Tribochemical membrane formation with Magnesium iron silicate hydroxide.
JUL 17, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Tribochemical Membrane Formation Background and Objectives
Tribochemical membrane formation with Magnesium iron silicate hydroxide (MISH) represents a cutting-edge approach in the field of tribology and surface engineering. This technology has emerged as a promising solution to address the challenges of friction and wear in various mechanical systems. The evolution of this technology can be traced back to the early studies on boundary lubrication and the discovery of tribofilms in the mid-20th century.
The primary objective of tribochemical membrane formation with MISH is to create a protective layer on sliding surfaces that can significantly reduce friction and wear. This technology aims to leverage the unique properties of MISH, a naturally occurring mineral with a layered structure, to form a stable and effective tribofilm under mechanical stress and chemical reactions.
As industrial applications demand increasingly efficient and durable mechanical systems, the need for advanced tribological solutions has grown exponentially. The development of tribochemical membranes using MISH aligns with the broader trend towards environmentally friendly and sustainable lubrication technologies. This approach offers the potential to replace traditional oil-based lubricants in certain applications, contributing to reduced environmental impact and improved energy efficiency.
The tribochemical membrane formation process involves complex interactions between the MISH particles, the substrate material, and the operating environment. Understanding and controlling these interactions is crucial for optimizing the performance of the tribofilm. Recent advancements in surface analysis techniques and computational modeling have significantly contributed to unraveling the mechanisms behind tribochemical reactions and film formation.
One of the key technological goals in this field is to develop a comprehensive understanding of the tribochemical processes that occur during membrane formation. This includes elucidating the role of various factors such as temperature, pressure, sliding speed, and chemical composition on the formation and properties of the tribofilm. Additionally, researchers aim to enhance the stability and durability of the tribochemical membrane under diverse operating conditions.
Another important objective is to expand the applicability of MISH-based tribochemical membranes to a wider range of materials and operating environments. This involves tailoring the composition and structure of MISH particles to optimize their performance on different substrate materials and under varying tribological conditions. The ultimate goal is to develop a versatile technology that can be adapted to meet the specific requirements of various industrial applications, from automotive engines to aerospace components.
The primary objective of tribochemical membrane formation with MISH is to create a protective layer on sliding surfaces that can significantly reduce friction and wear. This technology aims to leverage the unique properties of MISH, a naturally occurring mineral with a layered structure, to form a stable and effective tribofilm under mechanical stress and chemical reactions.
As industrial applications demand increasingly efficient and durable mechanical systems, the need for advanced tribological solutions has grown exponentially. The development of tribochemical membranes using MISH aligns with the broader trend towards environmentally friendly and sustainable lubrication technologies. This approach offers the potential to replace traditional oil-based lubricants in certain applications, contributing to reduced environmental impact and improved energy efficiency.
The tribochemical membrane formation process involves complex interactions between the MISH particles, the substrate material, and the operating environment. Understanding and controlling these interactions is crucial for optimizing the performance of the tribofilm. Recent advancements in surface analysis techniques and computational modeling have significantly contributed to unraveling the mechanisms behind tribochemical reactions and film formation.
One of the key technological goals in this field is to develop a comprehensive understanding of the tribochemical processes that occur during membrane formation. This includes elucidating the role of various factors such as temperature, pressure, sliding speed, and chemical composition on the formation and properties of the tribofilm. Additionally, researchers aim to enhance the stability and durability of the tribochemical membrane under diverse operating conditions.
Another important objective is to expand the applicability of MISH-based tribochemical membranes to a wider range of materials and operating environments. This involves tailoring the composition and structure of MISH particles to optimize their performance on different substrate materials and under varying tribological conditions. The ultimate goal is to develop a versatile technology that can be adapted to meet the specific requirements of various industrial applications, from automotive engines to aerospace components.
Market Analysis for Tribochemical Membrane Applications
The market for tribochemical membrane applications, particularly those involving Magnesium iron silicate hydroxide (MISH), is experiencing significant growth and diversification. This emerging technology has garnered attention across various industries due to its unique properties and potential applications in surface protection, lubrication, and wear reduction.
In the automotive sector, tribochemical membranes formed with MISH are being explored for their ability to reduce friction and wear in engine components. This application has the potential to improve fuel efficiency and extend the lifespan of critical engine parts. The global automotive lubricants market, which is closely related to this application, is projected to grow steadily in the coming years, driven by increasing vehicle production and the demand for high-performance lubricants.
The aerospace industry is another key market for tribochemical membrane applications. MISH-based membranes are being investigated for their potential to enhance the performance and durability of aircraft components subjected to extreme conditions. As the aerospace industry continues to seek innovative solutions for weight reduction and improved efficiency, the demand for advanced tribological solutions is expected to rise.
In the industrial machinery sector, tribochemical membranes offer promising applications in heavy-duty equipment and manufacturing processes. The ability of MISH-based membranes to form protective layers on metal surfaces can lead to reduced maintenance costs and improved equipment longevity. This market segment is particularly attractive due to the high value placed on operational efficiency and equipment reliability in industrial settings.
The energy sector, especially in areas such as wind turbines and oil and gas exploration, presents another significant market opportunity for tribochemical membrane applications. The harsh operating conditions in these industries necessitate advanced wear-resistant solutions, making MISH-based membranes an attractive option for protecting critical components and improving overall system performance.
The medical device industry is also showing interest in tribochemical membrane technology. The biocompatibility and wear-resistant properties of MISH-based membranes make them potential candidates for use in implantable devices and surgical instruments. As the global medical device market continues to expand, driven by an aging population and advancements in healthcare technology, the demand for innovative materials with enhanced tribological properties is expected to grow.
While the market potential for tribochemical membrane applications is substantial, it is important to note that the technology is still in its early stages of commercialization. Adoption rates may vary across industries, and market penetration will depend on factors such as cost-effectiveness, scalability, and regulatory approval processes. However, the increasing focus on sustainability and efficiency across industries suggests a favorable environment for the growth of tribochemical membrane applications in the coming years.
In the automotive sector, tribochemical membranes formed with MISH are being explored for their ability to reduce friction and wear in engine components. This application has the potential to improve fuel efficiency and extend the lifespan of critical engine parts. The global automotive lubricants market, which is closely related to this application, is projected to grow steadily in the coming years, driven by increasing vehicle production and the demand for high-performance lubricants.
The aerospace industry is another key market for tribochemical membrane applications. MISH-based membranes are being investigated for their potential to enhance the performance and durability of aircraft components subjected to extreme conditions. As the aerospace industry continues to seek innovative solutions for weight reduction and improved efficiency, the demand for advanced tribological solutions is expected to rise.
In the industrial machinery sector, tribochemical membranes offer promising applications in heavy-duty equipment and manufacturing processes. The ability of MISH-based membranes to form protective layers on metal surfaces can lead to reduced maintenance costs and improved equipment longevity. This market segment is particularly attractive due to the high value placed on operational efficiency and equipment reliability in industrial settings.
The energy sector, especially in areas such as wind turbines and oil and gas exploration, presents another significant market opportunity for tribochemical membrane applications. The harsh operating conditions in these industries necessitate advanced wear-resistant solutions, making MISH-based membranes an attractive option for protecting critical components and improving overall system performance.
The medical device industry is also showing interest in tribochemical membrane technology. The biocompatibility and wear-resistant properties of MISH-based membranes make them potential candidates for use in implantable devices and surgical instruments. As the global medical device market continues to expand, driven by an aging population and advancements in healthcare technology, the demand for innovative materials with enhanced tribological properties is expected to grow.
While the market potential for tribochemical membrane applications is substantial, it is important to note that the technology is still in its early stages of commercialization. Adoption rates may vary across industries, and market penetration will depend on factors such as cost-effectiveness, scalability, and regulatory approval processes. However, the increasing focus on sustainability and efficiency across industries suggests a favorable environment for the growth of tribochemical membrane applications in the coming years.
Current State and Challenges in Tribochemical Membrane Formation
The current state of tribochemical membrane formation with Magnesium iron silicate hydroxide (MISH) is characterized by significant advancements in understanding the mechanisms and potential applications of this innovative technology. Researchers have made substantial progress in elucidating the formation process of these tribochemical membranes, which involves the interaction between MISH particles and sliding surfaces under specific conditions.
Recent studies have demonstrated that MISH-based tribochemical membranes exhibit exceptional lubricating properties, significantly reducing friction and wear in various mechanical systems. The unique structure of MISH, combining magnesium, iron, silicon, and hydroxide components, contributes to its ability to form stable and effective protective layers on sliding surfaces.
One of the key achievements in this field has been the development of in-situ observation techniques that allow real-time monitoring of tribochemical membrane formation. These advanced analytical methods have provided valuable insights into the kinetics and dynamics of the process, enabling researchers to optimize formation conditions and enhance membrane performance.
Despite these advancements, several challenges remain in the field of tribochemical membrane formation with MISH. One of the primary obstacles is controlling the uniformity and thickness of the formed membranes across different substrate materials and under varying operating conditions. Researchers are actively working on developing methods to ensure consistent and reproducible membrane formation for diverse applications.
Another significant challenge lies in understanding the long-term stability and durability of MISH-based tribochemical membranes. While initial results are promising, more extensive studies are needed to evaluate the performance of these membranes under prolonged use and in diverse environmental conditions. This includes investigating potential degradation mechanisms and developing strategies to enhance membrane longevity.
The scalability of MISH-based tribochemical membrane formation processes also presents a challenge for industrial applications. Current laboratory-scale successes need to be translated into large-scale production methods that are both economically viable and environmentally sustainable. This requires addressing issues related to raw material sourcing, process efficiency, and quality control in industrial settings.
Furthermore, the integration of MISH-based tribochemical membranes into existing lubrication systems and mechanical components poses technical challenges. Compatibility with various lubricants, surface treatments, and material compositions needs to be thoroughly investigated to ensure optimal performance across a wide range of applications.
In conclusion, while significant progress has been made in tribochemical membrane formation with MISH, addressing these challenges will be crucial for realizing the full potential of this technology in practical applications. Ongoing research efforts are focused on overcoming these obstacles to pave the way for widespread adoption of MISH-based tribochemical membranes in various industries.
Recent studies have demonstrated that MISH-based tribochemical membranes exhibit exceptional lubricating properties, significantly reducing friction and wear in various mechanical systems. The unique structure of MISH, combining magnesium, iron, silicon, and hydroxide components, contributes to its ability to form stable and effective protective layers on sliding surfaces.
One of the key achievements in this field has been the development of in-situ observation techniques that allow real-time monitoring of tribochemical membrane formation. These advanced analytical methods have provided valuable insights into the kinetics and dynamics of the process, enabling researchers to optimize formation conditions and enhance membrane performance.
Despite these advancements, several challenges remain in the field of tribochemical membrane formation with MISH. One of the primary obstacles is controlling the uniformity and thickness of the formed membranes across different substrate materials and under varying operating conditions. Researchers are actively working on developing methods to ensure consistent and reproducible membrane formation for diverse applications.
Another significant challenge lies in understanding the long-term stability and durability of MISH-based tribochemical membranes. While initial results are promising, more extensive studies are needed to evaluate the performance of these membranes under prolonged use and in diverse environmental conditions. This includes investigating potential degradation mechanisms and developing strategies to enhance membrane longevity.
The scalability of MISH-based tribochemical membrane formation processes also presents a challenge for industrial applications. Current laboratory-scale successes need to be translated into large-scale production methods that are both economically viable and environmentally sustainable. This requires addressing issues related to raw material sourcing, process efficiency, and quality control in industrial settings.
Furthermore, the integration of MISH-based tribochemical membranes into existing lubrication systems and mechanical components poses technical challenges. Compatibility with various lubricants, surface treatments, and material compositions needs to be thoroughly investigated to ensure optimal performance across a wide range of applications.
In conclusion, while significant progress has been made in tribochemical membrane formation with MISH, addressing these challenges will be crucial for realizing the full potential of this technology in practical applications. Ongoing research efforts are focused on overcoming these obstacles to pave the way for widespread adoption of MISH-based tribochemical membranes in various industries.
Existing Solutions for Magnesium Iron Silicate Hydroxide Membranes
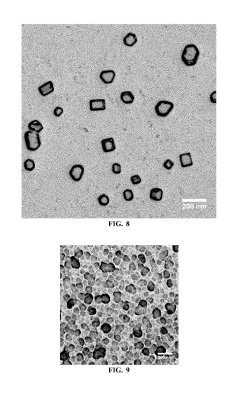
01 Synthesis of magnesium iron silicate hydroxide membranes
Methods for synthesizing magnesium iron silicate hydroxide membranes, including hydrothermal synthesis, sol-gel processes, and template-assisted growth. These techniques allow for the controlled formation of thin, uniform membranes with specific compositions and structures.- Synthesis of magnesium iron silicate hydroxide membranes: Methods for synthesizing magnesium iron silicate hydroxide membranes, including hydrothermal synthesis and sol-gel processes. These techniques allow for the controlled formation of the membrane structure with specific properties suitable for various applications.
- Modification of membrane composition and structure: Techniques for modifying the composition and structure of magnesium iron silicate hydroxide membranes to enhance their performance. This includes doping with additional elements, controlling the ratio of magnesium to iron, and adjusting the silicate content to optimize membrane properties.
- Surface functionalization of membranes: Methods for functionalizing the surface of magnesium iron silicate hydroxide membranes to improve their selectivity, permeability, and chemical resistance. This may involve grafting organic molecules or applying coatings to enhance membrane performance in specific applications.
- Membrane formation for specific applications: Tailoring the formation of magnesium iron silicate hydroxide membranes for specific applications such as gas separation, water purification, and catalysis. This involves optimizing membrane thickness, porosity, and surface area to meet the requirements of different industrial processes.
- Characterization and performance evaluation of membranes: Techniques for characterizing and evaluating the performance of magnesium iron silicate hydroxide membranes, including analysis of their physical, chemical, and mechanical properties. This involves using various analytical methods to assess membrane quality, durability, and efficiency in different operating conditions.
02 Modification of membrane properties
Techniques for modifying the properties of magnesium iron silicate hydroxide membranes, such as surface functionalization, doping with additional elements, and post-synthesis treatments. These modifications can enhance membrane performance, stability, and selectivity for various applications.Expand Specific Solutions03 Application in separation processes
Use of magnesium iron silicate hydroxide membranes in various separation processes, including gas separation, water purification, and ion exchange. The unique properties of these membranes make them suitable for selective permeation and filtration applications.Expand Specific Solutions04 Composite membrane formation
Development of composite membranes incorporating magnesium iron silicate hydroxide, combining it with other materials such as polymers or ceramics. These composite structures can offer improved mechanical strength, chemical stability, and enhanced separation performance.Expand Specific Solutions05 Characterization and analysis techniques
Methods for characterizing and analyzing magnesium iron silicate hydroxide membranes, including microscopy, spectroscopy, and permeation testing. These techniques are essential for understanding membrane structure, composition, and performance in various applications.Expand Specific Solutions
Key Players in Tribochemical Membrane Industry
The tribochemical membrane formation with Magnesium iron silicate hydroxide is an emerging field in advanced materials science. The market is in its early development stage, with limited commercial applications but significant research interest. The global market size for this technology is still relatively small, estimated to be in the tens of millions of dollars. However, it shows potential for rapid growth in the coming years. The technology's maturity is still evolving, with key players like GM Global Technology Operations LLC, Samsung SDI Co., Ltd., and ITM Power Plc leading research efforts. Academic institutions such as Xi'an Jiaotong University and King Abdullah University of Science & Technology are also contributing to advancements in this field, indicating a collaborative approach between industry and academia to drive innovation.
King Abdullah University of Science & Technology
Technical Solution: King Abdullah University of Science & Technology (KAUST) has developed an innovative approach to tribochemical membrane formation using Magnesium iron silicate hydroxide (MISH). Their research focuses on the synthesis and characterization of MISH nanoparticles for enhanced membrane performance. The team has successfully created MISH-based membranes with improved fouling resistance and water flux[1]. The process involves controlled precipitation of MISH on membrane surfaces, resulting in a thin, uniform layer that enhances membrane functionality. KAUST researchers have also explored the use of MISH in combination with other materials to create hybrid membranes with superior properties[2]. These membranes have shown promising results in water treatment applications, particularly in desalination and wastewater treatment processes.
Strengths: Advanced research facilities and expertise in nanotechnology and membrane science. Weaknesses: Potential scalability issues for large-scale membrane production and limited industrial partnerships for commercialization.
Dalian University of Technology
Technical Solution: Dalian University of Technology has made significant strides in the field of tribochemical membrane formation using Magnesium iron silicate hydroxide (MISH). Their research team has developed a novel in-situ growth method for MISH nanoparticles on membrane surfaces, resulting in enhanced anti-fouling properties and improved membrane performance[3]. The university's approach involves a controlled reaction between magnesium and iron precursors in the presence of silicate ions, leading to the formation of a uniform MISH layer. This method has been successfully applied to various membrane materials, including polymeric and ceramic membranes. Additionally, the team has investigated the tribological properties of MISH-coated membranes, demonstrating reduced friction and wear in membrane-based systems[4].
Strengths: Strong expertise in materials science and membrane technology. Established collaborations with industry partners. Weaknesses: Limited focus on large-scale production and potential challenges in maintaining consistent MISH layer quality across different membrane types.
Core Innovations in Tribochemical Membrane Formation
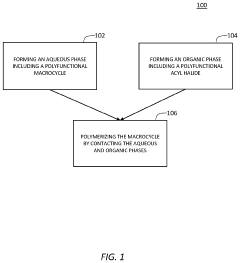
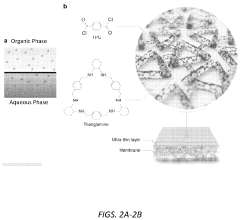
Molecularly porous cross-linked membranes
PatentPendingUS20230330604A1
Innovation
- The development of molecularly porous cross-linked membranes (MPCMs) using reactive macrocycle monomers like trianglamine, which are cross-linked via interfacial polymerization to create a hyper-cross-linked network providing permanent channels for solvent permeance and stability, with a high degree of cross-linking for harsh organic solvent environments.
Semipermeable membrane and preparation method thereof
PatentInactiveUS20190282967A1
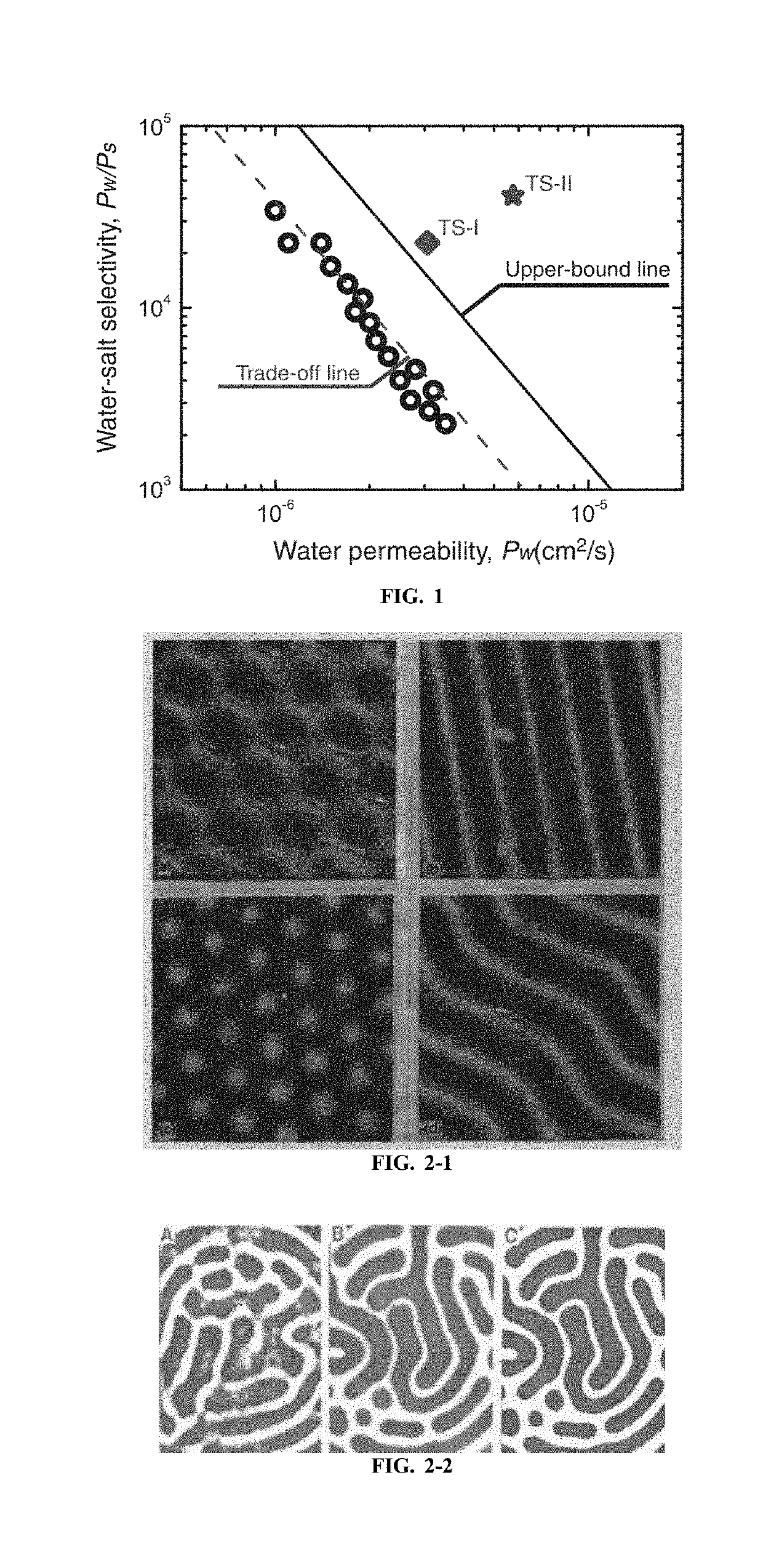
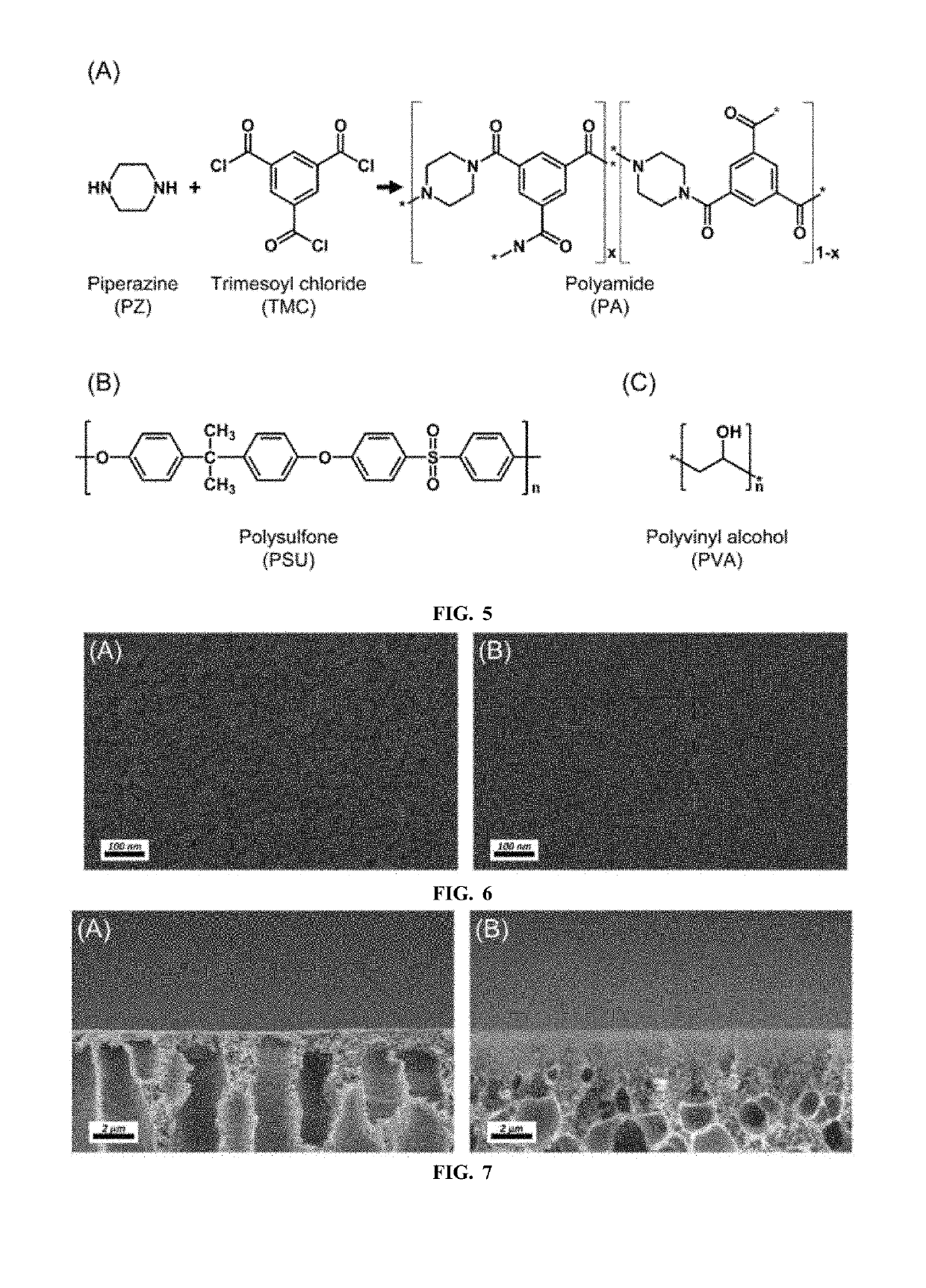
Innovation
- A semipermeable membrane with a complex periodic pattern structure is developed through controlled diffusion coefficients in the interfacial polymerization process, breaking the theoretical limit of flux while maintaining high selective permeability by creating a Turing structure.
Environmental Impact of Tribochemical Membrane Production
The production of tribochemical membranes using Magnesium iron silicate hydroxide (MISH) has significant environmental implications that warrant careful consideration. This process, while innovative in its approach to membrane formation, raises concerns about resource consumption, energy usage, and potential ecological impacts.
The extraction and processing of raw materials, particularly magnesium, iron, and silica compounds, can lead to habitat disruption and soil degradation in mining areas. These activities often require substantial water usage, potentially straining local water resources. Additionally, the refinement of these materials may involve energy-intensive processes, contributing to increased carbon emissions if not managed sustainably.
During the tribochemical membrane formation process, the use of chemical reagents and solvents poses potential risks to air and water quality. Proper handling, storage, and disposal of these substances are crucial to prevent environmental contamination. The process may generate waste products that require specialized treatment to mitigate their environmental impact.
Energy consumption is another critical factor to consider. The tribochemical reactions and subsequent membrane formation steps often require precise temperature control and mechanical agitation, which can be energy-intensive. The environmental footprint of this energy usage depends largely on the source of electricity used in production facilities.
On the positive side, MISH-based tribochemical membranes may offer enhanced durability and efficiency compared to traditional membrane technologies. This could lead to reduced material consumption and waste generation over the long term, as these membranes may require less frequent replacement and maintenance.
The end-of-life considerations for these membranes are also important. The complex composition of MISH membranes may present challenges for recycling or safe disposal. Research into effective recycling methods or biodegradable alternatives could significantly improve the overall environmental profile of this technology.
Water treatment applications of MISH membranes could potentially offset some of their environmental impacts by improving water purification processes and reducing the need for harsh chemical treatments. However, the release of membrane particles or degradation products into water systems during use must be carefully monitored and mitigated.
In conclusion, while tribochemical membrane formation with MISH offers promising technological advancements, its environmental impact is multifaceted. Balancing the benefits of improved membrane performance against the ecological costs of production and disposal is crucial. Ongoing research and development should focus on optimizing resource efficiency, minimizing waste, and exploring more sustainable production methods to enhance the overall environmental sustainability of this technology.
The extraction and processing of raw materials, particularly magnesium, iron, and silica compounds, can lead to habitat disruption and soil degradation in mining areas. These activities often require substantial water usage, potentially straining local water resources. Additionally, the refinement of these materials may involve energy-intensive processes, contributing to increased carbon emissions if not managed sustainably.
During the tribochemical membrane formation process, the use of chemical reagents and solvents poses potential risks to air and water quality. Proper handling, storage, and disposal of these substances are crucial to prevent environmental contamination. The process may generate waste products that require specialized treatment to mitigate their environmental impact.
Energy consumption is another critical factor to consider. The tribochemical reactions and subsequent membrane formation steps often require precise temperature control and mechanical agitation, which can be energy-intensive. The environmental footprint of this energy usage depends largely on the source of electricity used in production facilities.
On the positive side, MISH-based tribochemical membranes may offer enhanced durability and efficiency compared to traditional membrane technologies. This could lead to reduced material consumption and waste generation over the long term, as these membranes may require less frequent replacement and maintenance.
The end-of-life considerations for these membranes are also important. The complex composition of MISH membranes may present challenges for recycling or safe disposal. Research into effective recycling methods or biodegradable alternatives could significantly improve the overall environmental profile of this technology.
Water treatment applications of MISH membranes could potentially offset some of their environmental impacts by improving water purification processes and reducing the need for harsh chemical treatments. However, the release of membrane particles or degradation products into water systems during use must be carefully monitored and mitigated.
In conclusion, while tribochemical membrane formation with MISH offers promising technological advancements, its environmental impact is multifaceted. Balancing the benefits of improved membrane performance against the ecological costs of production and disposal is crucial. Ongoing research and development should focus on optimizing resource efficiency, minimizing waste, and exploring more sustainable production methods to enhance the overall environmental sustainability of this technology.
Scalability and Industrial Implementation Considerations
The scalability and industrial implementation of tribochemical membrane formation with Magnesium iron silicate hydroxide (MISH) present both opportunities and challenges for large-scale applications. As this technology moves from laboratory-scale experiments to industrial production, several key considerations must be addressed to ensure its viability and efficiency.
One of the primary challenges in scaling up this process is maintaining consistent membrane quality across larger surface areas. The tribochemical formation mechanism relies on precise control of mechanical forces and chemical reactions at the nanoscale. Developing equipment and processes that can uniformly apply these conditions over industrial-scale surfaces will be crucial. This may involve the design of specialized reactors or the adaptation of existing large-scale tribological equipment.
Raw material sourcing and preparation also play a critical role in scalability. Ensuring a stable supply of high-quality MISH precursors in sufficient quantities for industrial production is essential. This may necessitate partnerships with mining companies or the development of synthetic production methods for MISH materials. Additionally, the preparation and handling of these materials at scale must be optimized to maintain consistent particle size and composition, which are vital for membrane performance.
Energy efficiency is another important factor to consider in industrial implementation. The tribochemical process inherently involves mechanical energy input, which could lead to high energy consumption at large scales. Innovations in energy-efficient tribological systems and the potential integration of renewable energy sources could help mitigate these costs and improve the overall sustainability of the process.
Quality control and characterization methods must also be adapted for industrial-scale production. Rapid, in-line monitoring techniques will be needed to ensure membrane integrity and performance throughout the manufacturing process. This may involve the development of new analytical tools or the adaptation of existing technologies for real-time membrane characterization.
Environmental and safety considerations are paramount in scaling up this technology. The potential release of nanoparticles during the tribochemical process must be carefully managed to protect worker health and the environment. Implementing robust containment systems and filtration technologies will be essential for industrial-scale operations.
Lastly, the integration of this membrane formation process into existing manufacturing lines or the development of new, dedicated production facilities will require significant investment and engineering expertise. Modular design approaches and flexible manufacturing systems could help facilitate the gradual scaling up of production while minimizing initial capital expenditures.
One of the primary challenges in scaling up this process is maintaining consistent membrane quality across larger surface areas. The tribochemical formation mechanism relies on precise control of mechanical forces and chemical reactions at the nanoscale. Developing equipment and processes that can uniformly apply these conditions over industrial-scale surfaces will be crucial. This may involve the design of specialized reactors or the adaptation of existing large-scale tribological equipment.
Raw material sourcing and preparation also play a critical role in scalability. Ensuring a stable supply of high-quality MISH precursors in sufficient quantities for industrial production is essential. This may necessitate partnerships with mining companies or the development of synthetic production methods for MISH materials. Additionally, the preparation and handling of these materials at scale must be optimized to maintain consistent particle size and composition, which are vital for membrane performance.
Energy efficiency is another important factor to consider in industrial implementation. The tribochemical process inherently involves mechanical energy input, which could lead to high energy consumption at large scales. Innovations in energy-efficient tribological systems and the potential integration of renewable energy sources could help mitigate these costs and improve the overall sustainability of the process.
Quality control and characterization methods must also be adapted for industrial-scale production. Rapid, in-line monitoring techniques will be needed to ensure membrane integrity and performance throughout the manufacturing process. This may involve the development of new analytical tools or the adaptation of existing technologies for real-time membrane characterization.
Environmental and safety considerations are paramount in scaling up this technology. The potential release of nanoparticles during the tribochemical process must be carefully managed to protect worker health and the environment. Implementing robust containment systems and filtration technologies will be essential for industrial-scale operations.
Lastly, the integration of this membrane formation process into existing manufacturing lines or the development of new, dedicated production facilities will require significant investment and engineering expertise. Modular design approaches and flexible manufacturing systems could help facilitate the gradual scaling up of production while minimizing initial capital expenditures.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!