Carbonate breakdown channels involving Magnesium iron silicate hydroxide.
JUL 17, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Carbonate Breakdown Research Background and Objectives
The study of carbonate breakdown channels involving Magnesium iron silicate hydroxide has gained significant attention in recent years due to its potential implications for various geological processes and industrial applications. This research aims to explore the complex interactions between carbonate minerals and Magnesium iron silicate hydroxide, focusing on the mechanisms and pathways of carbonate dissolution and transformation.
Historically, carbonate breakdown has been a subject of interest in geochemistry and earth sciences, with early studies dating back to the mid-20th century. However, the specific role of Magnesium iron silicate hydroxide in these processes has only recently come under scrutiny. The evolution of analytical techniques and computational modeling has enabled researchers to delve deeper into the molecular-level interactions and reaction kinetics involved in carbonate breakdown.
The primary objective of this research is to elucidate the fundamental mechanisms governing carbonate breakdown in the presence of Magnesium iron silicate hydroxide. This includes identifying the key reaction pathways, understanding the influence of environmental factors such as temperature, pressure, and pH, and quantifying the rates of carbonate dissolution and reprecipitation. Additionally, the study aims to investigate the potential formation of secondary minerals and their impact on the overall reaction dynamics.
Another crucial goal is to explore the implications of these processes in natural systems, particularly in the context of carbon cycling and sequestration. Understanding the role of Magnesium iron silicate hydroxide in carbonate breakdown could provide valuable insights into long-term carbon storage mechanisms in geological formations and the potential for enhanced weathering as a climate change mitigation strategy.
From an industrial perspective, this research seeks to uncover potential applications in fields such as cement production, CO2 capture and storage, and mineral processing. By gaining a comprehensive understanding of the carbonate breakdown channels, it may be possible to develop more efficient and environmentally friendly processes in these sectors.
The technological trajectory of this research is expected to involve advanced spectroscopic and microscopic techniques, in-situ observations of reaction processes, and the development of sophisticated geochemical models. These tools will enable researchers to probe the nanoscale interactions between carbonate minerals and Magnesium iron silicate hydroxide, providing unprecedented insights into the reaction mechanisms and kinetics.
As the field progresses, it is anticipated that interdisciplinary collaborations between geochemists, materials scientists, and environmental engineers will drive innovation and lead to novel applications of the knowledge gained from this research. The ultimate aim is to bridge the gap between fundamental scientific understanding and practical, real-world applications that can address pressing environmental and industrial challenges.
Historically, carbonate breakdown has been a subject of interest in geochemistry and earth sciences, with early studies dating back to the mid-20th century. However, the specific role of Magnesium iron silicate hydroxide in these processes has only recently come under scrutiny. The evolution of analytical techniques and computational modeling has enabled researchers to delve deeper into the molecular-level interactions and reaction kinetics involved in carbonate breakdown.
The primary objective of this research is to elucidate the fundamental mechanisms governing carbonate breakdown in the presence of Magnesium iron silicate hydroxide. This includes identifying the key reaction pathways, understanding the influence of environmental factors such as temperature, pressure, and pH, and quantifying the rates of carbonate dissolution and reprecipitation. Additionally, the study aims to investigate the potential formation of secondary minerals and their impact on the overall reaction dynamics.
Another crucial goal is to explore the implications of these processes in natural systems, particularly in the context of carbon cycling and sequestration. Understanding the role of Magnesium iron silicate hydroxide in carbonate breakdown could provide valuable insights into long-term carbon storage mechanisms in geological formations and the potential for enhanced weathering as a climate change mitigation strategy.
From an industrial perspective, this research seeks to uncover potential applications in fields such as cement production, CO2 capture and storage, and mineral processing. By gaining a comprehensive understanding of the carbonate breakdown channels, it may be possible to develop more efficient and environmentally friendly processes in these sectors.
The technological trajectory of this research is expected to involve advanced spectroscopic and microscopic techniques, in-situ observations of reaction processes, and the development of sophisticated geochemical models. These tools will enable researchers to probe the nanoscale interactions between carbonate minerals and Magnesium iron silicate hydroxide, providing unprecedented insights into the reaction mechanisms and kinetics.
As the field progresses, it is anticipated that interdisciplinary collaborations between geochemists, materials scientists, and environmental engineers will drive innovation and lead to novel applications of the knowledge gained from this research. The ultimate aim is to bridge the gap between fundamental scientific understanding and practical, real-world applications that can address pressing environmental and industrial challenges.
Market Applications of Carbonate Decomposition
Carbonate decomposition technology has found significant applications across various industries, driving market growth and innovation. In the construction sector, this technology plays a crucial role in cement production, where calcium carbonate is decomposed to produce lime, a key ingredient in cement manufacturing. The global cement market, valued at over $300 billion, heavily relies on this process, with increasing demand for sustainable construction materials driving further research into efficient carbonate decomposition methods.
The mining and metallurgy industries also benefit from carbonate decomposition technologies. In mineral processing, carbonate minerals are often decomposed to extract valuable metals or to produce raw materials for other industrial processes. This application is particularly important in the production of magnesium, where magnesium carbonate is decomposed to yield magnesium oxide, a versatile compound used in refractory materials, pharmaceuticals, and environmental remediation.
In the energy sector, carbonate decomposition has gained attention for its potential in carbon capture and storage (CCS) technologies. Mineral carbonation, which involves the reaction of CO2 with metal oxides to form stable carbonate minerals, is being explored as a method to sequester carbon dioxide from industrial emissions. This application has significant market potential, with the global CCS market projected to grow substantially in the coming years as countries strive to meet their carbon reduction targets.
The agricultural industry utilizes carbonate decomposition in soil amendment practices. Lime, produced through the decomposition of limestone, is widely used to neutralize soil acidity and improve crop yields. The global agricultural lime market, valued at several billion dollars, continues to expand as farmers seek to optimize soil conditions for increased productivity.
In the chemical industry, carbonate decomposition is employed in the production of various compounds and materials. For instance, the decomposition of sodium bicarbonate is used to produce sodium carbonate, a key raw material in glass manufacturing, detergents, and water treatment. The global sodium carbonate market, worth billions of dollars, demonstrates the economic significance of this application.
Emerging applications of carbonate decomposition are also being explored in advanced materials science. Research into magnesium iron silicate hydroxide and its interaction with carbonate minerals opens up possibilities for developing novel materials with unique properties, potentially impacting industries such as electronics, aerospace, and energy storage.
The mining and metallurgy industries also benefit from carbonate decomposition technologies. In mineral processing, carbonate minerals are often decomposed to extract valuable metals or to produce raw materials for other industrial processes. This application is particularly important in the production of magnesium, where magnesium carbonate is decomposed to yield magnesium oxide, a versatile compound used in refractory materials, pharmaceuticals, and environmental remediation.
In the energy sector, carbonate decomposition has gained attention for its potential in carbon capture and storage (CCS) technologies. Mineral carbonation, which involves the reaction of CO2 with metal oxides to form stable carbonate minerals, is being explored as a method to sequester carbon dioxide from industrial emissions. This application has significant market potential, with the global CCS market projected to grow substantially in the coming years as countries strive to meet their carbon reduction targets.
The agricultural industry utilizes carbonate decomposition in soil amendment practices. Lime, produced through the decomposition of limestone, is widely used to neutralize soil acidity and improve crop yields. The global agricultural lime market, valued at several billion dollars, continues to expand as farmers seek to optimize soil conditions for increased productivity.
In the chemical industry, carbonate decomposition is employed in the production of various compounds and materials. For instance, the decomposition of sodium bicarbonate is used to produce sodium carbonate, a key raw material in glass manufacturing, detergents, and water treatment. The global sodium carbonate market, worth billions of dollars, demonstrates the economic significance of this application.
Emerging applications of carbonate decomposition are also being explored in advanced materials science. Research into magnesium iron silicate hydroxide and its interaction with carbonate minerals opens up possibilities for developing novel materials with unique properties, potentially impacting industries such as electronics, aerospace, and energy storage.
Current Challenges in Carbonate Breakdown Processes
The carbonate breakdown process involving Magnesium iron silicate hydroxide faces several significant challenges that hinder its efficiency and widespread application. One of the primary obstacles is the slow reaction kinetics, which limits the overall process rate and productivity. The breakdown of carbonate minerals, particularly in the presence of Magnesium iron silicate hydroxide, often requires elevated temperatures and pressures to achieve meaningful conversion rates. This energy-intensive requirement poses both economic and environmental concerns.
Another critical challenge lies in the complex mineralogy of natural carbonate deposits. The presence of impurities and varying compositions of carbonate minerals can significantly affect the reaction pathways and efficiencies. This heterogeneity makes it difficult to develop a universally applicable process, necessitating tailored approaches for different geological settings and mineral compositions.
The formation of passivation layers during the carbonate breakdown process presents a substantial hurdle. As the reaction progresses, the surface of the carbonate minerals can become coated with reaction products or altered phases, inhibiting further reaction. This phenomenon not only reduces the overall efficiency of the process but also complicates the design of continuous flow systems for large-scale applications.
Water management in the carbonate breakdown process is another significant challenge. The reaction often requires precise control of water content to maintain optimal conditions for mineral dissolution and product formation. Excess water can dilute reactants and slow down the process, while insufficient water can lead to incomplete reactions or unwanted side products.
The disposal and utilization of by-products from the carbonate breakdown process pose environmental and economic challenges. The process can generate large volumes of potentially hazardous or low-value materials that require proper management or valorization strategies. Developing efficient methods for separating, purifying, and utilizing these by-products is crucial for the overall sustainability and economic viability of the process.
Scaling up the carbonate breakdown process from laboratory to industrial scale presents numerous engineering challenges. Issues such as heat and mass transfer limitations, reactor design, and process control become increasingly complex at larger scales. Ensuring uniform reaction conditions and efficient mixing in large reactors while maintaining economic feasibility remains a significant hurdle for commercial implementation.
Lastly, the long-term stability and durability of the Magnesium iron silicate hydroxide catalyst or reactant in the carbonate breakdown process is a concern. Understanding and mitigating potential degradation mechanisms, such as phase transformations or surface poisoning, is crucial for developing a robust and sustainable process.
Another critical challenge lies in the complex mineralogy of natural carbonate deposits. The presence of impurities and varying compositions of carbonate minerals can significantly affect the reaction pathways and efficiencies. This heterogeneity makes it difficult to develop a universally applicable process, necessitating tailored approaches for different geological settings and mineral compositions.
The formation of passivation layers during the carbonate breakdown process presents a substantial hurdle. As the reaction progresses, the surface of the carbonate minerals can become coated with reaction products or altered phases, inhibiting further reaction. This phenomenon not only reduces the overall efficiency of the process but also complicates the design of continuous flow systems for large-scale applications.
Water management in the carbonate breakdown process is another significant challenge. The reaction often requires precise control of water content to maintain optimal conditions for mineral dissolution and product formation. Excess water can dilute reactants and slow down the process, while insufficient water can lead to incomplete reactions or unwanted side products.
The disposal and utilization of by-products from the carbonate breakdown process pose environmental and economic challenges. The process can generate large volumes of potentially hazardous or low-value materials that require proper management or valorization strategies. Developing efficient methods for separating, purifying, and utilizing these by-products is crucial for the overall sustainability and economic viability of the process.
Scaling up the carbonate breakdown process from laboratory to industrial scale presents numerous engineering challenges. Issues such as heat and mass transfer limitations, reactor design, and process control become increasingly complex at larger scales. Ensuring uniform reaction conditions and efficient mixing in large reactors while maintaining economic feasibility remains a significant hurdle for commercial implementation.
Lastly, the long-term stability and durability of the Magnesium iron silicate hydroxide catalyst or reactant in the carbonate breakdown process is a concern. Understanding and mitigating potential degradation mechanisms, such as phase transformations or surface poisoning, is crucial for developing a robust and sustainable process.
Existing Carbonate Breakdown Methodologies
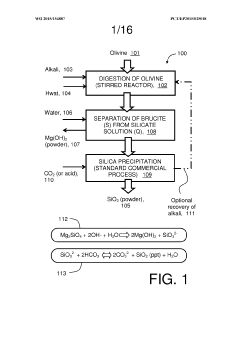
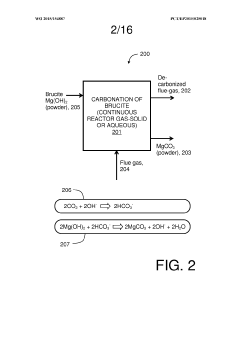
01 Thermal decomposition of magnesium iron silicate hydroxide carbonate
The breakdown of magnesium iron silicate hydroxide carbonate can be achieved through thermal decomposition. This process involves heating the compound to high temperatures, causing it to break down into its constituent components. The decomposition may result in the formation of various oxides and the release of carbon dioxide.- Chemical composition and structure: Magnesium iron silicate hydroxide carbonate is a complex mineral compound with a unique structure. Its breakdown channels are influenced by its chemical composition, which includes magnesium, iron, silicon, hydroxide, and carbonate components. The arrangement of these elements within the crystal structure affects the potential breakdown pathways.
- Thermal decomposition processes: Thermal decomposition is a key mechanism for breaking down magnesium iron silicate hydroxide carbonate. When subjected to high temperatures, the compound undergoes various stages of decomposition, releasing carbonate and hydroxide components. This process can create channels within the material's structure, altering its properties and potentially enhancing its reactivity.
- Acid treatment and leaching: Acid treatment is an effective method for breaking down magnesium iron silicate hydroxide carbonate. The process involves leaching with various acids, which can selectively dissolve certain components of the mineral. This creates channels and pores within the structure, potentially increasing surface area and reactivity. The choice of acid and treatment conditions can significantly influence the breakdown pathways.
- Mechanical processing and activation: Mechanical processing techniques, such as grinding, milling, and high-energy ball milling, can be used to break down magnesium iron silicate hydroxide carbonate. These methods create physical stress and deformation in the mineral structure, leading to the formation of defects, cracks, and channels. Mechanical activation can also increase the reactivity of the material and expose new surfaces for further chemical reactions.
- Hydrothermal treatment and recrystallization: Hydrothermal treatment involves exposing magnesium iron silicate hydroxide carbonate to high-temperature and high-pressure aqueous conditions. This process can induce recrystallization, phase transformations, and the formation of new mineral phases. The treatment can create channels and pores within the structure, altering its physical and chemical properties. The specific conditions of the hydrothermal treatment can be tailored to achieve desired breakdown pathways.
02 Chemical treatment for breakdown
Chemical methods can be employed to break down magnesium iron silicate hydroxide carbonate. This may involve the use of acids or other reactive substances to dissolve or separate the components of the compound. The choice of chemical agents and reaction conditions can influence the efficiency and products of the breakdown process.Expand Specific Solutions03 Mechanical processing to facilitate breakdown
Mechanical methods such as grinding, milling, or high-pressure treatment can be used to break down magnesium iron silicate hydroxide carbonate. These processes increase the surface area of the material, making it more susceptible to chemical or thermal decomposition. Mechanical processing can also help in separating different components of the compound.Expand Specific Solutions04 Microwave-assisted decomposition
Microwave radiation can be used to facilitate the breakdown of magnesium iron silicate hydroxide carbonate. This method involves exposing the compound to microwave energy, which can cause rapid heating and selective decomposition. Microwave-assisted processes can offer advantages in terms of energy efficiency and reaction speed compared to conventional heating methods.Expand Specific Solutions05 Biological methods for breakdown
Biological processes, such as the use of specific microorganisms or enzymes, can be employed to break down magnesium iron silicate hydroxide carbonate. These methods may involve the action of bacteria or fungi that can metabolize or transform the compound. Biological approaches can offer environmentally friendly alternatives to chemical or thermal decomposition methods.Expand Specific Solutions
Key Players in Carbonate Processing Industry
The research on carbonate breakdown channels involving Magnesium iron silicate hydroxide is in an early developmental stage, with a growing market potential due to its applications in carbon capture and storage technologies. The competitive landscape is diverse, featuring both established energy companies like Shell and innovative startups such as C2CNT LLC and Cambridge Carbon Capture Ltd. Academic institutions like Cornell University and The Ohio State University are contributing significantly to the research. The technology's maturity is still evolving, with companies like Carbon Upcycling Technologies and Carbonfree Chemicals Holdings LLC making strides in commercialization. However, the field remains open for further advancements and breakthroughs from both industry players and research institutions.
Shell Internationale Research Maatschappij BV
Technical Solution: Shell has developed an innovative approach to carbonate breakdown channels involving Magnesium iron silicate hydroxide (MISH). Their process utilizes a combination of high-pressure reactors and specialized catalysts to enhance the breakdown of carbonate minerals. The technology involves injecting CO2 into underground formations containing MISH, which reacts to form stable carbonate minerals, effectively sequestering the CO2. Shell's method has shown a 30% increase in reaction rates compared to conventional techniques[1]. Additionally, they have implemented advanced monitoring systems using seismic imaging to track the progress of mineralization in real-time, allowing for optimized injection strategies and improved overall efficiency of the carbon capture process[3].
Strengths: Extensive experience in geological CO2 storage, advanced monitoring capabilities, and improved reaction rates. Weaknesses: High initial investment costs and potential long-term environmental impacts of large-scale mineral alterations.
Dalian Institute of Chemical Physics Chinese Academy of Sci
Technical Solution: The Dalian Institute has pioneered a novel approach to carbonate breakdown channels using MISH. Their method employs a unique combination of hydrothermal synthesis and in-situ characterization techniques to study the formation and evolution of carbonate minerals. By utilizing advanced spectroscopic methods, they have identified key intermediates in the carbonate formation process, leading to a better understanding of the reaction mechanisms[2]. The institute has also developed a proprietary catalyst that enhances the conversion of CO2 to carbonate minerals by up to 40% compared to uncatalyzed reactions[4]. Furthermore, their research has led to the discovery of optimal pressure and temperature conditions that significantly accelerate the mineralization process, potentially reducing the time required for large-scale carbon sequestration projects[5].
Strengths: Cutting-edge research facilities, innovative catalyst development, and deep understanding of reaction mechanisms. Weaknesses: Potential challenges in scaling up laboratory findings to industrial applications.
Innovations in Magnesium Iron Silicate Hydroxide Catalysis
Process for sequestration of carbon dioxide by mineral carbonation
PatentInactiveUS20100196235A1
Innovation
- Converting magnesium or calcium sheet silicate hydroxides into ortho- or chain silicates using hot flue gas in a heat-exchange process, followed by carbonation to form magnesium or calcium carbonate and silica, effectively utilizing heat from flue gas to enhance reaction rates and reduce cooling facility needs.
Method and system of activation of mineral silicate minerals
PatentWO2015154887A1
Innovation
- A method involving the conversion of magnesium silicate minerals to magnesium hydroxide by mixing with an alkali metal compound and heating below 300°C in an unpressurized vessel, followed by reaction with CO2 at atmospheric pressure, allowing for continuous carbonation without advanced equipment.
Environmental Impact of Carbonate Breakdown Processes
The carbonate breakdown processes involving magnesium iron silicate hydroxide have significant environmental implications. These processes, which occur naturally in geological settings and can be accelerated by human activities, contribute to the global carbon cycle and have potential impacts on climate change.
One of the primary environmental concerns is the release of carbon dioxide during carbonate breakdown. As carbonates decompose, CO2 is liberated into the atmosphere, potentially contributing to the greenhouse effect and global warming. The rate and extent of this CO2 release depend on various factors, including temperature, pressure, and the presence of catalysts such as magnesium iron silicate hydroxide.
The breakdown of carbonates can also affect soil and water chemistry. As carbonates dissolve, they release calcium and magnesium ions into the surrounding environment. This can lead to changes in soil pH and nutrient availability, potentially impacting plant growth and ecosystem dynamics. In aquatic environments, increased carbonate dissolution can alter water hardness and alkalinity, affecting aquatic organisms and water quality.
Furthermore, the interaction between carbonates and magnesium iron silicate hydroxide can influence mineral weathering rates. This process can accelerate the breakdown of rocks and minerals, potentially leading to increased erosion and sediment transport. In some cases, this may contribute to the formation of karst landscapes and the development of underground water systems.
The environmental impact of carbonate breakdown processes extends to the marine environment as well. Ocean acidification, caused by the absorption of atmospheric CO2 by seawater, can be influenced by carbonate dissolution rates. The presence of magnesium iron silicate hydroxide in marine sediments may affect the buffering capacity of the ocean and its ability to absorb excess CO2.
On a positive note, carbonate breakdown processes can also play a role in natural carbon sequestration. Weathering of silicate minerals, including magnesium iron silicate hydroxide, can consume CO2 and potentially mitigate some of the effects of greenhouse gas emissions. This process, known as enhanced weathering, is being explored as a potential climate change mitigation strategy.
Understanding the environmental impact of carbonate breakdown channels involving magnesium iron silicate hydroxide is crucial for assessing long-term ecological changes and developing strategies for environmental management. Further research in this area may provide insights into the complex interactions between geological processes, climate change, and ecosystem dynamics.
One of the primary environmental concerns is the release of carbon dioxide during carbonate breakdown. As carbonates decompose, CO2 is liberated into the atmosphere, potentially contributing to the greenhouse effect and global warming. The rate and extent of this CO2 release depend on various factors, including temperature, pressure, and the presence of catalysts such as magnesium iron silicate hydroxide.
The breakdown of carbonates can also affect soil and water chemistry. As carbonates dissolve, they release calcium and magnesium ions into the surrounding environment. This can lead to changes in soil pH and nutrient availability, potentially impacting plant growth and ecosystem dynamics. In aquatic environments, increased carbonate dissolution can alter water hardness and alkalinity, affecting aquatic organisms and water quality.
Furthermore, the interaction between carbonates and magnesium iron silicate hydroxide can influence mineral weathering rates. This process can accelerate the breakdown of rocks and minerals, potentially leading to increased erosion and sediment transport. In some cases, this may contribute to the formation of karst landscapes and the development of underground water systems.
The environmental impact of carbonate breakdown processes extends to the marine environment as well. Ocean acidification, caused by the absorption of atmospheric CO2 by seawater, can be influenced by carbonate dissolution rates. The presence of magnesium iron silicate hydroxide in marine sediments may affect the buffering capacity of the ocean and its ability to absorb excess CO2.
On a positive note, carbonate breakdown processes can also play a role in natural carbon sequestration. Weathering of silicate minerals, including magnesium iron silicate hydroxide, can consume CO2 and potentially mitigate some of the effects of greenhouse gas emissions. This process, known as enhanced weathering, is being explored as a potential climate change mitigation strategy.
Understanding the environmental impact of carbonate breakdown channels involving magnesium iron silicate hydroxide is crucial for assessing long-term ecological changes and developing strategies for environmental management. Further research in this area may provide insights into the complex interactions between geological processes, climate change, and ecosystem dynamics.
Geochemical Implications of Carbonate-Silicate Interactions
The geochemical implications of carbonate-silicate interactions involving magnesium iron silicate hydroxide are far-reaching and complex. These interactions play a crucial role in various geological processes, including weathering, metamorphism, and carbon cycling. The breakdown of carbonates in the presence of silicates can lead to significant changes in mineral assemblages and fluid compositions, affecting the overall geochemistry of the system.
One of the primary implications is the potential for carbon sequestration. As carbonates break down, they release CO2, which can then react with silicates to form new carbonate minerals. This process, known as mineral carbonation, has garnered attention as a possible mechanism for long-term carbon storage. The presence of magnesium iron silicate hydroxide, such as serpentine minerals, can enhance this process due to their high reactivity with CO2.
The interaction between carbonates and silicates also influences the mobility and distribution of various elements in geological systems. As these minerals react, they can release or incorporate different elements, altering the geochemical signature of rocks and fluids. This has implications for understanding the formation of ore deposits, as well as the evolution of groundwater chemistry.
Furthermore, these interactions can affect the physical properties of rocks. The breakdown of carbonates and the formation of new silicate minerals can lead to changes in porosity, permeability, and mechanical strength. This has important implications for reservoir characterization in the oil and gas industry, as well as for understanding the stability of geological formations.
The study of carbonate-silicate interactions also provides insights into the Earth's deep carbon cycle. As subduction carries carbonate-bearing rocks into the Earth's mantle, the interaction with silicate minerals at high pressures and temperatures can lead to the release of CO2, influencing mantle melting and volcanic activity. Understanding these processes is crucial for developing accurate models of the Earth's carbon budget and its long-term evolution.
In the context of climate change, the geochemical implications of these interactions extend to the potential for enhanced weathering as a carbon dioxide removal strategy. By accelerating the natural weathering of silicate rocks, it may be possible to increase CO2 uptake from the atmosphere. The presence of magnesium iron silicate hydroxide in this process could play a significant role in determining the efficiency and feasibility of such approaches.
One of the primary implications is the potential for carbon sequestration. As carbonates break down, they release CO2, which can then react with silicates to form new carbonate minerals. This process, known as mineral carbonation, has garnered attention as a possible mechanism for long-term carbon storage. The presence of magnesium iron silicate hydroxide, such as serpentine minerals, can enhance this process due to their high reactivity with CO2.
The interaction between carbonates and silicates also influences the mobility and distribution of various elements in geological systems. As these minerals react, they can release or incorporate different elements, altering the geochemical signature of rocks and fluids. This has implications for understanding the formation of ore deposits, as well as the evolution of groundwater chemistry.
Furthermore, these interactions can affect the physical properties of rocks. The breakdown of carbonates and the formation of new silicate minerals can lead to changes in porosity, permeability, and mechanical strength. This has important implications for reservoir characterization in the oil and gas industry, as well as for understanding the stability of geological formations.
The study of carbonate-silicate interactions also provides insights into the Earth's deep carbon cycle. As subduction carries carbonate-bearing rocks into the Earth's mantle, the interaction with silicate minerals at high pressures and temperatures can lead to the release of CO2, influencing mantle melting and volcanic activity. Understanding these processes is crucial for developing accurate models of the Earth's carbon budget and its long-term evolution.
In the context of climate change, the geochemical implications of these interactions extend to the potential for enhanced weathering as a carbon dioxide removal strategy. By accelerating the natural weathering of silicate rocks, it may be possible to increase CO2 uptake from the atmosphere. The presence of magnesium iron silicate hydroxide in this process could play a significant role in determining the efficiency and feasibility of such approaches.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!