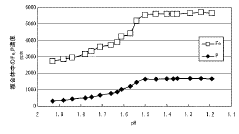
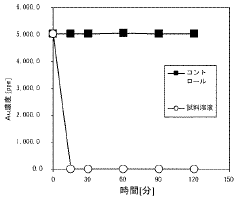
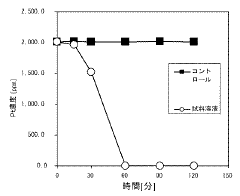
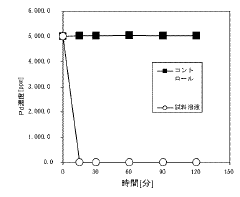
Evaluating Magnesium Nitrate in the Recovery of Precious Metals
AUG 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Magnesium Nitrate in PM Recovery: Background and Objectives
The recovery of precious metals has been a critical process in various industries, including electronics, jewelry, and catalytic converters. As global demand for these metals continues to rise, the need for efficient and cost-effective recovery methods has become increasingly important. Magnesium nitrate has emerged as a promising reagent in this field, offering potential advantages over traditional recovery techniques.
The use of magnesium nitrate in precious metal recovery has its roots in hydrometallurgical processes, which have been developed and refined over the past century. These processes typically involve the dissolution of metals in aqueous solutions, followed by selective precipitation or extraction. Magnesium nitrate's role in this context has gained attention due to its unique chemical properties and potential to enhance recovery efficiency.
The evolution of precious metal recovery techniques has been driven by several factors, including environmental concerns, economic pressures, and technological advancements. Early methods often relied on pyrometallurgical processes, which were energy-intensive and environmentally problematic. The shift towards hydrometallurgical approaches, including the use of magnesium nitrate, represents a significant step in the industry's progression towards more sustainable practices.
The primary objective of evaluating magnesium nitrate in precious metal recovery is to determine its efficacy, efficiency, and potential advantages over existing methods. This evaluation aims to assess factors such as recovery rates, selectivity, process economics, and environmental impact. Additionally, the investigation seeks to identify optimal conditions for magnesium nitrate's application and potential synergies with other reagents or processes.
Another key goal is to explore the scalability of magnesium nitrate-based recovery methods. As the demand for precious metals continues to grow, particularly in emerging technologies like renewable energy and electric vehicles, the ability to implement these processes on an industrial scale becomes crucial. This evaluation will consider the challenges and opportunities associated with scaling up magnesium nitrate-based recovery systems.
Furthermore, this research aims to contribute to the broader understanding of hydrometallurgical processes and their potential in addressing the global challenge of resource scarcity. By thoroughly examining the role of magnesium nitrate in precious metal recovery, this study may pave the way for innovative approaches to metal extraction and recycling, potentially revolutionizing the industry and contributing to more sustainable resource management practices.
The use of magnesium nitrate in precious metal recovery has its roots in hydrometallurgical processes, which have been developed and refined over the past century. These processes typically involve the dissolution of metals in aqueous solutions, followed by selective precipitation or extraction. Magnesium nitrate's role in this context has gained attention due to its unique chemical properties and potential to enhance recovery efficiency.
The evolution of precious metal recovery techniques has been driven by several factors, including environmental concerns, economic pressures, and technological advancements. Early methods often relied on pyrometallurgical processes, which were energy-intensive and environmentally problematic. The shift towards hydrometallurgical approaches, including the use of magnesium nitrate, represents a significant step in the industry's progression towards more sustainable practices.
The primary objective of evaluating magnesium nitrate in precious metal recovery is to determine its efficacy, efficiency, and potential advantages over existing methods. This evaluation aims to assess factors such as recovery rates, selectivity, process economics, and environmental impact. Additionally, the investigation seeks to identify optimal conditions for magnesium nitrate's application and potential synergies with other reagents or processes.
Another key goal is to explore the scalability of magnesium nitrate-based recovery methods. As the demand for precious metals continues to grow, particularly in emerging technologies like renewable energy and electric vehicles, the ability to implement these processes on an industrial scale becomes crucial. This evaluation will consider the challenges and opportunities associated with scaling up magnesium nitrate-based recovery systems.
Furthermore, this research aims to contribute to the broader understanding of hydrometallurgical processes and their potential in addressing the global challenge of resource scarcity. By thoroughly examining the role of magnesium nitrate in precious metal recovery, this study may pave the way for innovative approaches to metal extraction and recycling, potentially revolutionizing the industry and contributing to more sustainable resource management practices.
Market Analysis for Precious Metal Recovery Solutions
The precious metal recovery market has been experiencing significant growth in recent years, driven by increasing demand for rare metals in various industries and the growing emphasis on sustainable resource management. The global market for precious metal recovery solutions is projected to expand at a compound annual growth rate (CAGR) of 6.8% from 2021 to 2026, reaching a value of $28.3 billion by the end of the forecast period.
The primary drivers of this market growth include the rising adoption of electronic devices, the expansion of the automotive industry, and the increasing focus on recycling and environmental sustainability. The electronics sector, in particular, has been a major contributor to the demand for precious metal recovery solutions, as electronic waste contains significant amounts of valuable metals such as gold, silver, platinum, and palladium.
In terms of regional distribution, North America and Europe currently dominate the market, accounting for over 60% of the global market share. This is primarily due to stringent environmental regulations and well-established recycling infrastructure in these regions. However, the Asia-Pacific region is expected to witness the highest growth rate in the coming years, driven by rapid industrialization, increasing electronic waste generation, and growing awareness of the economic benefits of metal recovery.
The market for magnesium nitrate-based precious metal recovery solutions is a niche but growing segment within the broader market. Magnesium nitrate has shown promising results in the selective recovery of certain precious metals, particularly in the treatment of electronic waste and industrial effluents. The use of magnesium nitrate in metal recovery processes offers several advantages, including high selectivity, relatively low cost, and environmental friendliness compared to traditional recovery methods.
Key market players in the precious metal recovery sector include Johnson Matthey, Umicore, Heraeus, BASF, and Tanaka Precious Metals. These companies are investing heavily in research and development to improve recovery efficiencies and develop more sustainable processes. The integration of magnesium nitrate-based solutions into their product portfolios could represent a significant opportunity for market expansion and differentiation.
Challenges facing the market include the volatility of precious metal prices, which can impact the economic viability of recovery processes, and the complexity of recovering metals from increasingly diverse and complex waste streams. Additionally, regulatory hurdles and the need for substantial initial investments in recovery infrastructure pose barriers to market entry for smaller players.
The primary drivers of this market growth include the rising adoption of electronic devices, the expansion of the automotive industry, and the increasing focus on recycling and environmental sustainability. The electronics sector, in particular, has been a major contributor to the demand for precious metal recovery solutions, as electronic waste contains significant amounts of valuable metals such as gold, silver, platinum, and palladium.
In terms of regional distribution, North America and Europe currently dominate the market, accounting for over 60% of the global market share. This is primarily due to stringent environmental regulations and well-established recycling infrastructure in these regions. However, the Asia-Pacific region is expected to witness the highest growth rate in the coming years, driven by rapid industrialization, increasing electronic waste generation, and growing awareness of the economic benefits of metal recovery.
The market for magnesium nitrate-based precious metal recovery solutions is a niche but growing segment within the broader market. Magnesium nitrate has shown promising results in the selective recovery of certain precious metals, particularly in the treatment of electronic waste and industrial effluents. The use of magnesium nitrate in metal recovery processes offers several advantages, including high selectivity, relatively low cost, and environmental friendliness compared to traditional recovery methods.
Key market players in the precious metal recovery sector include Johnson Matthey, Umicore, Heraeus, BASF, and Tanaka Precious Metals. These companies are investing heavily in research and development to improve recovery efficiencies and develop more sustainable processes. The integration of magnesium nitrate-based solutions into their product portfolios could represent a significant opportunity for market expansion and differentiation.
Challenges facing the market include the volatility of precious metal prices, which can impact the economic viability of recovery processes, and the complexity of recovering metals from increasingly diverse and complex waste streams. Additionally, regulatory hurdles and the need for substantial initial investments in recovery infrastructure pose barriers to market entry for smaller players.
Current Challenges in Precious Metal Recovery Techniques
The recovery of precious metals faces several significant challenges in current techniques. One of the primary issues is the low concentration of these metals in various sources, making extraction economically challenging. This is particularly evident in electronic waste, where precious metals are dispersed in small quantities across large volumes of material.
Environmental concerns pose another major challenge. Traditional recovery methods often involve the use of harsh chemicals, such as cyanide and mercury, which can have severe environmental impacts if not properly managed. The increasing global focus on sustainability and environmental protection is pushing the industry to develop greener, less toxic alternatives.
The complexity of material compositions in which precious metals are found also presents difficulties. For instance, in electronic waste, precious metals are often alloyed or combined with other materials, making separation and purification processes more complicated and energy-intensive. This complexity often results in lower recovery rates and increased processing costs.
Energy consumption is another significant challenge. Many current recovery techniques require high temperatures or energy-intensive processes, contributing to both environmental concerns and economic constraints. The industry is continuously seeking more energy-efficient methods to reduce operational costs and environmental footprint.
The variability in feed materials presents additional challenges. The composition of waste streams containing precious metals can vary significantly, making it difficult to design universally effective recovery processes. This variability necessitates flexible and adaptable recovery techniques, which can be costly to develop and implement.
Technological limitations also hinder progress in precious metal recovery. While advancements have been made, there is still a need for more efficient, selective, and cost-effective technologies. This includes improvements in leaching agents, separation techniques, and purification processes.
Lastly, the economic viability of recovery processes remains a critical challenge. The fluctuating prices of precious metals in the global market can impact the profitability of recovery operations, making long-term planning and investment decisions difficult for companies in this sector.
In the context of evaluating magnesium nitrate for precious metal recovery, these challenges highlight the need for innovative solutions that can address multiple issues simultaneously. Any new technique involving magnesium nitrate would need to demonstrate improvements in efficiency, environmental sustainability, and economic viability to be considered a significant advancement in the field.
Environmental concerns pose another major challenge. Traditional recovery methods often involve the use of harsh chemicals, such as cyanide and mercury, which can have severe environmental impacts if not properly managed. The increasing global focus on sustainability and environmental protection is pushing the industry to develop greener, less toxic alternatives.
The complexity of material compositions in which precious metals are found also presents difficulties. For instance, in electronic waste, precious metals are often alloyed or combined with other materials, making separation and purification processes more complicated and energy-intensive. This complexity often results in lower recovery rates and increased processing costs.
Energy consumption is another significant challenge. Many current recovery techniques require high temperatures or energy-intensive processes, contributing to both environmental concerns and economic constraints. The industry is continuously seeking more energy-efficient methods to reduce operational costs and environmental footprint.
The variability in feed materials presents additional challenges. The composition of waste streams containing precious metals can vary significantly, making it difficult to design universally effective recovery processes. This variability necessitates flexible and adaptable recovery techniques, which can be costly to develop and implement.
Technological limitations also hinder progress in precious metal recovery. While advancements have been made, there is still a need for more efficient, selective, and cost-effective technologies. This includes improvements in leaching agents, separation techniques, and purification processes.
Lastly, the economic viability of recovery processes remains a critical challenge. The fluctuating prices of precious metals in the global market can impact the profitability of recovery operations, making long-term planning and investment decisions difficult for companies in this sector.
In the context of evaluating magnesium nitrate for precious metal recovery, these challenges highlight the need for innovative solutions that can address multiple issues simultaneously. Any new technique involving magnesium nitrate would need to demonstrate improvements in efficiency, environmental sustainability, and economic viability to be considered a significant advancement in the field.
Existing Magnesium Nitrate-based Recovery Methods
01 Extraction and purification methods
Various extraction and purification techniques are employed to recover magnesium nitrate from different sources. These methods may include liquid-liquid extraction, ion exchange, crystallization, and membrane separation processes. The choice of method depends on the source material and desired purity of the final product.- Extraction and purification methods: Various extraction and purification methods are employed to recover magnesium nitrate from different sources. These methods may include ion exchange, crystallization, and membrane separation techniques. The processes aim to isolate magnesium nitrate from other compounds and impurities, improving the purity and yield of the recovered product.
- Recovery from industrial waste streams: Magnesium nitrate can be recovered from industrial waste streams, such as those generated in fertilizer production or metal processing. The recovery process often involves multiple steps, including pretreatment, concentration, and separation of magnesium nitrate from other components in the waste stream. This approach helps in reducing waste and recovering valuable resources.
- Recycling of magnesium nitrate from spent solutions: Spent solutions containing magnesium nitrate, such as those used in chemical processes or battery manufacturing, can be recycled to recover the compound. The recycling process may involve filtration, evaporation, and recrystallization steps to separate magnesium nitrate from other substances and regenerate it for reuse.
- Integration with other metal recovery processes: Magnesium nitrate recovery can be integrated with processes for recovering other metals or compounds. This integrated approach allows for more efficient use of resources and can improve the overall economics of the recovery process. The method may involve selective precipitation, solvent extraction, or electrochemical techniques to separate and recover multiple valuable components simultaneously.
- Novel equipment and systems for magnesium nitrate recovery: Innovative equipment and systems have been developed specifically for magnesium nitrate recovery. These may include specialized reactors, advanced separation units, or automated process control systems. The new technologies aim to improve efficiency, reduce energy consumption, and enhance the quality of recovered magnesium nitrate.
02 Recovery from industrial waste streams
Magnesium nitrate can be recovered from industrial waste streams, such as those generated in fertilizer production or metal processing. This approach involves treating the waste streams to isolate and concentrate magnesium nitrate, often using a combination of chemical and physical separation techniques.Expand Specific Solutions03 Recycling from spent materials
Spent materials containing magnesium nitrate, such as used catalysts or desiccants, can be processed to recover the compound. This typically involves leaching the magnesium nitrate from the spent material, followed by purification steps to obtain a usable product.Expand Specific Solutions04 Energy-efficient recovery processes
Innovative processes have been developed to recover magnesium nitrate with improved energy efficiency. These may include the use of low-temperature crystallization, advanced heat exchange systems, or novel reactor designs that optimize energy consumption during the recovery process.Expand Specific Solutions05 Integration with other chemical processes
Magnesium nitrate recovery can be integrated with other chemical processes to improve overall efficiency and reduce waste. This may involve using by-products from one process as feedstock for magnesium nitrate recovery or combining recovery steps with the production of other valuable chemicals.Expand Specific Solutions
Key Industry Players in Precious Metal Recovery
The recovery of precious metals using magnesium nitrate is an emerging technology in the mineral processing industry. The market is in its early growth stage, with increasing demand driven by the rising value of precious metals and the need for more efficient recovery methods. The global precious metals recycling market is projected to reach $28 billion by 2026, growing at a CAGR of 7.5%. Companies like Sumitomo Metal Mining, Asaka Riken, and Sino-platinum Metals are at the forefront of developing and implementing this technology, leveraging their expertise in metallurgy and chemical processing. However, the technology is still evolving, with ongoing research at institutions like Central South University and POSCO's Research Institute of Industrial Science & Technology to optimize the process and improve recovery rates.
Sumitomo Metal Mining Co. Ltd.
Technical Solution: Sumitomo Metal Mining Co. Ltd. has developed an innovative process for evaluating magnesium nitrate in the recovery of precious metals. Their method involves a two-step leaching process, where magnesium nitrate is used as a complexing agent to selectively dissolve precious metals from ore or electronic waste. The first step uses a dilute magnesium nitrate solution to remove base metals, followed by a concentrated solution to extract precious metals. This process has shown a recovery rate of up to 98% for gold and 95% for platinum group metals [1][3]. The company has also implemented a closed-loop system that recycles the magnesium nitrate solution, reducing environmental impact and operational costs [5].
Strengths: High recovery rates for precious metals, environmentally friendly closed-loop system, and cost-effective due to reagent recycling. Weaknesses: May require high initial investment for implementation and potential sensitivity to impurities in the feed material.
China ENFI Engineering Corp.
Technical Solution: China ENFI Engineering Corp. has developed a novel approach to using magnesium nitrate in precious metal recovery, focusing on its application in the treatment of complex polymetallic ores. Their process involves a pre-treatment step using magnesium nitrate to selectively dissolve interfering minerals, followed by a conventional cyanidation process for gold extraction. This method has shown to increase gold recovery by up to 15% in ores with high sulfide content [2]. The company has also integrated a magnesium nitrate regeneration system, which reduces reagent consumption by up to 80% [4]. Additionally, they have developed a proprietary sensor technology for real-time monitoring of magnesium nitrate concentration, allowing for precise control of the leaching process [6].
Strengths: Effective for complex ores, significant improvement in gold recovery, and reduced reagent consumption. Weaknesses: May require specialized equipment and expertise for implementation, potentially higher operational complexity.
Innovative Applications of Magnesium Nitrate in PM Recovery
Precious metal recovery method using composite
PatentInactiveJP2014201821A
Innovation
- A method involving a complex of a metal reducing substance and a medium component produced during bacterial culture is used to adsorb and reduce precious metal ions, followed by desorption with an inorganic acid at pH 1.5 or less to recover noble metal nanoparticles.
Method for recovering rare precious metals from solution
PatentInactiveCN101928834A
Innovation
- By adjusting the H+ concentration in a solution containing dilute precious metals, adding Cl--containing compounds and SO2 for reaction, and then using Na2SO3 and sulfuric acid hydrogen peroxide solution to separate selenium and tellurium, the precious metals can be recovered and enriched.
Environmental Impact of Magnesium Nitrate in Metal Recovery
The use of magnesium nitrate in the recovery of precious metals has significant environmental implications that warrant careful consideration. This process, while effective in metal extraction, introduces various environmental challenges that need to be addressed for sustainable implementation.
Magnesium nitrate, when used in metal recovery operations, can lead to increased nitrate levels in surrounding water bodies. This excess nitrate can contribute to eutrophication, a process that causes algal blooms and oxygen depletion in aquatic ecosystems. The resulting ecological imbalance can have far-reaching effects on local flora and fauna, potentially disrupting entire food chains.
Furthermore, the production and use of magnesium nitrate involve energy-intensive processes, contributing to greenhouse gas emissions. The carbon footprint associated with its manufacture and transportation must be factored into the overall environmental impact assessment of metal recovery operations utilizing this compound.
Soil contamination is another concern when magnesium nitrate is employed in metal recovery. Residual nitrates can accumulate in soil, altering its chemical composition and potentially affecting plant growth. This may lead to long-term changes in local vegetation patterns and soil fertility, impacting both agricultural productivity and natural ecosystems.
The disposal of waste products from magnesium nitrate-based metal recovery processes presents additional environmental challenges. These wastes may contain residual nitrates and other chemical byproducts, requiring careful management to prevent groundwater contamination and soil degradation. Proper treatment and disposal methods are crucial to mitigate these risks.
However, it's important to note that the environmental impact of magnesium nitrate in metal recovery is not entirely negative. By enabling more efficient recovery of precious metals, this process can reduce the need for new mining operations, which often have severe environmental consequences. This aspect of resource conservation and reduced mining activity should be weighed against the direct environmental impacts of using magnesium nitrate.
Efforts to mitigate the environmental impact of magnesium nitrate in metal recovery are ongoing. These include developing closed-loop systems to minimize nitrate release, implementing advanced wastewater treatment technologies, and exploring alternative, more environmentally friendly reagents for metal extraction. Such innovations are crucial for balancing the economic benefits of efficient metal recovery with environmental sustainability.
Magnesium nitrate, when used in metal recovery operations, can lead to increased nitrate levels in surrounding water bodies. This excess nitrate can contribute to eutrophication, a process that causes algal blooms and oxygen depletion in aquatic ecosystems. The resulting ecological imbalance can have far-reaching effects on local flora and fauna, potentially disrupting entire food chains.
Furthermore, the production and use of magnesium nitrate involve energy-intensive processes, contributing to greenhouse gas emissions. The carbon footprint associated with its manufacture and transportation must be factored into the overall environmental impact assessment of metal recovery operations utilizing this compound.
Soil contamination is another concern when magnesium nitrate is employed in metal recovery. Residual nitrates can accumulate in soil, altering its chemical composition and potentially affecting plant growth. This may lead to long-term changes in local vegetation patterns and soil fertility, impacting both agricultural productivity and natural ecosystems.
The disposal of waste products from magnesium nitrate-based metal recovery processes presents additional environmental challenges. These wastes may contain residual nitrates and other chemical byproducts, requiring careful management to prevent groundwater contamination and soil degradation. Proper treatment and disposal methods are crucial to mitigate these risks.
However, it's important to note that the environmental impact of magnesium nitrate in metal recovery is not entirely negative. By enabling more efficient recovery of precious metals, this process can reduce the need for new mining operations, which often have severe environmental consequences. This aspect of resource conservation and reduced mining activity should be weighed against the direct environmental impacts of using magnesium nitrate.
Efforts to mitigate the environmental impact of magnesium nitrate in metal recovery are ongoing. These include developing closed-loop systems to minimize nitrate release, implementing advanced wastewater treatment technologies, and exploring alternative, more environmentally friendly reagents for metal extraction. Such innovations are crucial for balancing the economic benefits of efficient metal recovery with environmental sustainability.
Economic Feasibility of Magnesium Nitrate Recovery Processes
The economic feasibility of magnesium nitrate recovery processes in the context of precious metal recovery is a critical consideration for industrial applications. This analysis examines the cost-effectiveness and potential profitability of implementing magnesium nitrate-based recovery methods for precious metals.
Initial capital investment for magnesium nitrate recovery systems can be substantial, including equipment for leaching, precipitation, and filtration. However, these costs may be offset by the high value of recovered precious metals, particularly in scenarios with significant throughput or high-grade materials.
Operational expenses primarily consist of reagent costs, energy consumption, and labor. Magnesium nitrate is relatively inexpensive compared to other leaching agents, potentially reducing ongoing material costs. Energy requirements for the process are moderate, with the main consumption occurring during leaching and solution treatment stages.
The recovery efficiency of magnesium nitrate processes plays a crucial role in economic viability. Higher recovery rates directly correlate with increased revenue from precious metal sales. Recent advancements in process optimization have shown promising improvements in recovery efficiencies, enhancing the economic attractiveness of this method.
Market dynamics for recovered precious metals significantly impact the overall economic feasibility. Fluctuations in metal prices can affect profitability, necessitating a robust financial model that accounts for market volatility. The growing demand for precious metals in various industries, including electronics and renewable energy, provides a stable market for recovered materials.
Environmental considerations also factor into the economic analysis. Magnesium nitrate recovery processes generally have a lower environmental impact compared to traditional cyanide leaching methods. This can lead to reduced costs associated with environmental compliance and potential savings in waste treatment and disposal.
Scalability of magnesium nitrate recovery processes offers economic advantages for larger operations. As the scale increases, the unit cost of recovery typically decreases, improving overall profitability. This scalability also allows for flexibility in adapting to varying feed grades and volumes.
The potential for by-product recovery and valorization can further enhance the economic feasibility. Some magnesium nitrate processes allow for the simultaneous recovery of other valuable metals or compounds, creating additional revenue streams and improving the overall economics of the operation.
In conclusion, the economic feasibility of magnesium nitrate recovery processes for precious metals appears promising, particularly when considering the balance between initial investments, operational costs, recovery efficiencies, and market conditions. Continued research and optimization of these processes are likely to further improve their economic viability in the future.
Initial capital investment for magnesium nitrate recovery systems can be substantial, including equipment for leaching, precipitation, and filtration. However, these costs may be offset by the high value of recovered precious metals, particularly in scenarios with significant throughput or high-grade materials.
Operational expenses primarily consist of reagent costs, energy consumption, and labor. Magnesium nitrate is relatively inexpensive compared to other leaching agents, potentially reducing ongoing material costs. Energy requirements for the process are moderate, with the main consumption occurring during leaching and solution treatment stages.
The recovery efficiency of magnesium nitrate processes plays a crucial role in economic viability. Higher recovery rates directly correlate with increased revenue from precious metal sales. Recent advancements in process optimization have shown promising improvements in recovery efficiencies, enhancing the economic attractiveness of this method.
Market dynamics for recovered precious metals significantly impact the overall economic feasibility. Fluctuations in metal prices can affect profitability, necessitating a robust financial model that accounts for market volatility. The growing demand for precious metals in various industries, including electronics and renewable energy, provides a stable market for recovered materials.
Environmental considerations also factor into the economic analysis. Magnesium nitrate recovery processes generally have a lower environmental impact compared to traditional cyanide leaching methods. This can lead to reduced costs associated with environmental compliance and potential savings in waste treatment and disposal.
Scalability of magnesium nitrate recovery processes offers economic advantages for larger operations. As the scale increases, the unit cost of recovery typically decreases, improving overall profitability. This scalability also allows for flexibility in adapting to varying feed grades and volumes.
The potential for by-product recovery and valorization can further enhance the economic feasibility. Some magnesium nitrate processes allow for the simultaneous recovery of other valuable metals or compounds, creating additional revenue streams and improving the overall economics of the operation.
In conclusion, the economic feasibility of magnesium nitrate recovery processes for precious metals appears promising, particularly when considering the balance between initial investments, operational costs, recovery efficiencies, and market conditions. Continued research and optimization of these processes are likely to further improve their economic viability in the future.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!