Corp., DuPont de Nemours, Inc., and The Chemours Co. focusing on specialized applications. These companies are exploring alternative compounds and developing more environmentally friendly processes to leverage carbon tetrachloride's properties while addressing regulatory challenges. Research institutions like Central South University and Zhejiang University of Technology are also contributing to innovation in this field, potentially opening new avenues for product development.
Occidental Chemical Corp.
Technical Solution: Occidental Chemical Corp. has developed innovative processes for utilizing carbon tetrachloride in the production of chlorinated solvents and intermediates. Their approach involves a closed-loop system that minimizes environmental impact while maximizing product yield. The company has implemented advanced catalytic conversion techniques that allow for the efficient transformation of carbon tetrachloride into valuable chemical products[1]. Additionally, they have invested in state-of-the-art purification and recovery systems to ensure high-quality output and reduce waste[2]. Occidental's technology also incorporates sophisticated monitoring and control systems to maintain optimal reaction conditions and ensure process safety[3].
Strengths: Efficient conversion of carbon tetrachloride, reduced environmental impact, high product quality. Weaknesses: Potential regulatory challenges, limited applications outside chemical industry.
DuPont de Nemours, Inc.
Technical Solution: DuPont has developed a proprietary process for utilizing carbon tetrachloride as a key intermediate in the production of high-performance fluoropolymers. Their technology leverages the unique properties of carbon tetrachloride, such as its high reactivity and non-flammability, to create advanced materials with exceptional chemical resistance and thermal stability[4]. The process involves a series of controlled reactions that convert carbon tetrachloride into fluorinated monomers, which are then polymerized to form products like PTFE and FEP[5]. DuPont's approach also includes advanced containment and recycling systems to minimize environmental impact and maximize resource efficiency[6].
Strengths: Production of high-value fluoropolymers, established expertise in handling halogenated compounds. Weaknesses: Dependence on a controversial raw material, potential for stricter regulations.
Innovative Carbon Tetrachloride Properties Exploitation
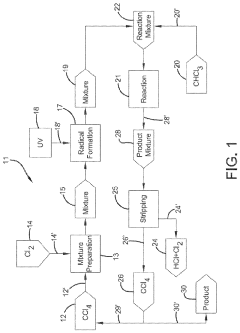
Photochlorination of partially-chlorinated chloromethanes to carbon tetrachloride
PatentActiveUS20240025823A1
Innovation
- A method involving the photochlorination of a chloromethanes stream containing chloroform, methyl chloride, and methylene chloride, combined with chlorine and additional carbon tetrachloride, and subjected to electromagnetic radiation to form carbon tetrachloride, achieving high conversion rates with reduced levels of unwanted chlorinated hydrocarbons.
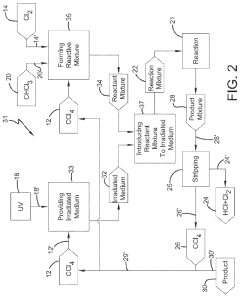
Chlorinolysis process for producing carbon tetrachloride
PatentActiveUS20210130266A1
Innovation
- A process involving a chlorination zone with chlorine, a C1 chlorinated compound, and a carbon/second chlorine source to produce a reaction mixture that favors the formation of carbon tetrachloride over perchloroethylene, using waste products as the carbon/second chlorine source to enhance efficiency and reduce impurity formation.
Environmental Impact and Regulations
Carbon tetrachloride, once widely used in various industrial applications, has been subject to stringent regulations due to its significant environmental impact. The Montreal Protocol, an international treaty designed to protect the ozone layer, has phased out the production and consumption of carbon tetrachloride in most countries. This regulatory framework has drastically limited the use of carbon tetrachloride in product development, particularly in consumer goods and industrial processes.
The environmental concerns surrounding carbon tetrachloride stem from its ozone-depleting properties and its persistence in the atmosphere. When released, it can remain in the air for decades, contributing to the depletion of the ozone layer and potentially exacerbating global warming. Additionally, carbon tetrachloride can contaminate soil and groundwater, posing risks to ecosystems and human health.
Despite these challenges, there are still limited applications where carbon tetrachloride's unique properties can be leveraged in product development, primarily in controlled laboratory settings or specific industrial processes. However, any such use must adhere to strict environmental regulations and safety protocols. Companies considering the use of carbon tetrachloride must conduct thorough environmental impact assessments and implement robust containment and disposal measures.
The regulatory landscape for carbon tetrachloride varies globally, with some countries maintaining more stringent controls than others. In the United States, for instance, the Environmental Protection Agency (EPA) has classified carbon tetrachloride as a hazardous air pollutant and strictly regulates its production, use, and disposal under the Clean Air Act and other environmental laws.
Product developers must consider alternative substances or technologies that can provide similar functionality without the associated environmental risks. This has led to increased research and development efforts focused on finding safer substitutes for carbon tetrachloride in various applications. Green chemistry initiatives have played a crucial role in this transition, promoting the design of chemical products and processes that reduce or eliminate the use and generation of hazardous substances.
In cases where the use of carbon tetrachloride is deemed necessary and permissible, companies must implement comprehensive environmental management systems. These systems should include regular monitoring of emissions, proper handling and storage protocols, and detailed plans for accidental release prevention and response. Furthermore, organizations must stay informed about evolving regulations and be prepared to adapt their practices accordingly.
The environmental concerns surrounding carbon tetrachloride stem from its ozone-depleting properties and its persistence in the atmosphere. When released, it can remain in the air for decades, contributing to the depletion of the ozone layer and potentially exacerbating global warming. Additionally, carbon tetrachloride can contaminate soil and groundwater, posing risks to ecosystems and human health.
Despite these challenges, there are still limited applications where carbon tetrachloride's unique properties can be leveraged in product development, primarily in controlled laboratory settings or specific industrial processes. However, any such use must adhere to strict environmental regulations and safety protocols. Companies considering the use of carbon tetrachloride must conduct thorough environmental impact assessments and implement robust containment and disposal measures.
The regulatory landscape for carbon tetrachloride varies globally, with some countries maintaining more stringent controls than others. In the United States, for instance, the Environmental Protection Agency (EPA) has classified carbon tetrachloride as a hazardous air pollutant and strictly regulates its production, use, and disposal under the Clean Air Act and other environmental laws.
Product developers must consider alternative substances or technologies that can provide similar functionality without the associated environmental risks. This has led to increased research and development efforts focused on finding safer substitutes for carbon tetrachloride in various applications. Green chemistry initiatives have played a crucial role in this transition, promoting the design of chemical products and processes that reduce or eliminate the use and generation of hazardous substances.
In cases where the use of carbon tetrachloride is deemed necessary and permissible, companies must implement comprehensive environmental management systems. These systems should include regular monitoring of emissions, proper handling and storage protocols, and detailed plans for accidental release prevention and response. Furthermore, organizations must stay informed about evolving regulations and be prepared to adapt their practices accordingly.
Safety Considerations in Product Development
When considering the use of carbon tetrachloride in product development, safety considerations are paramount due to the compound's known toxicity and environmental hazards. Product developers must prioritize the implementation of robust safety protocols throughout the entire lifecycle of products incorporating this substance.
Firstly, proper handling and storage procedures are critical. Carbon tetrachloride should be stored in tightly sealed containers in well-ventilated areas, away from sources of heat or ignition. Personal protective equipment (PPE), including chemical-resistant gloves, safety goggles, and respiratory protection, must be mandatory for all personnel working with the compound.
Exposure limits set by regulatory agencies, such as the Occupational Safety and Health Administration (OSHA), must be strictly adhered to. Regular air quality monitoring in work areas is essential to ensure that concentrations remain below permissible levels. Implementation of engineering controls, such as closed systems and local exhaust ventilation, can significantly reduce the risk of exposure.
Product designers must consider the potential for carbon tetrachloride to be released during the use phase of the product. Incorporating fail-safe mechanisms and leak detection systems can help prevent accidental releases. Additionally, clear labeling and user instructions are crucial to inform consumers about the presence of carbon tetrachloride and necessary precautions.
Environmental safety is another critical aspect. Given carbon tetrachloride's ozone-depleting properties and persistence in the environment, product developers must design with end-of-life considerations in mind. This includes developing recycling or safe disposal methods to prevent environmental contamination.
Risk assessment and management plans should be integral to the product development process. This involves identifying potential hazards, evaluating risks, and implementing appropriate control measures. Regular safety audits and reviews should be conducted to ensure ongoing compliance with safety standards and regulations.
Training programs for employees involved in the development, production, and handling of products containing carbon tetrachloride are essential. These programs should cover safe handling procedures, emergency response protocols, and the importance of adhering to safety guidelines.
Collaboration with toxicologists and environmental experts can provide valuable insights into minimizing the risks associated with carbon tetrachloride use. Their expertise can inform the development of safer alternatives or modifications to reduce the compound's hazardous properties while maintaining its beneficial characteristics for the product.
In conclusion, while carbon tetrachloride offers unique properties that may be advantageous in certain applications, its use in product development necessitates a comprehensive and stringent approach to safety. By implementing thorough safety measures, product developers can mitigate risks and ensure responsible use of this potentially hazardous substance.
Firstly, proper handling and storage procedures are critical. Carbon tetrachloride should be stored in tightly sealed containers in well-ventilated areas, away from sources of heat or ignition. Personal protective equipment (PPE), including chemical-resistant gloves, safety goggles, and respiratory protection, must be mandatory for all personnel working with the compound.
Exposure limits set by regulatory agencies, such as the Occupational Safety and Health Administration (OSHA), must be strictly adhered to. Regular air quality monitoring in work areas is essential to ensure that concentrations remain below permissible levels. Implementation of engineering controls, such as closed systems and local exhaust ventilation, can significantly reduce the risk of exposure.
Product designers must consider the potential for carbon tetrachloride to be released during the use phase of the product. Incorporating fail-safe mechanisms and leak detection systems can help prevent accidental releases. Additionally, clear labeling and user instructions are crucial to inform consumers about the presence of carbon tetrachloride and necessary precautions.
Environmental safety is another critical aspect. Given carbon tetrachloride's ozone-depleting properties and persistence in the environment, product developers must design with end-of-life considerations in mind. This includes developing recycling or safe disposal methods to prevent environmental contamination.
Risk assessment and management plans should be integral to the product development process. This involves identifying potential hazards, evaluating risks, and implementing appropriate control measures. Regular safety audits and reviews should be conducted to ensure ongoing compliance with safety standards and regulations.
Training programs for employees involved in the development, production, and handling of products containing carbon tetrachloride are essential. These programs should cover safe handling procedures, emergency response protocols, and the importance of adhering to safety guidelines.
Collaboration with toxicologists and environmental experts can provide valuable insights into minimizing the risks associated with carbon tetrachloride use. Their expertise can inform the development of safer alternatives or modifications to reduce the compound's hazardous properties while maintaining its beneficial characteristics for the product.
In conclusion, while carbon tetrachloride offers unique properties that may be advantageous in certain applications, its use in product development necessitates a comprehensive and stringent approach to safety. By implementing thorough safety measures, product developers can mitigate risks and ensure responsible use of this potentially hazardous substance.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!