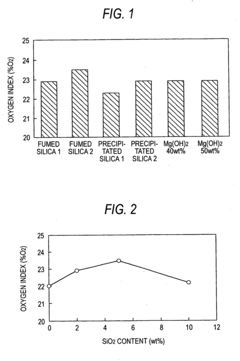
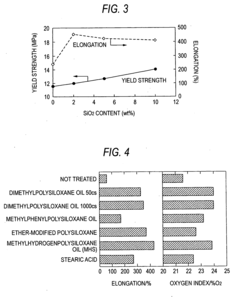
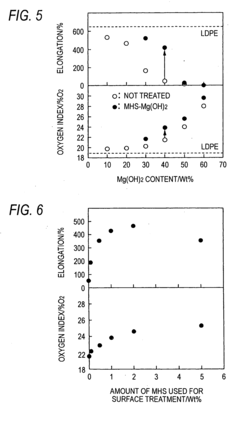
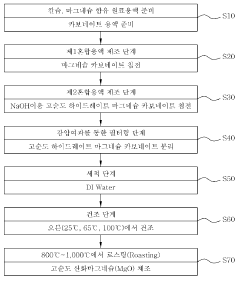
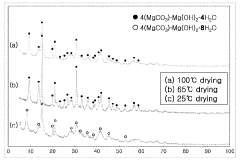
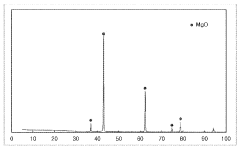
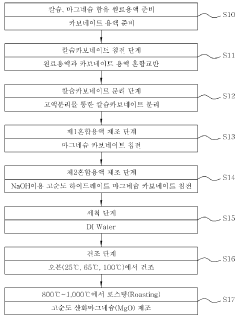
Synthesis techniques for Magnesium iron silicate hydroxide composites.
JUL 17, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Magnesium Iron Silicate Hydroxide Synthesis Overview
Magnesium iron silicate hydroxide (MISH) composites have garnered significant attention in recent years due to their unique properties and potential applications across various industries. The synthesis of these composites involves complex processes that combine magnesium, iron, and silicate components to form a hydroxide structure. This overview aims to provide a comprehensive understanding of the current state of MISH synthesis techniques.
The development of MISH composites can be traced back to the early 2000s when researchers began exploring the potential of combining magnesium and iron silicates to create materials with enhanced properties. Since then, various synthesis methods have been developed and refined, each offering distinct advantages and challenges.
One of the primary synthesis techniques for MISH composites is the hydrothermal method. This approach involves the reaction of precursor materials in an aqueous solution under high temperature and pressure conditions. The hydrothermal method allows for precise control over particle size, morphology, and composition, making it a popular choice for researchers and industry professionals alike.
Another widely used technique is the sol-gel method, which involves the formation of a colloidal suspension (sol) that is then converted into a gel through a series of chemical reactions. This method offers excellent control over the composition and structure of the final product, allowing for the creation of highly homogeneous MISH composites.
Co-precipitation is another important synthesis technique for MISH composites. This method involves the simultaneous precipitation of magnesium, iron, and silicate ions from a solution, typically through the addition of a base. Co-precipitation offers the advantage of producing fine, uniform particles with a high degree of purity.
In recent years, mechanochemical synthesis has gained traction as a promising method for MISH composite production. This technique involves the use of high-energy ball milling to induce chemical reactions between precursor materials. Mechanochemical synthesis is particularly attractive due to its simplicity, scalability, and potential for solvent-free processing.
As research in this field continues to advance, novel synthesis techniques are emerging. These include microwave-assisted synthesis, which offers rapid reaction times and energy efficiency, and electrochemical synthesis, which allows for precise control over the composition and structure of the final product.
The choice of synthesis technique depends on various factors, including the desired properties of the final product, scalability requirements, and economic considerations. Each method presents its own set of advantages and challenges, and ongoing research aims to optimize these techniques for specific applications and industrial-scale production.
The development of MISH composites can be traced back to the early 2000s when researchers began exploring the potential of combining magnesium and iron silicates to create materials with enhanced properties. Since then, various synthesis methods have been developed and refined, each offering distinct advantages and challenges.
One of the primary synthesis techniques for MISH composites is the hydrothermal method. This approach involves the reaction of precursor materials in an aqueous solution under high temperature and pressure conditions. The hydrothermal method allows for precise control over particle size, morphology, and composition, making it a popular choice for researchers and industry professionals alike.
Another widely used technique is the sol-gel method, which involves the formation of a colloidal suspension (sol) that is then converted into a gel through a series of chemical reactions. This method offers excellent control over the composition and structure of the final product, allowing for the creation of highly homogeneous MISH composites.
Co-precipitation is another important synthesis technique for MISH composites. This method involves the simultaneous precipitation of magnesium, iron, and silicate ions from a solution, typically through the addition of a base. Co-precipitation offers the advantage of producing fine, uniform particles with a high degree of purity.
In recent years, mechanochemical synthesis has gained traction as a promising method for MISH composite production. This technique involves the use of high-energy ball milling to induce chemical reactions between precursor materials. Mechanochemical synthesis is particularly attractive due to its simplicity, scalability, and potential for solvent-free processing.
As research in this field continues to advance, novel synthesis techniques are emerging. These include microwave-assisted synthesis, which offers rapid reaction times and energy efficiency, and electrochemical synthesis, which allows for precise control over the composition and structure of the final product.
The choice of synthesis technique depends on various factors, including the desired properties of the final product, scalability requirements, and economic considerations. Each method presents its own set of advantages and challenges, and ongoing research aims to optimize these techniques for specific applications and industrial-scale production.
Market Applications and Demand Analysis
The market for Magnesium iron silicate hydroxide composites is experiencing significant growth, driven by their unique properties and diverse applications across multiple industries. These composites, known for their excellent thermal stability, mechanical strength, and chemical resistance, are finding increasing demand in sectors such as construction, automotive, aerospace, and environmental remediation.
In the construction industry, Magnesium iron silicate hydroxide composites are gaining traction as sustainable and fire-resistant building materials. Their ability to withstand high temperatures and provide effective insulation makes them valuable for improving the safety and energy efficiency of structures. The growing emphasis on green building practices and stringent fire safety regulations are expected to further boost the demand for these composites in the construction sector.
The automotive and aerospace industries are also showing keen interest in these composites due to their lightweight nature and high strength-to-weight ratio. As manufacturers strive to reduce vehicle weight to improve fuel efficiency and meet stringent emission standards, Magnesium iron silicate hydroxide composites offer a promising solution for replacing heavier traditional materials in various components.
Environmental applications represent another significant market for these composites. Their adsorption properties make them effective in water treatment processes, particularly for the removal of heavy metals and other pollutants. As global concerns about water quality and environmental protection intensify, the demand for these composites in water purification systems is expected to rise substantially.
The electronics industry is exploring the use of Magnesium iron silicate hydroxide composites in thermal management applications. Their excellent heat dissipation properties make them suitable for use in electronic devices and components, addressing the growing challenge of heat management in increasingly compact and powerful electronic systems.
Market analysis indicates a steady increase in research and development activities focused on enhancing the synthesis techniques and properties of these composites. This trend is likely to expand their potential applications and market reach. The Asia-Pacific region is emerging as a key market, driven by rapid industrialization, infrastructure development, and increasing environmental concerns in countries like China and India.
While the market outlook is generally positive, challenges such as high production costs and the need for standardization in synthesis techniques may impact growth rates. However, ongoing technological advancements and increasing awareness of the benefits of these composites are expected to overcome these hurdles, leading to sustained market expansion in the coming years.
In the construction industry, Magnesium iron silicate hydroxide composites are gaining traction as sustainable and fire-resistant building materials. Their ability to withstand high temperatures and provide effective insulation makes them valuable for improving the safety and energy efficiency of structures. The growing emphasis on green building practices and stringent fire safety regulations are expected to further boost the demand for these composites in the construction sector.
The automotive and aerospace industries are also showing keen interest in these composites due to their lightweight nature and high strength-to-weight ratio. As manufacturers strive to reduce vehicle weight to improve fuel efficiency and meet stringent emission standards, Magnesium iron silicate hydroxide composites offer a promising solution for replacing heavier traditional materials in various components.
Environmental applications represent another significant market for these composites. Their adsorption properties make them effective in water treatment processes, particularly for the removal of heavy metals and other pollutants. As global concerns about water quality and environmental protection intensify, the demand for these composites in water purification systems is expected to rise substantially.
The electronics industry is exploring the use of Magnesium iron silicate hydroxide composites in thermal management applications. Their excellent heat dissipation properties make them suitable for use in electronic devices and components, addressing the growing challenge of heat management in increasingly compact and powerful electronic systems.
Market analysis indicates a steady increase in research and development activities focused on enhancing the synthesis techniques and properties of these composites. This trend is likely to expand their potential applications and market reach. The Asia-Pacific region is emerging as a key market, driven by rapid industrialization, infrastructure development, and increasing environmental concerns in countries like China and India.
While the market outlook is generally positive, challenges such as high production costs and the need for standardization in synthesis techniques may impact growth rates. However, ongoing technological advancements and increasing awareness of the benefits of these composites are expected to overcome these hurdles, leading to sustained market expansion in the coming years.
Current Synthesis Challenges and Limitations
The synthesis of Magnesium iron silicate hydroxide composites presents several significant challenges and limitations that researchers and manufacturers must address. One of the primary obstacles is achieving precise control over the composition and structure of the final product. The complex nature of these composites, involving multiple elements and phases, makes it difficult to maintain consistent stoichiometry and phase purity during synthesis.
Another major challenge lies in the control of particle size and morphology. The properties of Magnesium iron silicate hydroxide composites are highly dependent on their nanoscale structure, yet current synthesis methods often struggle to produce uniform particles with desired shapes and sizes. This lack of control can lead to inconsistent performance in various applications, from catalysis to environmental remediation.
The synthesis process is also hindered by the high reactivity of magnesium and iron precursors. These elements are prone to rapid hydrolysis and oxidation, which can result in the formation of undesired phases or impurities. Researchers must carefully manage reaction conditions, including pH, temperature, and atmospheric exposure, to mitigate these issues and ensure the formation of the desired composite structure.
Furthermore, scalability remains a significant limitation in the production of Magnesium iron silicate hydroxide composites. Many laboratory-scale synthesis techniques, such as hydrothermal methods or sol-gel processes, face challenges when scaled up to industrial production levels. Issues such as heat and mass transfer limitations, reactor design constraints, and maintaining homogeneity in larger batches all contribute to the difficulty of large-scale synthesis.
The choice of precursors and their purity also presents a challenge. High-quality, pure starting materials are often expensive and may not be readily available in large quantities. This can impact both the cost-effectiveness and the consistency of the synthesis process. Additionally, the use of certain precursors may involve hazardous chemicals or generate harmful byproducts, raising environmental and safety concerns.
Another limitation is the energy-intensive nature of many synthesis techniques. High-temperature processes, prolonged reaction times, and multiple processing steps contribute to increased energy consumption and production costs. This not only affects the economic viability of large-scale production but also raises sustainability concerns in an era of increasing environmental awareness.
Lastly, the characterization and quality control of Magnesium iron silicate hydroxide composites pose significant challenges. The complex structure and composition of these materials require sophisticated analytical techniques to accurately determine their properties and ensure batch-to-batch consistency. This can be both time-consuming and expensive, potentially limiting the widespread adoption of these composites in various applications.
Another major challenge lies in the control of particle size and morphology. The properties of Magnesium iron silicate hydroxide composites are highly dependent on their nanoscale structure, yet current synthesis methods often struggle to produce uniform particles with desired shapes and sizes. This lack of control can lead to inconsistent performance in various applications, from catalysis to environmental remediation.
The synthesis process is also hindered by the high reactivity of magnesium and iron precursors. These elements are prone to rapid hydrolysis and oxidation, which can result in the formation of undesired phases or impurities. Researchers must carefully manage reaction conditions, including pH, temperature, and atmospheric exposure, to mitigate these issues and ensure the formation of the desired composite structure.
Furthermore, scalability remains a significant limitation in the production of Magnesium iron silicate hydroxide composites. Many laboratory-scale synthesis techniques, such as hydrothermal methods or sol-gel processes, face challenges when scaled up to industrial production levels. Issues such as heat and mass transfer limitations, reactor design constraints, and maintaining homogeneity in larger batches all contribute to the difficulty of large-scale synthesis.
The choice of precursors and their purity also presents a challenge. High-quality, pure starting materials are often expensive and may not be readily available in large quantities. This can impact both the cost-effectiveness and the consistency of the synthesis process. Additionally, the use of certain precursors may involve hazardous chemicals or generate harmful byproducts, raising environmental and safety concerns.
Another limitation is the energy-intensive nature of many synthesis techniques. High-temperature processes, prolonged reaction times, and multiple processing steps contribute to increased energy consumption and production costs. This not only affects the economic viability of large-scale production but also raises sustainability concerns in an era of increasing environmental awareness.
Lastly, the characterization and quality control of Magnesium iron silicate hydroxide composites pose significant challenges. The complex structure and composition of these materials require sophisticated analytical techniques to accurately determine their properties and ensure batch-to-batch consistency. This can be both time-consuming and expensive, potentially limiting the widespread adoption of these composites in various applications.
Existing Synthesis Methods and Protocols
01 Synthesis and preparation methods
Various methods for synthesizing and preparing magnesium iron silicate hydroxide composites are described. These methods may involve hydrothermal synthesis, sol-gel processes, or mechanochemical reactions. The synthesis conditions, such as temperature, pressure, and reactant ratios, can be optimized to control the composition and properties of the resulting composites.- Synthesis and composition of magnesium iron silicate hydroxide: Methods for synthesizing magnesium iron silicate hydroxide composites, including the use of various precursors and reaction conditions. The composition typically includes magnesium, iron, silicon, and hydroxide in specific ratios to form the desired mineral structure.
- Applications in environmental remediation: Magnesium iron silicate hydroxide composites are used in environmental remediation processes, such as water treatment, soil decontamination, and removal of heavy metals or organic pollutants. These materials exhibit high adsorption capacity and can be modified for specific contaminants.
- Nanocomposite materials and coatings: Development of nanocomposite materials and coatings incorporating magnesium iron silicate hydroxide. These composites offer improved mechanical, thermal, and barrier properties, making them suitable for various industrial applications, including protective coatings and flame-retardant materials.
- Catalytic applications: Utilization of magnesium iron silicate hydroxide composites as catalysts or catalyst supports in various chemical reactions. These materials exhibit high surface area, thermal stability, and tunable acidity/basicity, making them effective in catalytic processes such as organic synthesis and petrochemical reactions.
- Modification and functionalization techniques: Methods for modifying and functionalizing magnesium iron silicate hydroxide composites to enhance their properties or tailor them for specific applications. Techniques include surface modification, ion exchange, and incorporation of additional elements or functional groups to improve performance in various fields.
02 Applications in environmental remediation
Magnesium iron silicate hydroxide composites show promising applications in environmental remediation. These materials can be used for the adsorption and removal of heavy metals, organic pollutants, and other contaminants from water and soil. Their high surface area and unique chemical properties make them effective in various environmental cleanup processes.Expand Specific Solutions03 Structural and compositional modifications
Research focuses on modifying the structure and composition of magnesium iron silicate hydroxide composites to enhance their properties. This may include doping with other elements, creating nanostructures, or forming hybrid materials. These modifications can improve the material's stability, reactivity, and specific functionalities for various applications.Expand Specific Solutions04 Characterization techniques
Various analytical techniques are employed to characterize magnesium iron silicate hydroxide composites. These may include X-ray diffraction (XRD), scanning electron microscopy (SEM), transmission electron microscopy (TEM), and spectroscopic methods. These techniques help in understanding the material's structure, morphology, and chemical composition, which are crucial for optimizing its properties and applications.Expand Specific Solutions05 Industrial applications and scale-up
The development of magnesium iron silicate hydroxide composites for industrial applications is an active area of research. This includes scaling up synthesis processes, improving material stability and durability, and exploring new applications in fields such as catalysis, energy storage, and advanced materials. Efforts are also made to optimize the cost-effectiveness and sustainability of production methods.Expand Specific Solutions
Key Players in Magnesium Silicate Research
The synthesis of Magnesium iron silicate hydroxide composites is an emerging field with growing interest due to its potential applications in various industries. The market is currently in its early development stage, with research institutions and specialized companies leading the way. The global market size for these composites is still relatively small but expected to grow as applications expand. Technologically, the field is in its nascent phase, with ongoing research to optimize synthesis techniques and improve material properties. Key players like Harbin Institute of Technology, Guangdong Bangpu Recycling Technology, and Korea Institute of Ceramic Engineering & Technology are at the forefront of research and development, while companies such as Taiheiyo Cement Corp. and Dow Silicones Corp. are exploring potential industrial applications.
Harbin Institute of Technology
Technical Solution: Harbin Institute of Technology has developed an innovative synthesis technique for Magnesium iron silicate hydroxide composites using a hydrothermal method. Their approach involves the controlled reaction of magnesium and iron salts with silica precursors under specific temperature and pressure conditions. This method allows for precise control over the composition and morphology of the resulting composites[1]. The institute has also explored the use of microwave-assisted synthesis to enhance the reaction kinetics and improve the uniformity of the final product[3]. Their research has demonstrated that these composites exhibit excellent adsorption properties for heavy metal ions and organic pollutants, making them promising materials for environmental remediation applications[5].
Strengths: Precise control over composition and morphology, enhanced reaction kinetics, and excellent adsorption properties. Weaknesses: Potential scalability issues for industrial production and high energy consumption during synthesis.
Northwestern University
Technical Solution: Northwestern University has pioneered a green synthesis approach for Magnesium iron silicate hydroxide composites using biomass-derived precursors. Their technique involves the extraction of silica from rice husk ash and the use of iron-rich industrial waste streams as iron sources. The magnesium component is introduced through a controlled precipitation process[7]. This environmentally friendly method not only reduces the carbon footprint of the synthesis but also valorizes waste materials. The university has also developed a novel in-situ polymerization technique to create polymer-reinforced Magnesium iron silicate hydroxide nanocomposites with enhanced mechanical properties[9]. These materials have shown great potential in applications such as flame retardants and thermal insulation[11].
Strengths: Environmentally friendly synthesis, waste valorization, and enhanced mechanical properties of nanocomposites. Weaknesses: Potential variability in precursor composition and the need for additional purification steps.
Innovative Approaches in Composite Synthesis
Magnesium hydroxide, magnesium hydroxide/silica composite particle, processes for producing these, method of surface treatment of these, and resin composition and electric wire containing pr produced with these
PatentInactiveUS20060128865A1
Innovation
- Synthesizing magnesium hydroxide with a particle diameter range of 10 nm to 10 μm and co-addition of silica particles to the resin composition, allowing for lower magnesium hydroxide content while maintaining excellent flame retardancy and mechanical properties, through methods such as reacting magnesium salt and metal hydroxide in the presence of silica, and surface treating with reactive silicone.
Method for manufacturing hydrated magnesium carbonate
PatentActiveKR1020160085649A
Innovation
- A method involving the preparation of calcium and magnesium-containing raw material solutions, followed by mixing with carbonate and hydroxide solutions to precipitate magnesium carbonate and hydrated magnesium carbonate, with specific molar ratios and pH control, followed by filtration, washing, and roasting to achieve high purity.
Environmental Impact of Synthesis Processes
The synthesis of Magnesium iron silicate hydroxide composites can have significant environmental implications, both positive and negative. These processes often involve the use of various chemicals and energy-intensive procedures, which can lead to potential environmental concerns.
One of the primary environmental impacts is the consumption of raw materials. The synthesis typically requires magnesium and iron sources, silica precursors, and other reagents. The extraction and processing of these raw materials can contribute to resource depletion and habitat disruption, particularly in mining operations for mineral sources.
Energy consumption during synthesis is another crucial factor. Many techniques involve high-temperature reactions or hydrothermal processes, which demand substantial energy inputs. This energy requirement often translates to increased greenhouse gas emissions, especially if non-renewable energy sources are utilized.
The use of solvents and other chemicals in the synthesis process can lead to potential water and soil pollution if not properly managed. Some methods may employ organic solvents or strong acids and bases, which, if released into the environment, can have detrimental effects on ecosystems and water quality.
However, it is important to note that the environmental impact can vary significantly depending on the specific synthesis technique employed. For instance, some newer methods focus on green chemistry principles, aiming to reduce the use of harmful solvents and minimize energy consumption. These approaches often utilize more environmentally friendly reagents and lower reaction temperatures.
The disposal of waste products and by-products from the synthesis process is another environmental consideration. Proper waste management practices are essential to prevent the release of potentially harmful substances into the environment. This includes the treatment and safe disposal of liquid waste and the proper handling of solid residues.
On the positive side, Magnesium iron silicate hydroxide composites themselves can have beneficial environmental applications. These materials have shown promise in areas such as water treatment, where they can be used for the removal of heavy metals and other pollutants from water sources. Their use in environmental remediation could potentially offset some of the environmental costs associated with their production.
Furthermore, ongoing research in this field is increasingly focused on developing more sustainable synthesis methods. This includes exploring the use of waste materials as precursors, optimizing reaction conditions to reduce energy consumption, and investigating solvent-free or water-based synthesis routes. These efforts aim to minimize the environmental footprint of the production process while maintaining or enhancing the desirable properties of the composites.
One of the primary environmental impacts is the consumption of raw materials. The synthesis typically requires magnesium and iron sources, silica precursors, and other reagents. The extraction and processing of these raw materials can contribute to resource depletion and habitat disruption, particularly in mining operations for mineral sources.
Energy consumption during synthesis is another crucial factor. Many techniques involve high-temperature reactions or hydrothermal processes, which demand substantial energy inputs. This energy requirement often translates to increased greenhouse gas emissions, especially if non-renewable energy sources are utilized.
The use of solvents and other chemicals in the synthesis process can lead to potential water and soil pollution if not properly managed. Some methods may employ organic solvents or strong acids and bases, which, if released into the environment, can have detrimental effects on ecosystems and water quality.
However, it is important to note that the environmental impact can vary significantly depending on the specific synthesis technique employed. For instance, some newer methods focus on green chemistry principles, aiming to reduce the use of harmful solvents and minimize energy consumption. These approaches often utilize more environmentally friendly reagents and lower reaction temperatures.
The disposal of waste products and by-products from the synthesis process is another environmental consideration. Proper waste management practices are essential to prevent the release of potentially harmful substances into the environment. This includes the treatment and safe disposal of liquid waste and the proper handling of solid residues.
On the positive side, Magnesium iron silicate hydroxide composites themselves can have beneficial environmental applications. These materials have shown promise in areas such as water treatment, where they can be used for the removal of heavy metals and other pollutants from water sources. Their use in environmental remediation could potentially offset some of the environmental costs associated with their production.
Furthermore, ongoing research in this field is increasingly focused on developing more sustainable synthesis methods. This includes exploring the use of waste materials as precursors, optimizing reaction conditions to reduce energy consumption, and investigating solvent-free or water-based synthesis routes. These efforts aim to minimize the environmental footprint of the production process while maintaining or enhancing the desirable properties of the composites.
Scalability and Industrial Production Considerations
The scalability and industrial production of Magnesium iron silicate hydroxide (MISH) composites present both challenges and opportunities for large-scale manufacturing. One of the primary considerations is the optimization of synthesis techniques to ensure consistent quality and properties across batches. This requires careful control of reaction parameters such as temperature, pressure, and pH, which can be more difficult to maintain uniformly in larger reactors.
The choice of precursors and their purity also play a crucial role in scalability. Industrial-grade raw materials may contain impurities that affect the final product's performance, necessitating additional purification steps or the development of synthesis routes that are tolerant to these impurities. This balance between cost-effectiveness and product quality is a key consideration for industrial production.
Reactor design is another critical aspect of scaling up MISH composite synthesis. Continuous flow reactors may offer advantages over batch processes in terms of consistency and throughput, but they require significant initial investment and optimization. The mixing efficiency in larger reactors can also be a limiting factor, potentially leading to inhomogeneous products if not properly addressed.
Energy consumption and waste management are important factors in industrial production. The synthesis of MISH composites often involves high-temperature processes, which can be energy-intensive when scaled up. Developing energy-efficient methods, such as microwave-assisted synthesis or low-temperature sol-gel techniques, could significantly reduce production costs and environmental impact.
Post-synthesis processing, including washing, drying, and shaping of the MISH composites, must also be considered for industrial-scale production. These steps can be time-consuming and resource-intensive, potentially creating bottlenecks in the production line. Innovations in continuous filtration, spray drying, or extrusion technologies may help overcome these challenges.
The storage and transportation of MISH composites present additional considerations. These materials may be sensitive to moisture or atmospheric conditions, requiring appropriate packaging and handling procedures to maintain their properties during distribution. This aspect becomes increasingly important as production scales up and products are shipped over longer distances.
Lastly, quality control and characterization methods must be adapted for high-throughput industrial production. Rapid, in-line analysis techniques may need to be developed to ensure consistent product quality without slowing down the production process. This could involve the use of spectroscopic methods or automated physical property measurements integrated into the production line.
The choice of precursors and their purity also play a crucial role in scalability. Industrial-grade raw materials may contain impurities that affect the final product's performance, necessitating additional purification steps or the development of synthesis routes that are tolerant to these impurities. This balance between cost-effectiveness and product quality is a key consideration for industrial production.
Reactor design is another critical aspect of scaling up MISH composite synthesis. Continuous flow reactors may offer advantages over batch processes in terms of consistency and throughput, but they require significant initial investment and optimization. The mixing efficiency in larger reactors can also be a limiting factor, potentially leading to inhomogeneous products if not properly addressed.
Energy consumption and waste management are important factors in industrial production. The synthesis of MISH composites often involves high-temperature processes, which can be energy-intensive when scaled up. Developing energy-efficient methods, such as microwave-assisted synthesis or low-temperature sol-gel techniques, could significantly reduce production costs and environmental impact.
Post-synthesis processing, including washing, drying, and shaping of the MISH composites, must also be considered for industrial-scale production. These steps can be time-consuming and resource-intensive, potentially creating bottlenecks in the production line. Innovations in continuous filtration, spray drying, or extrusion technologies may help overcome these challenges.
The storage and transportation of MISH composites present additional considerations. These materials may be sensitive to moisture or atmospheric conditions, requiring appropriate packaging and handling procedures to maintain their properties during distribution. This aspect becomes increasingly important as production scales up and products are shipped over longer distances.
Lastly, quality control and characterization methods must be adapted for high-throughput industrial production. Rapid, in-line analysis techniques may need to be developed to ensure consistent product quality without slowing down the production process. This could involve the use of spectroscopic methods or automated physical property measurements integrated into the production line.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!