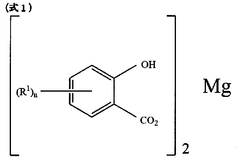
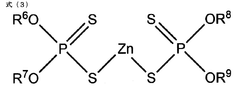
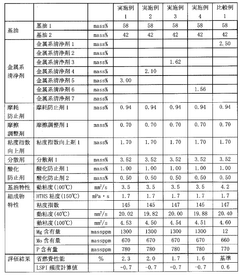
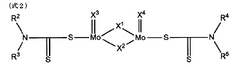Magnesium iron silicate hydroxide's influence on lubrication viscosity.
JUL 17, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Background and Objectives
Magnesium iron silicate hydroxide, also known as sepiolite, has garnered significant attention in the field of lubrication technology due to its unique structural properties and potential to influence viscosity. This naturally occurring clay mineral has a fibrous morphology and high surface area, which contribute to its exceptional adsorption and rheological characteristics.
The development of advanced lubricants has become increasingly crucial in various industries, including automotive, aerospace, and manufacturing. As machinery and equipment become more sophisticated, the demand for lubricants that can withstand extreme conditions while maintaining optimal performance has grown exponentially. In this context, the exploration of novel additives that can enhance lubricant properties has become a key focus of research and development efforts.
The study of magnesium iron silicate hydroxide's influence on lubrication viscosity is rooted in the broader field of tribology, which encompasses the science of friction, wear, and lubrication. Over the past decades, researchers have made significant strides in understanding the fundamental mechanisms of lubrication and the factors that affect viscosity. However, the complex interactions between lubricant additives and base oils continue to present challenges and opportunities for innovation.
The primary objective of investigating magnesium iron silicate hydroxide's impact on lubrication viscosity is to develop more efficient and durable lubricant formulations. By understanding how this mineral affects the rheological properties of lubricants, researchers aim to create products that can maintain optimal viscosity across a wide range of temperatures and pressures. This could lead to improved energy efficiency, reduced wear on mechanical components, and extended equipment lifespan.
Furthermore, the exploration of magnesium iron silicate hydroxide as a lubricant additive aligns with the growing trend towards environmentally friendly and sustainable lubrication solutions. As a naturally occurring mineral, it presents a potentially more eco-friendly alternative to some synthetic additives currently in use. This aspect of the research is particularly relevant given the increasing global emphasis on reducing environmental impact across all industrial sectors.
The technological evolution in this field is expected to have far-reaching implications for various industries. Improved lubricants could lead to significant advancements in automotive engine efficiency, aerospace applications, and industrial machinery performance. Additionally, the insights gained from studying magnesium iron silicate hydroxide's influence on viscosity could potentially be applied to other areas of materials science and nanotechnology.
As we delve deeper into this topic, it is essential to consider the multifaceted nature of the research. The investigation encompasses not only the chemical and physical properties of magnesium iron silicate hydroxide but also its interactions with different types of base oils and other additives. This comprehensive approach is crucial for developing a thorough understanding of its potential applications and limitations in lubrication technology.
The development of advanced lubricants has become increasingly crucial in various industries, including automotive, aerospace, and manufacturing. As machinery and equipment become more sophisticated, the demand for lubricants that can withstand extreme conditions while maintaining optimal performance has grown exponentially. In this context, the exploration of novel additives that can enhance lubricant properties has become a key focus of research and development efforts.
The study of magnesium iron silicate hydroxide's influence on lubrication viscosity is rooted in the broader field of tribology, which encompasses the science of friction, wear, and lubrication. Over the past decades, researchers have made significant strides in understanding the fundamental mechanisms of lubrication and the factors that affect viscosity. However, the complex interactions between lubricant additives and base oils continue to present challenges and opportunities for innovation.
The primary objective of investigating magnesium iron silicate hydroxide's impact on lubrication viscosity is to develop more efficient and durable lubricant formulations. By understanding how this mineral affects the rheological properties of lubricants, researchers aim to create products that can maintain optimal viscosity across a wide range of temperatures and pressures. This could lead to improved energy efficiency, reduced wear on mechanical components, and extended equipment lifespan.
Furthermore, the exploration of magnesium iron silicate hydroxide as a lubricant additive aligns with the growing trend towards environmentally friendly and sustainable lubrication solutions. As a naturally occurring mineral, it presents a potentially more eco-friendly alternative to some synthetic additives currently in use. This aspect of the research is particularly relevant given the increasing global emphasis on reducing environmental impact across all industrial sectors.
The technological evolution in this field is expected to have far-reaching implications for various industries. Improved lubricants could lead to significant advancements in automotive engine efficiency, aerospace applications, and industrial machinery performance. Additionally, the insights gained from studying magnesium iron silicate hydroxide's influence on viscosity could potentially be applied to other areas of materials science and nanotechnology.
As we delve deeper into this topic, it is essential to consider the multifaceted nature of the research. The investigation encompasses not only the chemical and physical properties of magnesium iron silicate hydroxide but also its interactions with different types of base oils and other additives. This comprehensive approach is crucial for developing a thorough understanding of its potential applications and limitations in lubrication technology.
Market Analysis
The market for magnesium iron silicate hydroxide (MISH) in lubrication applications has been experiencing steady growth due to its unique properties and increasing demand for high-performance lubricants. The global lubricants market, valued at approximately $150 billion in 2020, is projected to reach $180 billion by 2025, with a compound annual growth rate of 3.7%. Within this market, the demand for additives that enhance viscosity and thermal stability is particularly strong.
MISH has gained significant attention in the automotive and industrial sectors, where lubricants play a crucial role in machinery performance and longevity. The automotive lubricants segment, which accounts for about 40% of the total lubricants market, is a key driver for MISH demand. As vehicle manufacturers strive to meet stringent emission standards and improve fuel efficiency, there is a growing need for lubricants that can maintain viscosity under extreme conditions.
The industrial lubricants segment, representing approximately 35% of the market, is another major consumer of MISH-enhanced products. Industries such as manufacturing, mining, and power generation require lubricants that can withstand high temperatures and pressures, making MISH an attractive additive option. The aerospace and marine sectors are also emerging as potential growth areas for MISH-based lubricants due to their demanding operational environments.
Geographically, Asia-Pacific leads the lubricants market, accounting for over 40% of global consumption. The rapid industrialization and increasing automotive production in countries like China and India are driving the demand for high-performance lubricants. North America and Europe follow, with a combined market share of about 35%. These regions are particularly interested in environmentally friendly and long-lasting lubricant solutions, aligning well with the properties of MISH.
The market for MISH in lubrication applications is also influenced by broader trends in the industry. There is a growing emphasis on bio-based and sustainable lubricants, which presents both opportunities and challenges for MISH. While it is a naturally occurring mineral, its compatibility with bio-based formulations is an area of ongoing research and development.
Furthermore, the increasing focus on energy efficiency and reduced maintenance costs in various industries is expected to boost the demand for lubricants with enhanced viscosity properties. MISH's ability to improve the viscosity index and thermal stability of lubricants positions it favorably in this market trend. However, the market also faces challenges such as fluctuating raw material prices and the need for continuous innovation to meet evolving industry requirements.
MISH has gained significant attention in the automotive and industrial sectors, where lubricants play a crucial role in machinery performance and longevity. The automotive lubricants segment, which accounts for about 40% of the total lubricants market, is a key driver for MISH demand. As vehicle manufacturers strive to meet stringent emission standards and improve fuel efficiency, there is a growing need for lubricants that can maintain viscosity under extreme conditions.
The industrial lubricants segment, representing approximately 35% of the market, is another major consumer of MISH-enhanced products. Industries such as manufacturing, mining, and power generation require lubricants that can withstand high temperatures and pressures, making MISH an attractive additive option. The aerospace and marine sectors are also emerging as potential growth areas for MISH-based lubricants due to their demanding operational environments.
Geographically, Asia-Pacific leads the lubricants market, accounting for over 40% of global consumption. The rapid industrialization and increasing automotive production in countries like China and India are driving the demand for high-performance lubricants. North America and Europe follow, with a combined market share of about 35%. These regions are particularly interested in environmentally friendly and long-lasting lubricant solutions, aligning well with the properties of MISH.
The market for MISH in lubrication applications is also influenced by broader trends in the industry. There is a growing emphasis on bio-based and sustainable lubricants, which presents both opportunities and challenges for MISH. While it is a naturally occurring mineral, its compatibility with bio-based formulations is an area of ongoing research and development.
Furthermore, the increasing focus on energy efficiency and reduced maintenance costs in various industries is expected to boost the demand for lubricants with enhanced viscosity properties. MISH's ability to improve the viscosity index and thermal stability of lubricants positions it favorably in this market trend. However, the market also faces challenges such as fluctuating raw material prices and the need for continuous innovation to meet evolving industry requirements.
Technical Challenges
The development of magnesium iron silicate hydroxide (MISH) as a lubricant additive faces several technical challenges that hinder its widespread adoption and optimal performance. One of the primary obstacles is the difficulty in achieving uniform dispersion of MISH particles within the lubricant matrix. The tendency of these particles to agglomerate can lead to inconsistent viscosity and reduced lubricating effectiveness.
Another significant challenge lies in maintaining the stability of MISH-enhanced lubricants over extended periods and under varying operating conditions. The potential for particle settling or separation from the base oil can compromise the long-term performance and reliability of the lubricant system. This issue is particularly pronounced in applications involving high temperatures or extreme pressure conditions.
The interaction between MISH particles and other lubricant additives presents a complex challenge. Ensuring compatibility and synergistic effects rather than antagonistic interactions requires extensive research and formulation optimization. This complexity increases the difficulty of developing standardized MISH-based lubricant formulations suitable for a wide range of applications.
Controlling the particle size distribution of MISH additives is crucial for optimizing their influence on lubrication viscosity. However, achieving consistent and precise particle sizes during production and maintaining this distribution during lubricant use remains a technical hurdle. The relationship between particle size, concentration, and viscosity effects is not fully understood, necessitating further investigation.
The potential impact of MISH additives on lubricant degradation and equipment wear over time is another area of concern. While MISH shows promise in enhancing lubrication properties, its long-term effects on lubricant oxidation stability and potential abrasive wear on mechanical components require thorough evaluation and mitigation strategies.
Environmental and health considerations pose additional challenges. As regulations become more stringent, ensuring that MISH-based lubricants meet environmental standards and do not pose health risks during production, use, and disposal is critical. This includes addressing potential leaching of magnesium or iron ions into the environment and assessing any respiratory risks associated with MISH particle inhalation during lubricant application or maintenance.
Lastly, the scalability of MISH production and its integration into existing lubricant manufacturing processes present significant technical and economic challenges. Developing cost-effective methods for large-scale synthesis of MISH with consistent quality and properties is essential for its commercial viability as a lubricant additive.
Another significant challenge lies in maintaining the stability of MISH-enhanced lubricants over extended periods and under varying operating conditions. The potential for particle settling or separation from the base oil can compromise the long-term performance and reliability of the lubricant system. This issue is particularly pronounced in applications involving high temperatures or extreme pressure conditions.
The interaction between MISH particles and other lubricant additives presents a complex challenge. Ensuring compatibility and synergistic effects rather than antagonistic interactions requires extensive research and formulation optimization. This complexity increases the difficulty of developing standardized MISH-based lubricant formulations suitable for a wide range of applications.
Controlling the particle size distribution of MISH additives is crucial for optimizing their influence on lubrication viscosity. However, achieving consistent and precise particle sizes during production and maintaining this distribution during lubricant use remains a technical hurdle. The relationship between particle size, concentration, and viscosity effects is not fully understood, necessitating further investigation.
The potential impact of MISH additives on lubricant degradation and equipment wear over time is another area of concern. While MISH shows promise in enhancing lubrication properties, its long-term effects on lubricant oxidation stability and potential abrasive wear on mechanical components require thorough evaluation and mitigation strategies.
Environmental and health considerations pose additional challenges. As regulations become more stringent, ensuring that MISH-based lubricants meet environmental standards and do not pose health risks during production, use, and disposal is critical. This includes addressing potential leaching of magnesium or iron ions into the environment and assessing any respiratory risks associated with MISH particle inhalation during lubricant application or maintenance.
Lastly, the scalability of MISH production and its integration into existing lubricant manufacturing processes present significant technical and economic challenges. Developing cost-effective methods for large-scale synthesis of MISH with consistent quality and properties is essential for its commercial viability as a lubricant additive.
Current Solutions
01 Viscosity modification of magnesium iron silicate hydroxide
Magnesium iron silicate hydroxide can be used as a viscosity modifier in various applications. Its unique structure allows it to form gel-like networks in aqueous solutions, providing thixotropic properties. The viscosity can be adjusted by controlling the concentration and particle size of the material, making it suitable for use in cosmetics, paints, and other industrial products.- Viscosity modification of magnesium iron silicate hydroxide: Magnesium iron silicate hydroxide can be used as a viscosity modifier in various applications. Its unique structure allows for adjustable viscosity properties, making it suitable for use in cosmetics, pharmaceuticals, and industrial products. The viscosity can be controlled by altering the concentration and processing conditions of the material.
- Rheological properties of magnesium iron silicate hydroxide suspensions: Suspensions containing magnesium iron silicate hydroxide exhibit complex rheological behavior. The viscosity of these suspensions can be influenced by factors such as particle size, concentration, pH, and the presence of additives. Understanding these properties is crucial for optimizing the performance of products containing this material.
- Application in drilling fluids: Magnesium iron silicate hydroxide is used in drilling fluids to control viscosity and provide thixotropic properties. Its ability to form gel-like structures under static conditions and flow under shear makes it valuable for maintaining well stability and suspending drill cuttings. The viscosity of these fluids can be adjusted to suit different drilling conditions.
- Synergistic effects with other additives: The viscosity of magnesium iron silicate hydroxide can be further modified by combining it with other additives. Synergistic effects have been observed with various polymers, surfactants, and inorganic compounds. These combinations can lead to enhanced viscosity control and improved stability in different formulations.
- Temperature and shear-dependent viscosity: The viscosity of magnesium iron silicate hydroxide-based systems shows dependence on temperature and shear rate. This behavior is important in applications where the material is subjected to varying conditions. Understanding these relationships allows for better prediction and control of viscosity in different operating environments.
02 Rheological properties of magnesium iron silicate hydroxide suspensions
The rheological behavior of magnesium iron silicate hydroxide suspensions is influenced by factors such as pH, temperature, and shear rate. These suspensions often exhibit shear-thinning behavior, where viscosity decreases with increasing shear rate. Understanding these properties is crucial for optimizing the performance of products containing this material in various applications.Expand Specific Solutions03 Surface modification of magnesium iron silicate hydroxide for viscosity control
Surface modification techniques can be applied to magnesium iron silicate hydroxide particles to alter their interactions with surrounding media. This can lead to improved dispersion, stability, and viscosity control in various formulations. Methods such as organic coating or chemical grafting can be employed to tailor the material's properties for specific applications.Expand Specific Solutions04 Synergistic effects with other viscosity modifiers
Magnesium iron silicate hydroxide can be combined with other viscosity modifiers to achieve synergistic effects. These combinations can result in enhanced rheological properties, improved stability, and better control over the final product's viscosity. Examples of complementary materials include cellulose derivatives, synthetic polymers, and other clay minerals.Expand Specific Solutions05 Applications utilizing magnesium iron silicate hydroxide's viscosity properties
The unique viscosity properties of magnesium iron silicate hydroxide make it suitable for various applications. It can be used in drilling fluids to control rheology and prevent fluid loss, in cosmetics to provide texture and stability, and in construction materials to improve workability and prevent segregation. Its versatility and effectiveness in controlling viscosity have led to its widespread use across multiple industries.Expand Specific Solutions
Key Industry Players
The competitive landscape for magnesium iron silicate hydroxide's influence on lubrication viscosity is in an early development stage, with a growing market as industries seek improved lubricant performance. The technology is still maturing, with major players like China Petroleum & Chemical Corp., ENEOS Corp., and Chevron Japan Ltd. investing in research and development. These companies, along with others such as Afton Chemical Corp. and ExxonMobil Technology & Engineering Co., are exploring the potential of this material to enhance lubricant properties. The market size is expanding as automotive, industrial, and energy sectors recognize the benefits of improved lubrication viscosity. However, the technology's full potential and widespread adoption are yet to be realized, indicating significant room for growth and innovation in this field.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed advanced lubricant formulations incorporating magnesium iron silicate hydroxide (MISH) to enhance lubrication viscosity. Their research focuses on the synergistic effects of MISH with other additives to create high-performance lubricants. Sinopec's approach involves dispersing nano-sized MISH particles in base oils, which form a stable colloidal suspension. This suspension significantly improves the viscosity index and shear stability of the lubricant[1]. The company has also explored the tribological properties of MISH-enhanced lubricants, demonstrating reduced friction and wear in high-temperature and high-pressure conditions[2].
Strengths: Extensive research capabilities, access to large-scale production facilities, and a strong market presence in the petrochemical industry. Weaknesses: Potential environmental concerns associated with nanoparticle additives and the need for further long-term stability studies of MISH-enhanced lubricants.
Afton Chemical Corp.
Technical Solution: Afton Chemical Corp. has developed a proprietary technology that utilizes magnesium iron silicate hydroxide (MISH) as a viscosity modifier in lubricant formulations. Their approach involves surface modification of MISH particles to enhance compatibility with base oils and other additives. Afton's research has shown that MISH can provide excellent thickening efficiency and shear stability, particularly in high-temperature applications[3]. The company has also investigated the potential of MISH to act as a friction modifier, reducing energy losses in automotive and industrial machinery. Afton's formulations have demonstrated improved oxidation resistance and deposit control when compared to conventional viscosity modifiers[4].
Strengths: Strong expertise in additive chemistry, established relationships with major lubricant manufacturers, and a global research and development network. Weaknesses: Potential higher costs associated with specialized MISH production and modification processes.
Core Innovations
Lubricant composition for internal combustion engine
PatentWO2022250018A1
Innovation
- A lubricating oil composition with a specific HTHS viscosity range at 150°C, containing magnesium salicylate as a metal-based detergent, and a molybdenum-based friction modifier, which also includes a viscosity index improver, to achieve both fuel efficiency and reduced LSPI frequency.
Magnesium oxide powder, vulcanizing agent composition for rubber, rubber composition, and production method for magnesium oxide powder
PatentPendingUS20250162897A1
Innovation
- Adjusting the particle size distribution and citric acid activity of magnesium oxide powder to specific ranges (D50 ≤ 10 μm, D90/D10 ≤ 10, citric acid activity between 500 seconds and 2,500 seconds) enhances vulcanization acceleration while maintaining hydration resistance.
Environmental Impact
The environmental impact of magnesium iron silicate hydroxide's influence on lubrication viscosity is a critical consideration in the development and application of this technology. As lubricants play a crucial role in various industrial processes and mechanical systems, understanding the environmental implications of their composition and performance is essential for sustainable development.
Magnesium iron silicate hydroxide, when used as an additive in lubricants, can significantly affect the viscosity and overall performance of the lubricating fluid. This influence on viscosity has both direct and indirect environmental consequences. One of the primary environmental benefits is the potential for improved energy efficiency in mechanical systems. By optimizing the viscosity of lubricants, friction between moving parts can be reduced, leading to lower energy consumption and, consequently, reduced greenhouse gas emissions from power generation.
However, the production and use of magnesium iron silicate hydroxide in lubricants also present environmental challenges. The mining and processing of raw materials required for its synthesis can lead to habitat disruption, soil erosion, and water pollution if not managed responsibly. Additionally, the manufacturing process may involve energy-intensive steps, contributing to carbon emissions and resource depletion.
The disposal of used lubricants containing magnesium iron silicate hydroxide is another significant environmental concern. Improper disposal can lead to soil and water contamination, potentially harming ecosystems and human health. However, the presence of this compound may also offer opportunities for more effective recycling and regeneration of used lubricants, potentially reducing the overall environmental footprint of lubricant life cycles.
In terms of biodegradability, the impact of magnesium iron silicate hydroxide on the breakdown of lubricants in the environment requires careful consideration. While some studies suggest that certain formulations may enhance biodegradability, others indicate potential persistence in ecosystems. This aspect necessitates further research to fully understand the long-term environmental fate of lubricants containing this compound.
The use of magnesium iron silicate hydroxide in lubricants may also have implications for air quality. By improving the efficiency of mechanical systems and reducing wear, it could potentially decrease particulate emissions from engines and industrial machinery. However, the potential for nanoparticle release during use and disposal needs to be thoroughly investigated to ensure that air quality is not negatively impacted.
In conclusion, while magnesium iron silicate hydroxide shows promise in enhancing lubrication viscosity and potentially improving energy efficiency, its environmental impact is complex and multifaceted. Balancing the benefits of improved mechanical performance against the potential environmental risks requires ongoing research, careful lifecycle assessments, and the development of sustainable practices in production, use, and disposal.
Magnesium iron silicate hydroxide, when used as an additive in lubricants, can significantly affect the viscosity and overall performance of the lubricating fluid. This influence on viscosity has both direct and indirect environmental consequences. One of the primary environmental benefits is the potential for improved energy efficiency in mechanical systems. By optimizing the viscosity of lubricants, friction between moving parts can be reduced, leading to lower energy consumption and, consequently, reduced greenhouse gas emissions from power generation.
However, the production and use of magnesium iron silicate hydroxide in lubricants also present environmental challenges. The mining and processing of raw materials required for its synthesis can lead to habitat disruption, soil erosion, and water pollution if not managed responsibly. Additionally, the manufacturing process may involve energy-intensive steps, contributing to carbon emissions and resource depletion.
The disposal of used lubricants containing magnesium iron silicate hydroxide is another significant environmental concern. Improper disposal can lead to soil and water contamination, potentially harming ecosystems and human health. However, the presence of this compound may also offer opportunities for more effective recycling and regeneration of used lubricants, potentially reducing the overall environmental footprint of lubricant life cycles.
In terms of biodegradability, the impact of magnesium iron silicate hydroxide on the breakdown of lubricants in the environment requires careful consideration. While some studies suggest that certain formulations may enhance biodegradability, others indicate potential persistence in ecosystems. This aspect necessitates further research to fully understand the long-term environmental fate of lubricants containing this compound.
The use of magnesium iron silicate hydroxide in lubricants may also have implications for air quality. By improving the efficiency of mechanical systems and reducing wear, it could potentially decrease particulate emissions from engines and industrial machinery. However, the potential for nanoparticle release during use and disposal needs to be thoroughly investigated to ensure that air quality is not negatively impacted.
In conclusion, while magnesium iron silicate hydroxide shows promise in enhancing lubrication viscosity and potentially improving energy efficiency, its environmental impact is complex and multifaceted. Balancing the benefits of improved mechanical performance against the potential environmental risks requires ongoing research, careful lifecycle assessments, and the development of sustainable practices in production, use, and disposal.
Regulatory Compliance
The regulatory landscape surrounding the use of magnesium iron silicate hydroxide in lubrication applications is complex and multifaceted. Compliance with various regulations is crucial for manufacturers and users of lubricants containing this compound. The primary regulatory bodies overseeing the use of such materials include the Environmental Protection Agency (EPA), the Occupational Safety and Health Administration (OSHA), and the Food and Drug Administration (FDA) in the United States, as well as their counterparts in other countries.
Environmental regulations play a significant role in the use of magnesium iron silicate hydroxide in lubricants. The EPA's Toxic Substances Control Act (TSCA) requires manufacturers to report new chemical substances and maintain records of their use. Additionally, the Clean Water Act and Clean Air Act may impose restrictions on the disposal and emissions related to the production and use of lubricants containing this compound.
Workplace safety regulations are another critical aspect of compliance. OSHA mandates that employers provide safe working conditions and proper training for employees handling lubricants containing magnesium iron silicate hydroxide. This includes providing appropriate personal protective equipment (PPE) and implementing safety protocols to minimize exposure risks.
In the context of lubricant applications in food processing or pharmaceutical industries, FDA regulations come into play. The FDA's Good Manufacturing Practices (GMP) guidelines may apply to the production and use of lubricants in these sectors, ensuring that the materials used meet stringent safety and quality standards.
Internationally, the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation in the European Union imposes strict requirements on the registration and use of chemical substances, including those used in lubricants. Manufacturers and importers must comply with REACH guidelines when bringing products containing magnesium iron silicate hydroxide into the EU market.
Transportation of lubricants containing this compound is subject to regulations set by the Department of Transportation (DOT) in the US and similar agencies worldwide. These regulations govern the packaging, labeling, and shipping of potentially hazardous materials.
Compliance with these regulations requires ongoing monitoring and adaptation to changes in legislation. Companies must invest in robust compliance programs, including regular audits, employee training, and documentation systems to ensure adherence to all applicable regulations. Failure to comply can result in significant fines, legal liabilities, and reputational damage.
As environmental and safety concerns continue to evolve, it is likely that regulations surrounding the use of magnesium iron silicate hydroxide in lubricants will become more stringent. Companies in this field must stay informed about regulatory developments and be prepared to adapt their practices accordingly to maintain compliance and ensure the safe and responsible use of these materials.
Environmental regulations play a significant role in the use of magnesium iron silicate hydroxide in lubricants. The EPA's Toxic Substances Control Act (TSCA) requires manufacturers to report new chemical substances and maintain records of their use. Additionally, the Clean Water Act and Clean Air Act may impose restrictions on the disposal and emissions related to the production and use of lubricants containing this compound.
Workplace safety regulations are another critical aspect of compliance. OSHA mandates that employers provide safe working conditions and proper training for employees handling lubricants containing magnesium iron silicate hydroxide. This includes providing appropriate personal protective equipment (PPE) and implementing safety protocols to minimize exposure risks.
In the context of lubricant applications in food processing or pharmaceutical industries, FDA regulations come into play. The FDA's Good Manufacturing Practices (GMP) guidelines may apply to the production and use of lubricants in these sectors, ensuring that the materials used meet stringent safety and quality standards.
Internationally, the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation in the European Union imposes strict requirements on the registration and use of chemical substances, including those used in lubricants. Manufacturers and importers must comply with REACH guidelines when bringing products containing magnesium iron silicate hydroxide into the EU market.
Transportation of lubricants containing this compound is subject to regulations set by the Department of Transportation (DOT) in the US and similar agencies worldwide. These regulations govern the packaging, labeling, and shipping of potentially hazardous materials.
Compliance with these regulations requires ongoing monitoring and adaptation to changes in legislation. Companies must invest in robust compliance programs, including regular audits, employee training, and documentation systems to ensure adherence to all applicable regulations. Failure to comply can result in significant fines, legal liabilities, and reputational damage.
As environmental and safety concerns continue to evolve, it is likely that regulations surrounding the use of magnesium iron silicate hydroxide in lubricants will become more stringent. Companies in this field must stay informed about regulatory developments and be prepared to adapt their practices accordingly to maintain compliance and ensure the safe and responsible use of these materials.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!