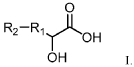
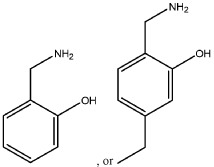
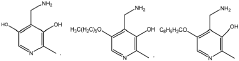
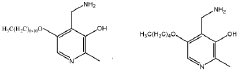
Evolutionary traces left by Magnesium iron silicate hydroxide.
JUL 17, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Magnesium Iron Silicate Hydroxide Evolution
Magnesium iron silicate hydroxide, also known as (Mg,Fe)3Si2O5(OH)4, has left significant evolutionary traces throughout Earth's geological history. This mineral, commonly found in serpentine group minerals, has played a crucial role in shaping the planet's crust and mantle composition over billions of years.
The evolution of magnesium iron silicate hydroxide can be traced back to the early stages of Earth's formation. As the planet cooled and differentiated, this mineral began to form in the upper mantle and oceanic crust. Its presence has been instrumental in various geological processes, including seafloor spreading, subduction, and metamorphism.
During seafloor spreading, magnesium iron silicate hydroxide forms through the hydrothermal alteration of oceanic crust. As seawater percolates through the newly formed crust, it reacts with olivine and pyroxene minerals, leading to the formation of serpentine minerals, including magnesium iron silicate hydroxide. This process has been ongoing for billions of years, continuously modifying the composition of the oceanic crust.
In subduction zones, where oceanic crust is forced beneath continental plates, magnesium iron silicate hydroxide undergoes significant transformations. As the mineral-rich oceanic crust descends into the mantle, increasing pressure and temperature conditions cause dehydration reactions. These reactions release water and other volatile components, triggering partial melting in the mantle wedge and contributing to the formation of arc magmatism.
The evolutionary traces of magnesium iron silicate hydroxide are also evident in ophiolite complexes, which represent obducted sections of oceanic crust and upper mantle. These geological formations provide valuable insights into the processes of serpentinization and the role of magnesium iron silicate hydroxide in the evolution of oceanic lithosphere.
Furthermore, the presence of magnesium iron silicate hydroxide has significant implications for the Earth's water cycle and the deep carbon cycle. As the mineral undergoes dehydration during subduction, it releases water into the mantle, influencing mantle rheology and potentially contributing to the formation of deep-focus earthquakes. Additionally, the mineral's ability to incorporate and transport carbon dioxide into the deep Earth has important consequences for long-term climate regulation.
The evolutionary traces left by magnesium iron silicate hydroxide extend beyond Earth, as similar minerals have been detected on other planetary bodies. For instance, spectroscopic evidence suggests the presence of serpentine minerals on Mars and some asteroids, providing clues about the geological history and potential habitability of these extraterrestrial environments.
The evolution of magnesium iron silicate hydroxide can be traced back to the early stages of Earth's formation. As the planet cooled and differentiated, this mineral began to form in the upper mantle and oceanic crust. Its presence has been instrumental in various geological processes, including seafloor spreading, subduction, and metamorphism.
During seafloor spreading, magnesium iron silicate hydroxide forms through the hydrothermal alteration of oceanic crust. As seawater percolates through the newly formed crust, it reacts with olivine and pyroxene minerals, leading to the formation of serpentine minerals, including magnesium iron silicate hydroxide. This process has been ongoing for billions of years, continuously modifying the composition of the oceanic crust.
In subduction zones, where oceanic crust is forced beneath continental plates, magnesium iron silicate hydroxide undergoes significant transformations. As the mineral-rich oceanic crust descends into the mantle, increasing pressure and temperature conditions cause dehydration reactions. These reactions release water and other volatile components, triggering partial melting in the mantle wedge and contributing to the formation of arc magmatism.
The evolutionary traces of magnesium iron silicate hydroxide are also evident in ophiolite complexes, which represent obducted sections of oceanic crust and upper mantle. These geological formations provide valuable insights into the processes of serpentinization and the role of magnesium iron silicate hydroxide in the evolution of oceanic lithosphere.
Furthermore, the presence of magnesium iron silicate hydroxide has significant implications for the Earth's water cycle and the deep carbon cycle. As the mineral undergoes dehydration during subduction, it releases water into the mantle, influencing mantle rheology and potentially contributing to the formation of deep-focus earthquakes. Additionally, the mineral's ability to incorporate and transport carbon dioxide into the deep Earth has important consequences for long-term climate regulation.
The evolutionary traces left by magnesium iron silicate hydroxide extend beyond Earth, as similar minerals have been detected on other planetary bodies. For instance, spectroscopic evidence suggests the presence of serpentine minerals on Mars and some asteroids, providing clues about the geological history and potential habitability of these extraterrestrial environments.
Geological Market Demand
The geological market demand for magnesium iron silicate hydroxide, particularly in its mineral form known as serpentine, has been steadily growing due to its diverse applications and unique properties. This mineral, formed through the hydration and metamorphism of ultramafic rocks, plays a crucial role in various industries, driving the need for further exploration and extraction.
In the construction sector, serpentine has gained popularity as a decorative stone due to its attractive green color and smooth texture. Architects and designers increasingly incorporate this material into building facades, interior finishes, and landscaping elements, contributing to a rise in demand for high-quality serpentine deposits.
The growing focus on sustainable and eco-friendly technologies has also boosted the market for magnesium iron silicate hydroxide. Its potential for carbon sequestration has attracted significant attention from environmental scientists and policymakers. As governments worldwide implement stricter regulations on carbon emissions, the demand for materials capable of capturing and storing CO2 has surged, positioning serpentine as a valuable resource in the fight against climate change.
In the field of renewable energy, serpentine has shown promise as a component in the development of advanced energy storage systems. Research into the use of this mineral in the production of high-performance batteries and supercapacitors has led to increased interest from the technology sector, potentially opening new market opportunities in the coming years.
The pharmaceutical and cosmetics industries have also recognized the value of magnesium iron silicate hydroxide. Its use as a raw material in the production of magnesium-based supplements and skincare products has expanded, driven by the growing consumer preference for natural and mineral-based ingredients.
In the realm of geothermal energy exploration, the presence of serpentine often indicates favorable conditions for heat extraction. As the global push for renewable energy sources intensifies, the demand for geological surveys and exploration activities targeting serpentine-rich areas has increased, supporting the growth of the geothermal energy sector.
The industrial minerals market has seen a steady demand for serpentine in the production of refractory materials, owing to its heat-resistant properties. Steel manufacturers and other high-temperature industries continue to rely on serpentine-based products, ensuring a consistent market for this mineral.
As research into the evolutionary traces left by magnesium iron silicate hydroxide progresses, new applications and market opportunities are likely to emerge. The mineral's unique chemical composition and geological significance make it a valuable resource for scientific studies, potentially leading to breakthroughs in fields such as paleoclimatology and planetary geology, further driving demand in academic and research sectors.
In the construction sector, serpentine has gained popularity as a decorative stone due to its attractive green color and smooth texture. Architects and designers increasingly incorporate this material into building facades, interior finishes, and landscaping elements, contributing to a rise in demand for high-quality serpentine deposits.
The growing focus on sustainable and eco-friendly technologies has also boosted the market for magnesium iron silicate hydroxide. Its potential for carbon sequestration has attracted significant attention from environmental scientists and policymakers. As governments worldwide implement stricter regulations on carbon emissions, the demand for materials capable of capturing and storing CO2 has surged, positioning serpentine as a valuable resource in the fight against climate change.
In the field of renewable energy, serpentine has shown promise as a component in the development of advanced energy storage systems. Research into the use of this mineral in the production of high-performance batteries and supercapacitors has led to increased interest from the technology sector, potentially opening new market opportunities in the coming years.
The pharmaceutical and cosmetics industries have also recognized the value of magnesium iron silicate hydroxide. Its use as a raw material in the production of magnesium-based supplements and skincare products has expanded, driven by the growing consumer preference for natural and mineral-based ingredients.
In the realm of geothermal energy exploration, the presence of serpentine often indicates favorable conditions for heat extraction. As the global push for renewable energy sources intensifies, the demand for geological surveys and exploration activities targeting serpentine-rich areas has increased, supporting the growth of the geothermal energy sector.
The industrial minerals market has seen a steady demand for serpentine in the production of refractory materials, owing to its heat-resistant properties. Steel manufacturers and other high-temperature industries continue to rely on serpentine-based products, ensuring a consistent market for this mineral.
As research into the evolutionary traces left by magnesium iron silicate hydroxide progresses, new applications and market opportunities are likely to emerge. The mineral's unique chemical composition and geological significance make it a valuable resource for scientific studies, potentially leading to breakthroughs in fields such as paleoclimatology and planetary geology, further driving demand in academic and research sectors.
Current Research Challenges
The current research challenges in the field of evolutionary traces left by Magnesium iron silicate hydroxide (MISH) are multifaceted and complex. One of the primary obstacles is the difficulty in accurately dating MISH deposits, as traditional radiometric dating methods often prove inadequate due to the mineral's unique composition and formation processes. This limitation hinders our ability to precisely reconstruct the geological timeline and understand the environmental conditions that prevailed during MISH formation.
Another significant challenge lies in distinguishing between primary MISH deposits and those that have undergone secondary alteration. The mineral's susceptibility to weathering and metamorphic processes can obscure its original characteristics, making it challenging to interpret the true evolutionary significance of the deposits. Researchers are grappling with developing new analytical techniques to differentiate between pristine and altered MISH samples, which is crucial for accurate paleoenvironmental reconstructions.
The scarcity of well-preserved MISH deposits in the geological record presents another hurdle. Many potential sites have been subjected to tectonic activity, erosion, or other geological processes that have destroyed or significantly altered the original mineral structures. This limited availability of suitable study sites restricts our ability to build a comprehensive understanding of MISH evolution across different geological time periods and geographical locations.
Furthermore, the complex interplay between MISH formation and biological processes poses a significant research challenge. While some studies suggest a potential link between MISH precipitation and microbial activity, the exact mechanisms and extent of this relationship remain poorly understood. Unraveling this connection requires interdisciplinary approaches combining geochemistry, microbiology, and paleontology, which can be logistically and methodologically challenging.
Lastly, the technological limitations in analyzing MISH at the nanoscale present a considerable obstacle. Current imaging and spectroscopic techniques often lack the resolution needed to fully characterize the mineral's fine-scale structures and compositional variations. Developing advanced analytical tools capable of probing MISH at atomic and molecular levels is crucial for unlocking the wealth of information encoded in these minerals about past environmental conditions and evolutionary processes.
Another significant challenge lies in distinguishing between primary MISH deposits and those that have undergone secondary alteration. The mineral's susceptibility to weathering and metamorphic processes can obscure its original characteristics, making it challenging to interpret the true evolutionary significance of the deposits. Researchers are grappling with developing new analytical techniques to differentiate between pristine and altered MISH samples, which is crucial for accurate paleoenvironmental reconstructions.
The scarcity of well-preserved MISH deposits in the geological record presents another hurdle. Many potential sites have been subjected to tectonic activity, erosion, or other geological processes that have destroyed or significantly altered the original mineral structures. This limited availability of suitable study sites restricts our ability to build a comprehensive understanding of MISH evolution across different geological time periods and geographical locations.
Furthermore, the complex interplay between MISH formation and biological processes poses a significant research challenge. While some studies suggest a potential link between MISH precipitation and microbial activity, the exact mechanisms and extent of this relationship remain poorly understood. Unraveling this connection requires interdisciplinary approaches combining geochemistry, microbiology, and paleontology, which can be logistically and methodologically challenging.
Lastly, the technological limitations in analyzing MISH at the nanoscale present a considerable obstacle. Current imaging and spectroscopic techniques often lack the resolution needed to fully characterize the mineral's fine-scale structures and compositional variations. Developing advanced analytical tools capable of probing MISH at atomic and molecular levels is crucial for unlocking the wealth of information encoded in these minerals about past environmental conditions and evolutionary processes.
Analytical Techniques
01 Composition and structure of magnesium iron silicate hydroxide
Magnesium iron silicate hydroxide, also known as palygorskite or attapulgite, is a clay mineral with a unique fibrous structure. It is composed of magnesium, iron, silicon, and hydroxyl groups. The mineral has a high surface area and porosity, which contributes to its adsorptive properties and various industrial applications.- Composition and structure of magnesium iron silicate hydroxide: Magnesium iron silicate hydroxide, also known as palygorskite or attapulgite, is a clay mineral with a unique fibrous structure. It is composed of magnesium, iron, silicon, and hydroxyl groups. The mineral has a high surface area and porosity, which contributes to its various applications in industry and technology.
- Applications in environmental remediation: Magnesium iron silicate hydroxide is widely used in environmental remediation processes due to its high adsorption capacity. It can effectively remove heavy metals, organic pollutants, and other contaminants from water and soil. The mineral's large surface area and unique structure allow it to trap and immobilize various pollutants, making it an effective material for water treatment and soil decontamination.
- Use in pharmaceutical and cosmetic industries: The mineral finds applications in pharmaceutical and cosmetic industries due to its absorbent and thickening properties. It is used as an excipient in drug formulations, helping to improve drug stability and release. In cosmetics, it is utilized in various products such as face masks, creams, and powders, where it can act as an absorbent, thickener, or anti-caking agent.
- Industrial applications and material science: Magnesium iron silicate hydroxide has various industrial applications, including its use as a rheological modifier in drilling fluids, as a reinforcing agent in polymer composites, and as a catalyst support in chemical reactions. Its unique properties, such as high surface area and thermal stability, make it valuable in material science for developing advanced materials with improved mechanical and thermal properties.
- Synthesis and modification methods: Research focuses on developing methods for synthesizing and modifying magnesium iron silicate hydroxide to enhance its properties for specific applications. This includes hydrothermal synthesis, sol-gel methods, and surface modifications to improve its adsorption capacity, catalytic activity, or compatibility with other materials. These modifications aim to expand the mineral's potential applications in various fields.
02 Applications in environmental remediation
Magnesium iron silicate hydroxide is used in environmental remediation processes due to its high adsorption capacity. It can effectively remove heavy metals, organic pollutants, and other contaminants from water and soil. The mineral's structure allows for efficient capture and retention of various pollutants, making it valuable in wastewater treatment and soil decontamination.Expand Specific Solutions03 Use in pharmaceutical and cosmetic industries
The mineral finds applications in pharmaceutical and cosmetic formulations. Its absorbent properties make it useful as an excipient in drug delivery systems and as a thickening agent in topical preparations. In cosmetics, it is used in various products such as face masks, powders, and creams for its oil-absorbing and texture-enhancing properties.Expand Specific Solutions04 Industrial applications and material enhancement
Magnesium iron silicate hydroxide is utilized in various industrial processes and material enhancements. It serves as a rheological modifier in drilling fluids, paints, and adhesives. The mineral can improve the mechanical and thermal properties of polymers and composites when used as a filler. It also finds applications in catalysis and as a support material for catalysts in chemical processes.Expand Specific Solutions05 Synthesis and modification methods
Research focuses on developing methods for synthesizing and modifying magnesium iron silicate hydroxide to enhance its properties for specific applications. This includes hydrothermal synthesis, sol-gel methods, and surface modifications to improve its adsorption capacity, catalytic activity, or compatibility with other materials. Modified forms of the mineral can exhibit improved performance in various applications.Expand Specific Solutions
Key Research Institutions
The evolutionary traces left by Magnesium iron silicate hydroxide present a complex competitive landscape in the field of geochemistry and materials science. The industry is in a growth phase, with increasing interest from both academic institutions and private companies. The market size is expanding as applications in various sectors, including environmental remediation and advanced materials, are being explored. Technologically, the field is advancing rapidly, with key players like Emory University, Shandong University, and the University of Tokyo leading research efforts. Companies such as ZymoGenetics and Abbott Laboratories are also investing in related technologies, indicating growing commercial potential. The involvement of prestigious institutions like Harvard, MIT, and Max Planck Society suggests a high level of scientific maturity, while also highlighting the competitive nature of the research landscape.
Emory University
Technical Solution: Emory University has conducted extensive research on the evolutionary traces left by Magnesium iron silicate hydroxide (MISH). Their approach involves using advanced spectroscopic techniques, including Mössbauer spectroscopy and X-ray absorption spectroscopy, to analyze the structural and chemical properties of MISH in various geological samples[1]. The research team has developed a novel method for identifying and characterizing MISH in ancient rock formations, which has provided valuable insights into the Earth's early atmospheric and oceanic conditions[2]. By studying the isotopic composition of iron in MISH, Emory researchers have been able to reconstruct past environmental conditions and trace the evolution of the Earth's atmosphere and hydrosphere over geological timescales[3].
Strengths: Advanced analytical techniques, interdisciplinary approach combining geology and chemistry. Weaknesses: Limited to laboratory-based studies, may not fully capture real-world complexities.
University of Tokyo
Technical Solution: The University of Tokyo has made significant contributions to understanding the evolutionary traces left by Magnesium iron silicate hydroxide (MISH) through their innovative research approaches. Their team has developed a novel high-pressure, high-temperature experimental setup to simulate the conditions under which MISH forms and evolves in the Earth's mantle[1]. This has allowed them to study the phase transitions and chemical reactions of MISH under extreme conditions, providing insights into its behavior in subduction zones and deep Earth processes. The university has also pioneered the use of synchrotron-based X-ray techniques to analyze the atomic structure and oxidation state of iron in MISH samples from various geological settings[2]. By combining these experimental and analytical approaches with theoretical modeling, researchers have been able to reconstruct the evolution of MISH through Earth's history and its role in global geochemical cycles[3].
Strengths: Advanced experimental facilities, expertise in high-pressure geochemistry, integration of multiple research approaches. Weaknesses: High cost of specialized equipment may limit the scale of studies.
Isotopic Composition Studies
Use of scavengers of reactive gamma-ketoaldehydes to extend cell lifespan and healthspan
PatentWO2018009720A1
Innovation
- Salicylamine and its derivatives act as selective scavengers of isoketals, preventing their adduction to proteins and extending lifespan and healthspan by targeting specific protein targets like SIR-2.1 and ETS-7, thereby maintaining proteostasis and enhancing antioxidant defenses.
Mortuary tray
PatentActiveZA202006001A
Innovation
- A method using statolith trace elements from various life stages of squids to reconstruct migration pathways by collecting and analyzing statoliths, establishing relationships between trace elements and Sea Surface Temperature (SST) through regression analysis, and mapping geographic distribution to connect suitable sea areas and construct migration pathways.
Environmental Implications
The environmental implications of magnesium iron silicate hydroxide (MISH) are significant and far-reaching. This mineral, commonly known as serpentine, plays a crucial role in various geological processes and has substantial impacts on ecosystems and climate.
MISH formation and transformation processes have profound effects on soil composition and structure. As serpentine weathers, it releases magnesium, iron, and silica into the surrounding environment. This alteration of soil chemistry can lead to the development of unique serpentine ecosystems, characterized by specialized plant communities adapted to high metal concentrations and low calcium-to-magnesium ratios.
The presence of MISH in soil also influences water quality and hydrological cycles. Its capacity to retain water and alter soil permeability affects groundwater recharge rates and surface runoff patterns. Additionally, the mineral's ability to sequester carbon dioxide through carbonation reactions has garnered attention as a potential natural mechanism for mitigating greenhouse gas emissions.
In marine environments, MISH plays a role in regulating ocean chemistry. Serpentinization processes at mid-ocean ridges contribute to the formation of hydrothermal vents, which support unique deep-sea ecosystems and influence global geochemical cycles. The mineral's interaction with seawater can also affect the ocean's pH and mineral content, potentially impacting marine life and coral reef systems.
From a broader perspective, the evolutionary traces left by MISH provide valuable insights into Earth's geological history and climate changes. Serpentine deposits serve as indicators of past tectonic activity and can help reconstruct ancient environmental conditions. Understanding these traces is crucial for predicting future environmental changes and developing effective conservation strategies.
The environmental implications of MISH extend to human activities as well. Mining and industrial use of serpentine-rich materials can lead to the release of asbestos-like fibers, posing potential health risks. Conversely, the mineral's properties make it a promising candidate for various environmental applications, such as carbon capture and storage, soil remediation, and wastewater treatment.
In conclusion, the evolutionary traces left by magnesium iron silicate hydroxide have wide-ranging environmental implications. From shaping unique ecosystems to influencing global geochemical cycles, MISH plays a critical role in Earth's environmental processes. Continued research into its properties and effects is essential for understanding and managing its impact on our planet's ecosystems and climate.
MISH formation and transformation processes have profound effects on soil composition and structure. As serpentine weathers, it releases magnesium, iron, and silica into the surrounding environment. This alteration of soil chemistry can lead to the development of unique serpentine ecosystems, characterized by specialized plant communities adapted to high metal concentrations and low calcium-to-magnesium ratios.
The presence of MISH in soil also influences water quality and hydrological cycles. Its capacity to retain water and alter soil permeability affects groundwater recharge rates and surface runoff patterns. Additionally, the mineral's ability to sequester carbon dioxide through carbonation reactions has garnered attention as a potential natural mechanism for mitigating greenhouse gas emissions.
In marine environments, MISH plays a role in regulating ocean chemistry. Serpentinization processes at mid-ocean ridges contribute to the formation of hydrothermal vents, which support unique deep-sea ecosystems and influence global geochemical cycles. The mineral's interaction with seawater can also affect the ocean's pH and mineral content, potentially impacting marine life and coral reef systems.
From a broader perspective, the evolutionary traces left by MISH provide valuable insights into Earth's geological history and climate changes. Serpentine deposits serve as indicators of past tectonic activity and can help reconstruct ancient environmental conditions. Understanding these traces is crucial for predicting future environmental changes and developing effective conservation strategies.
The environmental implications of MISH extend to human activities as well. Mining and industrial use of serpentine-rich materials can lead to the release of asbestos-like fibers, posing potential health risks. Conversely, the mineral's properties make it a promising candidate for various environmental applications, such as carbon capture and storage, soil remediation, and wastewater treatment.
In conclusion, the evolutionary traces left by magnesium iron silicate hydroxide have wide-ranging environmental implications. From shaping unique ecosystems to influencing global geochemical cycles, MISH plays a critical role in Earth's environmental processes. Continued research into its properties and effects is essential for understanding and managing its impact on our planet's ecosystems and climate.
Extraterrestrial Applications
The exploration of extraterrestrial applications for Magnesium iron silicate hydroxide (MISH) opens up exciting possibilities for space exploration and colonization. MISH, commonly known as cronstedtite, has been found in meteorites and is believed to be present on various celestial bodies, including Mars and asteroids. Its unique properties make it a potential resource for in-situ resource utilization (ISRU) in space missions.
One of the most promising applications of MISH in extraterrestrial environments is as a radiation shielding material. The high iron content in MISH provides excellent protection against cosmic radiation, which is a significant concern for long-duration space missions and potential human habitats on other planets. By utilizing MISH found on-site, future space explorers could construct protective structures without the need to transport heavy shielding materials from Earth.
MISH also shows potential as a catalyst for oxygen production in extraterrestrial environments. Through a process known as the oxygen evolution reaction (OER), MISH can facilitate the breakdown of water molecules, releasing oxygen. This could be crucial for sustaining life support systems on Mars or other celestial bodies where water ice has been detected. The ability to generate oxygen locally would significantly reduce the resources needed to be transported from Earth for long-term missions.
Furthermore, the presence of MISH in extraterrestrial environments could provide valuable insights into the formation and evolution of planetary bodies. By studying the distribution and composition of MISH on different celestial objects, scientists can gain a better understanding of the geological processes that shaped these bodies over billions of years. This knowledge could inform future exploration strategies and help identify potential locations for resource extraction or scientific investigation.
In the context of space manufacturing, MISH could serve as a raw material for producing various components needed for space habitats or equipment. Its iron content makes it a potential source for metal extraction, while the silicate components could be used in the production of ceramics or glass-like materials. This on-site manufacturing capability would greatly enhance the self-sufficiency of extraterrestrial outposts and reduce the reliance on supply missions from Earth.
Lastly, the study of MISH in extraterrestrial environments may lead to the discovery of novel applications or properties that are not apparent under Earth conditions. The unique conditions of space, such as microgravity and extreme temperature variations, could reveal new characteristics or behaviors of MISH that could be exploited for technological advancements both in space and on Earth.
One of the most promising applications of MISH in extraterrestrial environments is as a radiation shielding material. The high iron content in MISH provides excellent protection against cosmic radiation, which is a significant concern for long-duration space missions and potential human habitats on other planets. By utilizing MISH found on-site, future space explorers could construct protective structures without the need to transport heavy shielding materials from Earth.
MISH also shows potential as a catalyst for oxygen production in extraterrestrial environments. Through a process known as the oxygen evolution reaction (OER), MISH can facilitate the breakdown of water molecules, releasing oxygen. This could be crucial for sustaining life support systems on Mars or other celestial bodies where water ice has been detected. The ability to generate oxygen locally would significantly reduce the resources needed to be transported from Earth for long-term missions.
Furthermore, the presence of MISH in extraterrestrial environments could provide valuable insights into the formation and evolution of planetary bodies. By studying the distribution and composition of MISH on different celestial objects, scientists can gain a better understanding of the geological processes that shaped these bodies over billions of years. This knowledge could inform future exploration strategies and help identify potential locations for resource extraction or scientific investigation.
In the context of space manufacturing, MISH could serve as a raw material for producing various components needed for space habitats or equipment. Its iron content makes it a potential source for metal extraction, while the silicate components could be used in the production of ceramics or glass-like materials. This on-site manufacturing capability would greatly enhance the self-sufficiency of extraterrestrial outposts and reduce the reliance on supply missions from Earth.
Lastly, the study of MISH in extraterrestrial environments may lead to the discovery of novel applications or properties that are not apparent under Earth conditions. The unique conditions of space, such as microgravity and extreme temperature variations, could reveal new characteristics or behaviors of MISH that could be exploited for technological advancements both in space and on Earth.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!