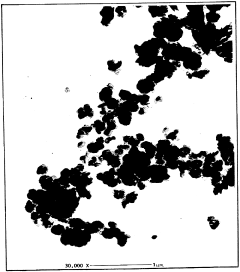
Exploring the crystalline structure of Magnesium iron silicate hydroxide.
JUL 17, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Magnesium Iron Silicate Hydroxide Overview
Magnesium iron silicate hydroxide, also known as (Mg,Fe)3Si2O5(OH)4, is a complex mineral compound that belongs to the serpentine group. This mineral is of significant interest in various scientific and industrial fields due to its unique crystalline structure and properties. The compound is primarily composed of magnesium, iron, silicon, oxygen, and hydroxyl groups, arranged in a layered structure that gives rise to its distinctive characteristics.
The crystalline structure of magnesium iron silicate hydroxide is characterized by alternating layers of tetrahedral silica sheets and octahedral brucite-like sheets. The tetrahedral layers consist of silicon atoms bonded to four oxygen atoms, while the octahedral layers contain magnesium and iron atoms coordinated with six oxygen or hydroxyl groups. This layered arrangement results in a 1:1 phyllosilicate structure, which is responsible for many of the mineral's physical and chemical properties.
One of the most notable features of magnesium iron silicate hydroxide is its ability to accommodate varying ratios of magnesium and iron within its structure. This compositional flexibility leads to a range of mineral varieties, including antigorite, lizardite, and chrysotile, each with slightly different structural arrangements and properties. The iron content, in particular, can significantly influence the mineral's magnetic and optical properties.
The crystalline structure of magnesium iron silicate hydroxide plays a crucial role in its geological significance. It is commonly found in metamorphic and igneous rocks, particularly in serpentinized ultramafic rocks. The formation of this mineral is often associated with hydrothermal alteration processes, where water-rich fluids interact with magnesium and iron-bearing silicate minerals at elevated temperatures and pressures.
Understanding the crystalline structure of magnesium iron silicate hydroxide is essential for various applications in materials science, geology, and environmental studies. Its layered structure contributes to its use as a potential catalyst support, adsorbent, and in nanocomposite materials. Additionally, the mineral's ability to incorporate and release water within its structure makes it relevant in studies of deep Earth processes and the global water cycle.
Recent advancements in analytical techniques, such as high-resolution transmission electron microscopy and synchrotron-based X-ray diffraction, have enabled researchers to gain deeper insights into the atomic-scale structure and defects within magnesium iron silicate hydroxide crystals. These studies have revealed complex polytypism and stacking disorder, which further contribute to the mineral's diverse properties and behaviors under different geological conditions.
The crystalline structure of magnesium iron silicate hydroxide is characterized by alternating layers of tetrahedral silica sheets and octahedral brucite-like sheets. The tetrahedral layers consist of silicon atoms bonded to four oxygen atoms, while the octahedral layers contain magnesium and iron atoms coordinated with six oxygen or hydroxyl groups. This layered arrangement results in a 1:1 phyllosilicate structure, which is responsible for many of the mineral's physical and chemical properties.
One of the most notable features of magnesium iron silicate hydroxide is its ability to accommodate varying ratios of magnesium and iron within its structure. This compositional flexibility leads to a range of mineral varieties, including antigorite, lizardite, and chrysotile, each with slightly different structural arrangements and properties. The iron content, in particular, can significantly influence the mineral's magnetic and optical properties.
The crystalline structure of magnesium iron silicate hydroxide plays a crucial role in its geological significance. It is commonly found in metamorphic and igneous rocks, particularly in serpentinized ultramafic rocks. The formation of this mineral is often associated with hydrothermal alteration processes, where water-rich fluids interact with magnesium and iron-bearing silicate minerals at elevated temperatures and pressures.
Understanding the crystalline structure of magnesium iron silicate hydroxide is essential for various applications in materials science, geology, and environmental studies. Its layered structure contributes to its use as a potential catalyst support, adsorbent, and in nanocomposite materials. Additionally, the mineral's ability to incorporate and release water within its structure makes it relevant in studies of deep Earth processes and the global water cycle.
Recent advancements in analytical techniques, such as high-resolution transmission electron microscopy and synchrotron-based X-ray diffraction, have enabled researchers to gain deeper insights into the atomic-scale structure and defects within magnesium iron silicate hydroxide crystals. These studies have revealed complex polytypism and stacking disorder, which further contribute to the mineral's diverse properties and behaviors under different geological conditions.
Geological Significance and Applications
Magnesium iron silicate hydroxide, also known as serpentine, plays a crucial role in geological processes and has significant applications across various industries. This mineral's unique crystalline structure, characterized by its layered silicate sheets, contributes to its importance in both natural and engineered environments.
In geological contexts, serpentine is a key component of the Earth's mantle and oceanic crust. Its formation is closely associated with the hydration of ultramafic rocks, particularly during seafloor spreading and subduction processes. The presence of serpentine in these settings provides valuable insights into the tectonic history and metamorphic conditions of a region. Geologists utilize the mineral as an indicator of past hydrothermal activity and can infer information about the temperature, pressure, and fluid composition during its formation.
The mineral's ability to incorporate and retain water within its crystal structure makes it a significant player in the global water cycle. Serpentine can hold up to 13% water by weight, influencing the transport of water and other volatiles from the Earth's surface to its interior through subduction zones. This process has implications for the long-term evolution of the planet's hydrosphere and atmosphere.
In terms of applications, serpentine's unique properties make it valuable in various industrial and environmental contexts. The mineral's high magnesium content and reactive surface area have led to its exploration as a potential carbon dioxide sequestration material. Through a process called mineral carbonation, serpentine can react with CO2 to form stable carbonate minerals, offering a promising avenue for mitigating greenhouse gas emissions.
The construction industry benefits from serpentine's use as a source of magnesium in the production of refractories and as an additive in certain types of cement. Its heat-resistant properties make it suitable for applications in high-temperature environments. Additionally, the fibrous variety of serpentine, known as chrysotile asbestos, was historically used extensively in construction and manufacturing due to its fire-resistant properties. However, its use has been largely phased out due to health concerns.
In the field of environmental remediation, serpentine's ability to adsorb heavy metals and organic contaminants has led to its application in water and soil treatment processes. The mineral's large surface area and cation exchange capacity make it effective in removing pollutants from contaminated sites, offering a natural and cost-effective solution for environmental cleanup efforts.
The study of serpentine's crystalline structure continues to drive innovation in materials science. Researchers are exploring biomimetic approaches inspired by the mineral's layered structure to develop new materials with enhanced mechanical properties and chemical resistance. These efforts could lead to advancements in areas such as protective coatings, energy storage materials, and advanced composites.
In geological contexts, serpentine is a key component of the Earth's mantle and oceanic crust. Its formation is closely associated with the hydration of ultramafic rocks, particularly during seafloor spreading and subduction processes. The presence of serpentine in these settings provides valuable insights into the tectonic history and metamorphic conditions of a region. Geologists utilize the mineral as an indicator of past hydrothermal activity and can infer information about the temperature, pressure, and fluid composition during its formation.
The mineral's ability to incorporate and retain water within its crystal structure makes it a significant player in the global water cycle. Serpentine can hold up to 13% water by weight, influencing the transport of water and other volatiles from the Earth's surface to its interior through subduction zones. This process has implications for the long-term evolution of the planet's hydrosphere and atmosphere.
In terms of applications, serpentine's unique properties make it valuable in various industrial and environmental contexts. The mineral's high magnesium content and reactive surface area have led to its exploration as a potential carbon dioxide sequestration material. Through a process called mineral carbonation, serpentine can react with CO2 to form stable carbonate minerals, offering a promising avenue for mitigating greenhouse gas emissions.
The construction industry benefits from serpentine's use as a source of magnesium in the production of refractories and as an additive in certain types of cement. Its heat-resistant properties make it suitable for applications in high-temperature environments. Additionally, the fibrous variety of serpentine, known as chrysotile asbestos, was historically used extensively in construction and manufacturing due to its fire-resistant properties. However, its use has been largely phased out due to health concerns.
In the field of environmental remediation, serpentine's ability to adsorb heavy metals and organic contaminants has led to its application in water and soil treatment processes. The mineral's large surface area and cation exchange capacity make it effective in removing pollutants from contaminated sites, offering a natural and cost-effective solution for environmental cleanup efforts.
The study of serpentine's crystalline structure continues to drive innovation in materials science. Researchers are exploring biomimetic approaches inspired by the mineral's layered structure to develop new materials with enhanced mechanical properties and chemical resistance. These efforts could lead to advancements in areas such as protective coatings, energy storage materials, and advanced composites.
Current Challenges in Structural Analysis
The structural analysis of Magnesium iron silicate hydroxide presents several significant challenges that researchers and scientists are currently grappling with. One of the primary difficulties lies in the complex nature of the mineral's crystalline structure, which can exhibit variations depending on the specific composition and formation conditions. This complexity makes it challenging to obtain accurate and consistent data across different samples.
Another major hurdle is the presence of impurities and defects within the crystal lattice. These imperfections can significantly affect the overall structure and properties of the material, making it difficult to isolate and study the pure form of Magnesium iron silicate hydroxide. Researchers must develop sophisticated techniques to account for these variations and their impact on the structural analysis.
The multi-layered nature of the mineral's structure also poses a considerable challenge. The intricate arrangement of silicate sheets and metal cations requires advanced analytical methods to fully characterize the three-dimensional structure. Traditional X-ray diffraction techniques, while useful, may not always provide sufficient resolution to capture all the nuances of the layered structure, especially in samples with high degrees of disorder.
Furthermore, the potential for structural changes under different environmental conditions adds another layer of complexity to the analysis. Factors such as temperature, pressure, and hydration state can induce subtle alterations in the crystal structure, necessitating in-situ studies under controlled conditions. This requirement often pushes the limits of current analytical instrumentation and methodologies.
The presence of multiple polymorphs and polytypes of Magnesium iron silicate hydroxide further complicates structural analysis efforts. Distinguishing between these closely related structural variants and understanding their formation mechanisms and stability regions remains a significant challenge in the field.
Lastly, the integration of experimental data with computational modeling presents its own set of challenges. While advanced simulation techniques have greatly enhanced our understanding of crystal structures, bridging the gap between theoretical predictions and experimental observations remains an ongoing effort. Researchers must continually refine their models to accurately represent the complex interactions within the Magnesium iron silicate hydroxide structure.
Another major hurdle is the presence of impurities and defects within the crystal lattice. These imperfections can significantly affect the overall structure and properties of the material, making it difficult to isolate and study the pure form of Magnesium iron silicate hydroxide. Researchers must develop sophisticated techniques to account for these variations and their impact on the structural analysis.
The multi-layered nature of the mineral's structure also poses a considerable challenge. The intricate arrangement of silicate sheets and metal cations requires advanced analytical methods to fully characterize the three-dimensional structure. Traditional X-ray diffraction techniques, while useful, may not always provide sufficient resolution to capture all the nuances of the layered structure, especially in samples with high degrees of disorder.
Furthermore, the potential for structural changes under different environmental conditions adds another layer of complexity to the analysis. Factors such as temperature, pressure, and hydration state can induce subtle alterations in the crystal structure, necessitating in-situ studies under controlled conditions. This requirement often pushes the limits of current analytical instrumentation and methodologies.
The presence of multiple polymorphs and polytypes of Magnesium iron silicate hydroxide further complicates structural analysis efforts. Distinguishing between these closely related structural variants and understanding their formation mechanisms and stability regions remains a significant challenge in the field.
Lastly, the integration of experimental data with computational modeling presents its own set of challenges. While advanced simulation techniques have greatly enhanced our understanding of crystal structures, bridging the gap between theoretical predictions and experimental observations remains an ongoing effort. Researchers must continually refine their models to accurately represent the complex interactions within the Magnesium iron silicate hydroxide structure.
Advanced Analytical Techniques
01 Crystal structure and composition
Magnesium iron silicate hydroxide, also known as talc or steatite, has a layered crystal structure. It is composed of magnesium, iron, silicon, and hydroxide ions arranged in a specific pattern. The crystal structure contributes to its unique properties, such as softness and lubricity.- Crystal structure and composition: Magnesium iron silicate hydroxide, also known as talc or steatite, has a layered crystal structure. It is composed of sheets of magnesium and iron ions coordinated with oxygen and hydroxyl groups, alternating with layers of silica tetrahedra. The specific arrangement of these layers and the ratio of magnesium to iron can vary, affecting the properties of the mineral.
- Synthesis and modification methods: Various methods have been developed to synthesize and modify magnesium iron silicate hydroxide crystals. These include hydrothermal synthesis, sol-gel processes, and solid-state reactions. Modifications can involve ion exchange, intercalation, or surface treatments to alter the crystal structure or introduce new functionalities.
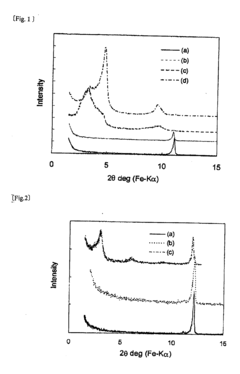
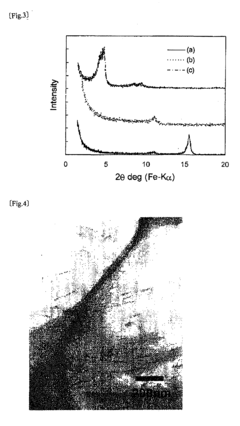
- Characterization techniques: Several analytical techniques are used to study the crystalline structure of magnesium iron silicate hydroxide. These include X-ray diffraction (XRD), transmission electron microscopy (TEM), and spectroscopic methods such as infrared and Raman spectroscopy. These techniques provide information on crystal lattice parameters, particle size, and chemical bonding within the structure.
- Influence of crystal structure on properties: The crystalline structure of magnesium iron silicate hydroxide significantly influences its physical and chemical properties. These include thermal stability, mechanical strength, adsorption capacity, and catalytic activity. Understanding the relationship between crystal structure and properties is crucial for optimizing the material for various applications.
- Applications based on crystal structure: The unique crystalline structure of magnesium iron silicate hydroxide makes it suitable for various applications. These include use as a filler in polymers, a catalyst support, an adsorbent for environmental remediation, and a raw material for ceramics. The layered structure also allows for potential applications in energy storage and nanocomposites.
02 Synthesis and preparation methods
Various methods are employed to synthesize magnesium iron silicate hydroxide with specific crystalline structures. These include hydrothermal synthesis, sol-gel methods, and solid-state reactions. The synthesis conditions, such as temperature, pressure, and pH, can be controlled to obtain desired crystal structures and properties.Expand Specific Solutions03 Characterization techniques
Several analytical techniques are used to study the crystalline structure of magnesium iron silicate hydroxide. These include X-ray diffraction (XRD), scanning electron microscopy (SEM), transmission electron microscopy (TEM), and spectroscopic methods such as Raman and infrared spectroscopy. These techniques provide information about crystal lattice parameters, morphology, and chemical bonding.Expand Specific Solutions04 Modifications and doping
The crystalline structure of magnesium iron silicate hydroxide can be modified through various methods, including ion exchange, intercalation, and doping with other elements. These modifications can alter the physical and chemical properties of the material, leading to enhanced performance in specific applications.Expand Specific Solutions05 Applications based on crystal structure
The unique crystalline structure of magnesium iron silicate hydroxide makes it suitable for various applications. These include use as a filler in polymers, ceramics, and cosmetics, as well as applications in catalysis, adsorption, and environmental remediation. The layered structure and surface properties contribute to its effectiveness in these applications.Expand Specific Solutions
Key Research Institutions and Scientists
The exploration of Magnesium iron silicate hydroxide's crystalline structure is in its early stages, with the market still developing. The global market for advanced materials research, including this compound, is estimated to be in the billions of dollars, driven by applications in various industries. The technology's maturity is evolving, with companies like BASF Corp., Tosoh Corp., and Eni SpA leading research efforts. Academic institutions such as the University of Queensland and Cornell University are also contributing to the field. National research organizations like the National Institute for Materials Science IAI and Consiglio Nazionale delle Ricerche are playing crucial roles in advancing the understanding of this material's structure and potential applications.
BASF Corp.
Technical Solution: BASF Corp. has developed advanced techniques for exploring the crystalline structure of Magnesium iron silicate hydroxide (MISH). They utilize high-resolution transmission electron microscopy (HRTEM) combined with X-ray diffraction (XRD) to analyze the atomic arrangement and interlayer spacing of MISH[1]. Their approach involves synthesizing MISH nanoparticles with controlled morphology and composition, allowing for precise manipulation of the crystal structure. BASF has also implemented in-situ characterization methods to study the dynamic behavior of MISH under various environmental conditions, providing insights into its structural stability and potential applications in catalysis and energy storage[3].
Strengths: Comprehensive characterization techniques, ability to synthesize tailored MISH structures. Weaknesses: Potentially high cost of advanced equipment, complexity in scaling up production.
National Institute for Materials Science IAI
Technical Solution: The National Institute for Materials Science (NIMS) has developed a multi-scale approach to exploring the crystalline structure of Magnesium iron silicate hydroxide. Their method combines advanced computational modeling with experimental techniques such as synchrotron-based X-ray absorption spectroscopy (XAS) and neutron diffraction[2]. NIMS researchers have created atomistic models of MISH using density functional theory (DFT) calculations, which are then validated through experimental data. This approach has led to the discovery of unique structural features in MISH, including the distribution of iron within the silicate layers and the role of hydroxyl groups in maintaining the crystal structure[4]. Additionally, NIMS has explored the potential of MISH as a precursor for novel magnetic materials by studying its transformation under controlled heat treatment[5].
Strengths: Integration of computational and experimental methods, access to advanced research facilities. Weaknesses: Primarily focused on fundamental research, which may limit immediate industrial applications.
Breakthrough Studies on Crystal Structure
Magnesium hydroxide having fine, plate-like crystalline structure and process therefor
PatentInactiveUS5143965A
Innovation
- A continuous process using ultrasonic mixing to precipitate magnesium hydroxide at controlled temperatures and alkaline material ratios, producing predominantly fine plate-like particles with a narrow size distribution, which can be coated with surfactants for improved performance.
Method for production of organic-inorganic complex, organic-inorganic complex, and polymeric composite material
PatentInactiveUS20090227715A1
Innovation
- A method involving heat treatment of non-swellable layered silicates to dehydrate them, followed by contact with a positively charged organic compound, facilitating the formation of organic-inorganic complexes and their incorporation into polymeric composites, which enhances the aspect ratio and properties of the nanocomposites.
Environmental Impact of Extraction
The extraction of Magnesium iron silicate hydroxide, commonly known as serpentine, can have significant environmental implications. The mining and processing of this mineral often involve large-scale operations that can lead to habitat destruction, soil erosion, and landscape alteration. Open-pit mining, a common method for serpentine extraction, can result in the removal of vast amounts of topsoil and vegetation, disrupting local ecosystems and potentially affecting biodiversity.
Water pollution is another critical concern associated with serpentine extraction. The mining process can release heavy metals and other contaminants into nearby water sources, potentially impacting aquatic life and downstream communities. Acid mine drainage, a phenomenon where sulfuric acid is produced when sulfide minerals are exposed to air and water, can further exacerbate water quality issues in the vicinity of mining operations.
Air quality can also be affected by serpentine extraction activities. Dust generated during mining and processing can contain asbestos-like fibers, which pose health risks to workers and nearby communities if inhaled. The use of heavy machinery and transportation vehicles in mining operations contributes to increased carbon emissions, potentially impacting local air quality and contributing to broader climate change concerns.
The extraction process often requires significant energy inputs, contributing to greenhouse gas emissions and resource depletion. The energy-intensive nature of serpentine mining and processing can have far-reaching environmental impacts beyond the immediate extraction site, particularly if fossil fuels are the primary energy source.
Waste management is another critical environmental challenge in serpentine extraction. The process generates substantial amounts of tailings and overburden, which must be properly managed to prevent environmental contamination. Improper disposal of these waste materials can lead to soil and water pollution, as well as potential long-term environmental liabilities.
Rehabilitation and reclamation of serpentine mining sites present unique challenges due to the mineral's chemical composition. The high magnesium content and low calcium levels in serpentine soils can make it difficult for vegetation to reestablish, potentially leading to long-term ecological impacts and delayed ecosystem recovery.
Efforts to mitigate the environmental impact of serpentine extraction include implementing more sustainable mining practices, such as improved waste management techniques, water recycling systems, and dust suppression measures. Additionally, research into alternative extraction methods and the development of more efficient processing technologies may help reduce the overall environmental footprint of serpentine mining operations in the future.
Water pollution is another critical concern associated with serpentine extraction. The mining process can release heavy metals and other contaminants into nearby water sources, potentially impacting aquatic life and downstream communities. Acid mine drainage, a phenomenon where sulfuric acid is produced when sulfide minerals are exposed to air and water, can further exacerbate water quality issues in the vicinity of mining operations.
Air quality can also be affected by serpentine extraction activities. Dust generated during mining and processing can contain asbestos-like fibers, which pose health risks to workers and nearby communities if inhaled. The use of heavy machinery and transportation vehicles in mining operations contributes to increased carbon emissions, potentially impacting local air quality and contributing to broader climate change concerns.
The extraction process often requires significant energy inputs, contributing to greenhouse gas emissions and resource depletion. The energy-intensive nature of serpentine mining and processing can have far-reaching environmental impacts beyond the immediate extraction site, particularly if fossil fuels are the primary energy source.
Waste management is another critical environmental challenge in serpentine extraction. The process generates substantial amounts of tailings and overburden, which must be properly managed to prevent environmental contamination. Improper disposal of these waste materials can lead to soil and water pollution, as well as potential long-term environmental liabilities.
Rehabilitation and reclamation of serpentine mining sites present unique challenges due to the mineral's chemical composition. The high magnesium content and low calcium levels in serpentine soils can make it difficult for vegetation to reestablish, potentially leading to long-term ecological impacts and delayed ecosystem recovery.
Efforts to mitigate the environmental impact of serpentine extraction include implementing more sustainable mining practices, such as improved waste management techniques, water recycling systems, and dust suppression measures. Additionally, research into alternative extraction methods and the development of more efficient processing technologies may help reduce the overall environmental footprint of serpentine mining operations in the future.
Computational Modeling Approaches
Computational modeling approaches have become indispensable tools in exploring the crystalline structure of Magnesium iron silicate hydroxide. These methods offer valuable insights into the atomic-scale properties and behavior of this complex mineral, complementing experimental techniques and advancing our understanding of its structural characteristics.
Density Functional Theory (DFT) calculations stand out as a primary computational approach for investigating the electronic structure and energetics of Magnesium iron silicate hydroxide. DFT simulations enable researchers to predict stable crystal structures, calculate formation energies, and analyze electronic properties with high accuracy. By employing various exchange-correlation functionals and accounting for van der Waals interactions, DFT studies have successfully reproduced experimental lattice parameters and provided detailed information on bond lengths and angles within the crystal structure.
Molecular Dynamics (MD) simulations offer another powerful tool for studying the dynamic behavior of Magnesium iron silicate hydroxide under different conditions. MD simulations allow researchers to explore thermal stability, phase transitions, and diffusion processes within the crystal lattice. By employing appropriate interatomic potentials and force fields, MD studies can reveal important insights into the structural evolution and mechanical properties of the mineral over extended time scales.
Ab initio Random Structure Searching (AIRSS) has emerged as a valuable technique for predicting novel crystal structures of Magnesium iron silicate hydroxide. This approach combines DFT calculations with intelligent structure generation algorithms to explore the energy landscape and identify thermodynamically stable configurations. AIRSS has been particularly useful in discovering new polymorphs and metastable phases that may be challenging to synthesize or observe experimentally.
Machine learning methods have recently gained traction in the computational modeling of complex materials like Magnesium iron silicate hydroxide. These approaches leverage large datasets of DFT calculations to develop accurate and computationally efficient interatomic potentials. Machine learning potentials can bridge the gap between the accuracy of quantum mechanical methods and the efficiency of classical force fields, enabling large-scale simulations of crystal growth, defect formation, and surface properties.
Spectroscopic simulations, such as the calculation of vibrational frequencies and X-ray absorption spectra, provide a crucial link between computational models and experimental observations. These simulations help interpret experimental data and validate theoretical predictions of the crystal structure. By comparing calculated spectra with experimental measurements, researchers can refine their structural models and gain deeper insights into the local atomic environment within Magnesium iron silicate hydroxide crystals.
Density Functional Theory (DFT) calculations stand out as a primary computational approach for investigating the electronic structure and energetics of Magnesium iron silicate hydroxide. DFT simulations enable researchers to predict stable crystal structures, calculate formation energies, and analyze electronic properties with high accuracy. By employing various exchange-correlation functionals and accounting for van der Waals interactions, DFT studies have successfully reproduced experimental lattice parameters and provided detailed information on bond lengths and angles within the crystal structure.
Molecular Dynamics (MD) simulations offer another powerful tool for studying the dynamic behavior of Magnesium iron silicate hydroxide under different conditions. MD simulations allow researchers to explore thermal stability, phase transitions, and diffusion processes within the crystal lattice. By employing appropriate interatomic potentials and force fields, MD studies can reveal important insights into the structural evolution and mechanical properties of the mineral over extended time scales.
Ab initio Random Structure Searching (AIRSS) has emerged as a valuable technique for predicting novel crystal structures of Magnesium iron silicate hydroxide. This approach combines DFT calculations with intelligent structure generation algorithms to explore the energy landscape and identify thermodynamically stable configurations. AIRSS has been particularly useful in discovering new polymorphs and metastable phases that may be challenging to synthesize or observe experimentally.
Machine learning methods have recently gained traction in the computational modeling of complex materials like Magnesium iron silicate hydroxide. These approaches leverage large datasets of DFT calculations to develop accurate and computationally efficient interatomic potentials. Machine learning potentials can bridge the gap between the accuracy of quantum mechanical methods and the efficiency of classical force fields, enabling large-scale simulations of crystal growth, defect formation, and surface properties.
Spectroscopic simulations, such as the calculation of vibrational frequencies and X-ray absorption spectra, provide a crucial link between computational models and experimental observations. These simulations help interpret experimental data and validate theoretical predictions of the crystal structure. By comparing calculated spectra with experimental measurements, researchers can refine their structural models and gain deeper insights into the local atomic environment within Magnesium iron silicate hydroxide crystals.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!