Emerging Directions in Carbon Tetrachloride Chemical Applications
JUL 3, 20258 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CCl4 Applications Evolution and Objectives
Carbon tetrachloride (CCl4) has undergone significant evolution in its applications since its discovery in the mid-19th century. Initially utilized as a solvent and cleaning agent, CCl4's usage expanded rapidly across various industries due to its non-flammability and effectiveness. However, the trajectory of its applications has been shaped by growing environmental and health concerns.
In the early 20th century, CCl4 found widespread use in fire extinguishers, dry cleaning, and as a precursor in the production of refrigerants. Its role in the manufacturing of chlorofluorocarbons (CFCs) became particularly prominent, driving increased production. However, the discovery of its ozone-depleting properties and potential carcinogenicity led to a dramatic shift in its application landscape.
The Montreal Protocol in 1987 marked a turning point, initiating the phase-out of ozone-depleting substances, including CCl4. This global agreement prompted a reevaluation of CCl4's applications and accelerated research into alternatives. Consequently, many traditional uses of CCl4 were discontinued or severely restricted in numerous countries.
Despite these challenges, the unique properties of CCl4 have continued to drive research into novel applications that minimize environmental impact. Recent years have seen emerging directions in CCl4 utilization, focusing on controlled, closed-system applications where its benefits can be leveraged without significant environmental release.
Current research objectives in CCl4 applications are multifaceted. One primary goal is to develop innovative synthesis routes that use CCl4 as a reagent in the production of high-value chemicals, particularly in the pharmaceutical and agrochemical industries. These processes aim to capitalize on CCl4's reactivity while ensuring minimal environmental exposure.
Another key objective is the exploration of CCl4's potential in advanced materials science. Researchers are investigating its role in the synthesis of nanomaterials and specialty polymers, where its unique chemical properties can be harnessed in controlled environments. Additionally, there is growing interest in CCl4's application in analytical chemistry, particularly as a solvent in specific spectroscopic techniques.
The evolution of CCl4 applications also encompasses efforts to remediate historical contamination. Objectives in this area include developing efficient methods for CCl4 detection in soil and groundwater, as well as innovative remediation technologies that can effectively neutralize or transform CCl4 in the environment.
Looking forward, the trajectory of CCl4 applications is likely to be characterized by a balance between leveraging its valuable chemical properties and adhering to strict environmental and safety protocols. The focus will remain on specialized, high-value applications where alternatives are less effective, coupled with ongoing research into mitigating its environmental impact.
In the early 20th century, CCl4 found widespread use in fire extinguishers, dry cleaning, and as a precursor in the production of refrigerants. Its role in the manufacturing of chlorofluorocarbons (CFCs) became particularly prominent, driving increased production. However, the discovery of its ozone-depleting properties and potential carcinogenicity led to a dramatic shift in its application landscape.
The Montreal Protocol in 1987 marked a turning point, initiating the phase-out of ozone-depleting substances, including CCl4. This global agreement prompted a reevaluation of CCl4's applications and accelerated research into alternatives. Consequently, many traditional uses of CCl4 were discontinued or severely restricted in numerous countries.
Despite these challenges, the unique properties of CCl4 have continued to drive research into novel applications that minimize environmental impact. Recent years have seen emerging directions in CCl4 utilization, focusing on controlled, closed-system applications where its benefits can be leveraged without significant environmental release.
Current research objectives in CCl4 applications are multifaceted. One primary goal is to develop innovative synthesis routes that use CCl4 as a reagent in the production of high-value chemicals, particularly in the pharmaceutical and agrochemical industries. These processes aim to capitalize on CCl4's reactivity while ensuring minimal environmental exposure.
Another key objective is the exploration of CCl4's potential in advanced materials science. Researchers are investigating its role in the synthesis of nanomaterials and specialty polymers, where its unique chemical properties can be harnessed in controlled environments. Additionally, there is growing interest in CCl4's application in analytical chemistry, particularly as a solvent in specific spectroscopic techniques.
The evolution of CCl4 applications also encompasses efforts to remediate historical contamination. Objectives in this area include developing efficient methods for CCl4 detection in soil and groundwater, as well as innovative remediation technologies that can effectively neutralize or transform CCl4 in the environment.
Looking forward, the trajectory of CCl4 applications is likely to be characterized by a balance between leveraging its valuable chemical properties and adhering to strict environmental and safety protocols. The focus will remain on specialized, high-value applications where alternatives are less effective, coupled with ongoing research into mitigating its environmental impact.
Market Demand Analysis for CCl4 Usage
The market demand for carbon tetrachloride (CCl4) has undergone significant shifts in recent years, driven by evolving regulatory landscapes and emerging applications. Historically, CCl4 was widely used as a solvent, cleaning agent, and refrigerant. However, its ozone-depleting properties led to severe restrictions under the Montreal Protocol, causing a sharp decline in traditional markets.
Despite these limitations, new niche applications have emerged, creating pockets of demand for CCl4. The pharmaceutical industry has shown renewed interest in CCl4 as a precursor in the synthesis of certain active pharmaceutical ingredients (APIs). This sector's demand is expected to grow steadily, albeit from a relatively small base, as novel drug development processes incorporate CCl4 in controlled environments.
In the semiconductor industry, high-purity CCl4 has found applications in plasma etching processes for manufacturing advanced microchips. As the demand for more sophisticated electronic devices continues to rise, this sector could become a significant consumer of CCl4, particularly in regions with robust semiconductor manufacturing capabilities.
The agrochemical sector presents another potential growth area for CCl4 usage. Some pesticide formulations utilize CCl4 as an intermediate, and ongoing research into more effective crop protection chemicals may uncover new roles for this compound. However, stringent environmental regulations pose challenges to widespread adoption in this field.
Geographically, the demand for CCl4 is shifting towards emerging economies, particularly in Asia-Pacific. Countries like China and India, with their growing industrial bases and less restrictive regulatory environments, are becoming key markets for CCl4 applications. This regional shift is reshaping global supply chains and production strategies for CCl4 manufacturers.
The overall market size for CCl4 remains relatively modest compared to its peak usage before environmental regulations. However, the compound's unique properties continue to attract interest from specialized industries. Market analysts project a compound annual growth rate (CAGR) in the low single digits for CCl4 demand over the next five years, primarily driven by high-tech and pharmaceutical applications.
Pricing trends for CCl4 have shown volatility, influenced by fluctuating raw material costs and changing supply-demand dynamics. The limited number of producers and the specialized nature of current applications contribute to price sensitivity in the market. Future demand and pricing will likely be heavily influenced by regulatory developments, particularly any changes in international agreements governing ozone-depleting substances.
Despite these limitations, new niche applications have emerged, creating pockets of demand for CCl4. The pharmaceutical industry has shown renewed interest in CCl4 as a precursor in the synthesis of certain active pharmaceutical ingredients (APIs). This sector's demand is expected to grow steadily, albeit from a relatively small base, as novel drug development processes incorporate CCl4 in controlled environments.
In the semiconductor industry, high-purity CCl4 has found applications in plasma etching processes for manufacturing advanced microchips. As the demand for more sophisticated electronic devices continues to rise, this sector could become a significant consumer of CCl4, particularly in regions with robust semiconductor manufacturing capabilities.
The agrochemical sector presents another potential growth area for CCl4 usage. Some pesticide formulations utilize CCl4 as an intermediate, and ongoing research into more effective crop protection chemicals may uncover new roles for this compound. However, stringent environmental regulations pose challenges to widespread adoption in this field.
Geographically, the demand for CCl4 is shifting towards emerging economies, particularly in Asia-Pacific. Countries like China and India, with their growing industrial bases and less restrictive regulatory environments, are becoming key markets for CCl4 applications. This regional shift is reshaping global supply chains and production strategies for CCl4 manufacturers.
The overall market size for CCl4 remains relatively modest compared to its peak usage before environmental regulations. However, the compound's unique properties continue to attract interest from specialized industries. Market analysts project a compound annual growth rate (CAGR) in the low single digits for CCl4 demand over the next five years, primarily driven by high-tech and pharmaceutical applications.
Pricing trends for CCl4 have shown volatility, influenced by fluctuating raw material costs and changing supply-demand dynamics. The limited number of producers and the specialized nature of current applications contribute to price sensitivity in the market. Future demand and pricing will likely be heavily influenced by regulatory developments, particularly any changes in international agreements governing ozone-depleting substances.
CCl4 Technical Challenges and Constraints
Carbon tetrachloride (CCl4) faces several significant technical challenges and constraints in its emerging chemical applications. One of the primary concerns is its high toxicity and potential environmental impact. CCl4 is known to be a potent ozone-depleting substance, which has led to strict regulations on its production and use under the Montreal Protocol. This regulatory environment severely limits its application in many industrial processes and consumer products.
Another major challenge is the development of safer and more environmentally friendly alternatives. Many industries that traditionally relied on CCl4 are now actively seeking substitutes that can provide similar performance without the associated health and environmental risks. This shift has created a need for extensive research and development to identify and validate new compounds or processes that can effectively replace CCl4 in various applications.
The stability of CCl4 presents both advantages and challenges. While its chemical stability makes it useful in certain applications, it also contributes to its persistence in the environment. This persistence raises concerns about long-term ecological effects and potential bioaccumulation in food chains. Addressing these environmental persistence issues requires innovative approaches to degradation or containment strategies.
From a technical standpoint, the use of CCl4 in emerging applications often requires specialized handling and containment systems. Its volatility and potential for atmospheric release necessitate advanced engineering controls and safety measures. This requirement for sophisticated infrastructure can significantly increase the cost and complexity of processes involving CCl4, limiting its adoption in new applications.
The analytical and detection challenges associated with CCl4 also pose significant constraints. Given its potential for environmental contamination, there is a critical need for sensitive and reliable detection methods. Developing and implementing these analytical techniques, especially for trace-level detection in complex environmental matrices, remains an ongoing challenge for researchers and regulatory bodies.
Furthermore, the historical use of CCl4 has left a legacy of contaminated sites worldwide. The remediation of these sites presents substantial technical challenges, particularly in addressing subsurface contamination. Innovative remediation technologies are needed to effectively clean up CCl4-contaminated soil and groundwater without causing further environmental damage.
In the realm of chemical synthesis, while CCl4 has been valuable as a solvent and reagent, finding ways to achieve similar chemical transformations without its use is a significant challenge. This requires the development of new synthetic routes and methodologies that can maintain or improve reaction efficiencies and yields while eliminating the need for CCl4.
Another major challenge is the development of safer and more environmentally friendly alternatives. Many industries that traditionally relied on CCl4 are now actively seeking substitutes that can provide similar performance without the associated health and environmental risks. This shift has created a need for extensive research and development to identify and validate new compounds or processes that can effectively replace CCl4 in various applications.
The stability of CCl4 presents both advantages and challenges. While its chemical stability makes it useful in certain applications, it also contributes to its persistence in the environment. This persistence raises concerns about long-term ecological effects and potential bioaccumulation in food chains. Addressing these environmental persistence issues requires innovative approaches to degradation or containment strategies.
From a technical standpoint, the use of CCl4 in emerging applications often requires specialized handling and containment systems. Its volatility and potential for atmospheric release necessitate advanced engineering controls and safety measures. This requirement for sophisticated infrastructure can significantly increase the cost and complexity of processes involving CCl4, limiting its adoption in new applications.
The analytical and detection challenges associated with CCl4 also pose significant constraints. Given its potential for environmental contamination, there is a critical need for sensitive and reliable detection methods. Developing and implementing these analytical techniques, especially for trace-level detection in complex environmental matrices, remains an ongoing challenge for researchers and regulatory bodies.
Furthermore, the historical use of CCl4 has left a legacy of contaminated sites worldwide. The remediation of these sites presents substantial technical challenges, particularly in addressing subsurface contamination. Innovative remediation technologies are needed to effectively clean up CCl4-contaminated soil and groundwater without causing further environmental damage.
In the realm of chemical synthesis, while CCl4 has been valuable as a solvent and reagent, finding ways to achieve similar chemical transformations without its use is a significant challenge. This requires the development of new synthetic routes and methodologies that can maintain or improve reaction efficiencies and yields while eliminating the need for CCl4.
Current CCl4 Application Solutions
01 Production and purification of carbon tetrachloride
Various methods for producing and purifying carbon tetrachloride are described. These include chemical synthesis processes, distillation techniques, and purification methods to obtain high-quality carbon tetrachloride for industrial and laboratory use.- Production and purification of carbon tetrachloride: Various methods for producing and purifying carbon tetrachloride are described. These include chemical synthesis processes, distillation techniques, and purification methods to obtain high-quality carbon tetrachloride for industrial and laboratory use.
- Applications of carbon tetrachloride in chemical processes: Carbon tetrachloride is utilized in various chemical processes as a solvent, reagent, or intermediate. It finds applications in organic synthesis, extraction processes, and as a precursor for other chlorinated compounds.
- Environmental and safety considerations: Due to its environmental impact and health hazards, research focuses on developing alternatives to carbon tetrachloride and methods for its safe handling, storage, and disposal. This includes techniques for detecting and monitoring carbon tetrachloride in various environments.
- Historical uses and patents: Early patents and historical documents describe various applications of carbon tetrachloride, including its use as a fire extinguishing agent, cleaning solvent, and in the production of refrigerants. These patents provide insight into the compound's industrial importance over time.
- Analytical methods involving carbon tetrachloride: Carbon tetrachloride is used in various analytical methods and laboratory techniques. This includes its application in spectroscopic studies, as a solvent for NMR spectroscopy, and in certain chemical analysis procedures.
02 Applications of carbon tetrachloride in chemical processes
Carbon tetrachloride is utilized in various chemical processes, including as a solvent, reagent, or intermediate in organic synthesis. It plays a role in the production of other chlorinated compounds and in certain industrial applications.Expand Specific Solutions03 Environmental and safety considerations
Due to its environmental impact and health hazards, research focuses on alternatives to carbon tetrachloride and methods for its safe handling, storage, and disposal. This includes developing eco-friendly substitutes and improving containment strategies.Expand Specific Solutions04 Detection and analysis methods
Various analytical techniques and methods have been developed for detecting and quantifying carbon tetrachloride in different matrices. These include spectroscopic methods, chromatographic techniques, and sensor-based approaches for environmental and quality control purposes.Expand Specific Solutions05 Historical uses and patents
Early patents and historical documents reveal the evolution of carbon tetrachloride's applications, including its use in fire extinguishers, dry cleaning, and as a refrigerant. These sources provide insight into the compound's industrial significance over time.Expand Specific Solutions
Key Industry Players in CCl4 Research
The carbon tetrachloride chemical applications market is in a transitional phase, with growing environmental concerns driving innovation in safer alternatives. The global market size is estimated to be around $100-150 million annually, with moderate growth projected. Technologically, the field is mature but evolving, as companies like DuPont, Occidental Chemical, and Honeywell International lead efforts to develop more sustainable processes and applications. Chinese firms like ZTE and Sinochem are also becoming increasingly competitive. Academic institutions such as the University of Tokyo and Max Planck Society are contributing cutting-edge research to advance the field. Overall, the industry is characterized by a mix of established players and new entrants seeking to capitalize on emerging opportunities in specialized applications and greener technologies.
DuPont de Nemours, Inc.
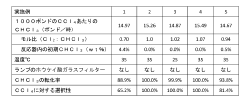
Technical Solution: DuPont has developed innovative applications for carbon tetrachloride in the production of advanced fluoropolymers. Their process involves using carbon tetrachloride as a chain transfer agent in the synthesis of high-performance fluoropolymers like PTFE and FEP. This method allows for precise control of molecular weight and end-group functionality, resulting in materials with enhanced thermal stability and chemical resistance[1]. DuPont has also explored the use of carbon tetrachloride in the production of chlorofluorocarbons (CFCs) alternatives, focusing on environmentally friendly options that maintain similar performance characteristics[2]. Additionally, they have invested in research to mitigate the environmental impact of carbon tetrachloride use, developing closed-loop systems and recycling technologies to minimize emissions and waste[3].
Strengths: Expertise in fluoropolymer synthesis, established infrastructure for handling chlorinated compounds, strong R&D capabilities. Weaknesses: Environmental concerns associated with carbon tetrachloride use, regulatory challenges, need for continuous innovation to maintain market position.
Honeywell International Technologies Ltd.
Technical Solution: Honeywell has focused on developing alternative applications for carbon tetrachloride in refrigeration and air conditioning systems. Their research has led to the creation of next-generation refrigerants that utilize carbon tetrachloride as a precursor, resulting in compounds with lower global warming potential (GWP) and improved energy efficiency[4]. Honeywell's approach involves modifying the molecular structure of carbon tetrachloride to create hydrofluoroolefins (HFOs), which offer similar cooling properties to traditional refrigerants but with significantly reduced environmental impact[5]. The company has also explored the use of carbon tetrachloride in the production of high-purity silicon for semiconductor applications, developing purification techniques that leverage the compound's unique properties[6].
Strengths: Strong presence in the HVAC industry, advanced R&D facilities, global distribution network. Weaknesses: Dependence on regulatory approvals for new refrigerants, competition from other alternative technologies, potential for market saturation.
Innovative CCl4 Usage Patents
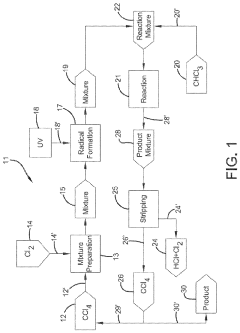
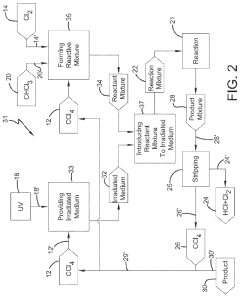
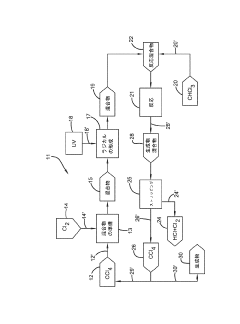
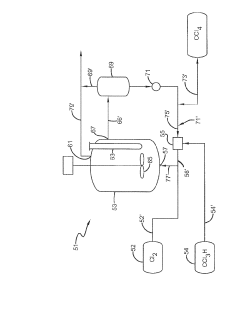
Photochlorination of partially-chlorinated chloromethanes to carbon tetrachloride
PatentActiveUS20240025823A1
Innovation
- A method involving the photochlorination of a chloromethanes stream containing chloroform, methyl chloride, and methylene chloride, combined with chlorine and additional carbon tetrachloride, and subjected to electromagnetic radiation to form carbon tetrachloride, achieving high conversion rates with reduced levels of unwanted chlorinated hydrocarbons.
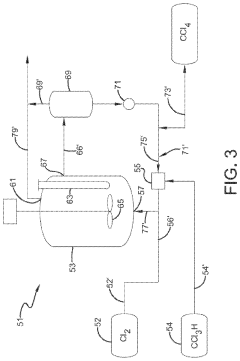
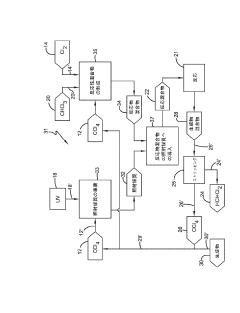
Producing carbon tetrachloride by photochlorination of chloroform
PatentActiveJP2024069268A
Innovation
- A method involving the photochlorination of chloroform with chlorine in the presence of electromagnetic radiation, maintaining a low concentration of chloroform and a stoichiometric concentration of chlorine, to produce carbon tetrachloride with high selectivity and minimize the formation of hexachloroethane.
Environmental Impact of CCl4 Use
The environmental impact of carbon tetrachloride (CCl4) use has been a significant concern for decades, primarily due to its ozone-depleting properties and potential toxicity. As a potent greenhouse gas, CCl4 contributes to global warming and climate change, with a global warming potential approximately 1,400 times that of carbon dioxide over a 100-year period.
In the atmosphere, CCl4 breaks down to release chlorine atoms, which catalyze the destruction of stratospheric ozone. This depletion of the ozone layer increases the amount of harmful ultraviolet radiation reaching the Earth's surface, posing risks to human health, ecosystems, and agricultural productivity. The Montreal Protocol, an international treaty designed to protect the ozone layer, has phased out the production and consumption of CCl4 for most uses since 2010.
Despite these regulations, CCl4 continues to persist in the environment due to its long atmospheric lifetime of approximately 26 years. Recent studies have shown that global emissions of CCl4 are higher than expected, suggesting ongoing production or unreported sources. This discrepancy highlights the need for improved monitoring and enforcement of international agreements.
In aquatic environments, CCl4 can accumulate in sediments and bioaccumulate in marine organisms, potentially affecting entire food chains. Its presence in groundwater and surface water poses risks to both human and ecological health. Soil contamination by CCl4 can lead to long-term environmental degradation and challenges in remediation efforts.
The toxicity of CCl4 to humans and wildlife is well-documented. Exposure can cause liver and kidney damage, and it is classified as a probable human carcinogen. Occupational exposure remains a concern in industries where CCl4 is still used as a feedstock or process agent.
As research into alternative chemicals and processes continues, the environmental impact of CCl4 use serves as a cautionary tale for the development and application of new chemical compounds. It underscores the importance of comprehensive environmental and health assessments before widespread adoption of industrial chemicals.
In the atmosphere, CCl4 breaks down to release chlorine atoms, which catalyze the destruction of stratospheric ozone. This depletion of the ozone layer increases the amount of harmful ultraviolet radiation reaching the Earth's surface, posing risks to human health, ecosystems, and agricultural productivity. The Montreal Protocol, an international treaty designed to protect the ozone layer, has phased out the production and consumption of CCl4 for most uses since 2010.
Despite these regulations, CCl4 continues to persist in the environment due to its long atmospheric lifetime of approximately 26 years. Recent studies have shown that global emissions of CCl4 are higher than expected, suggesting ongoing production or unreported sources. This discrepancy highlights the need for improved monitoring and enforcement of international agreements.
In aquatic environments, CCl4 can accumulate in sediments and bioaccumulate in marine organisms, potentially affecting entire food chains. Its presence in groundwater and surface water poses risks to both human and ecological health. Soil contamination by CCl4 can lead to long-term environmental degradation and challenges in remediation efforts.
The toxicity of CCl4 to humans and wildlife is well-documented. Exposure can cause liver and kidney damage, and it is classified as a probable human carcinogen. Occupational exposure remains a concern in industries where CCl4 is still used as a feedstock or process agent.
As research into alternative chemicals and processes continues, the environmental impact of CCl4 use serves as a cautionary tale for the development and application of new chemical compounds. It underscores the importance of comprehensive environmental and health assessments before widespread adoption of industrial chemicals.
CCl4 Regulatory Framework
The regulatory framework surrounding carbon tetrachloride (CCl4) has undergone significant changes in recent decades, reflecting growing concerns about its environmental and health impacts. Initially widely used in various industrial applications, CCl4 has been subject to increasingly stringent regulations due to its ozone-depleting properties and potential carcinogenicity.
At the international level, the Montreal Protocol on Substances that Deplete the Ozone Layer, signed in 1987, marked a turning point in CCl4 regulation. This agreement phased out the production and consumption of CCl4 for dispersive uses in developed countries by 1996 and in developing countries by 2010. However, the protocol allows for continued production and use of CCl4 as a feedstock for the manufacture of other chemicals, provided that it is not released into the atmosphere.
In the United States, the Environmental Protection Agency (EPA) has implemented strict controls on CCl4 under various legislative acts. The Clean Air Act Amendments of 1990 classified CCl4 as a hazardous air pollutant and mandated its phase-out in most applications. The Toxic Substances Control Act (TSCA) further regulates the manufacture, import, and use of CCl4, requiring reporting and risk assessments for any significant new uses.
The European Union has also imposed rigorous restrictions on CCl4 through the REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) regulation. Under REACH, CCl4 is classified as a substance of very high concern (SVHC) due to its carcinogenic properties, requiring authorization for specific uses and imposing strict safety measures.
Despite these regulations, CCl4 continues to play a role in certain industrial processes, particularly as a feedstock in the production of hydrofluorocarbons (HFCs) and other chemicals. This has led to ongoing debates about the balance between industrial needs and environmental protection. Recent studies suggesting higher-than-expected atmospheric concentrations of CCl4 have prompted calls for tighter controls and improved monitoring of its production and use.
As emerging applications for CCl4 in chemical synthesis and materials science are explored, regulatory bodies face the challenge of adapting their frameworks to address potential new uses while maintaining environmental safeguards. This evolving regulatory landscape necessitates continuous reassessment of CCl4 policies, taking into account scientific advancements, environmental impacts, and industrial requirements.
At the international level, the Montreal Protocol on Substances that Deplete the Ozone Layer, signed in 1987, marked a turning point in CCl4 regulation. This agreement phased out the production and consumption of CCl4 for dispersive uses in developed countries by 1996 and in developing countries by 2010. However, the protocol allows for continued production and use of CCl4 as a feedstock for the manufacture of other chemicals, provided that it is not released into the atmosphere.
In the United States, the Environmental Protection Agency (EPA) has implemented strict controls on CCl4 under various legislative acts. The Clean Air Act Amendments of 1990 classified CCl4 as a hazardous air pollutant and mandated its phase-out in most applications. The Toxic Substances Control Act (TSCA) further regulates the manufacture, import, and use of CCl4, requiring reporting and risk assessments for any significant new uses.
The European Union has also imposed rigorous restrictions on CCl4 through the REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) regulation. Under REACH, CCl4 is classified as a substance of very high concern (SVHC) due to its carcinogenic properties, requiring authorization for specific uses and imposing strict safety measures.
Despite these regulations, CCl4 continues to play a role in certain industrial processes, particularly as a feedstock in the production of hydrofluorocarbons (HFCs) and other chemicals. This has led to ongoing debates about the balance between industrial needs and environmental protection. Recent studies suggesting higher-than-expected atmospheric concentrations of CCl4 have prompted calls for tighter controls and improved monitoring of its production and use.
As emerging applications for CCl4 in chemical synthesis and materials science are explored, regulatory bodies face the challenge of adapting their frameworks to address potential new uses while maintaining environmental safeguards. This evolving regulatory landscape necessitates continuous reassessment of CCl4 policies, taking into account scientific advancements, environmental impacts, and industrial requirements.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!