Exploring Carboxylic Acid's Role in Advanced Diagnostics
JUL 31, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Carboxylic Acid Diagnostics: Background and Objectives
Carboxylic acids have long been recognized as crucial components in biological systems, playing vital roles in metabolism, energy production, and cellular signaling. In recent years, their potential in advanced diagnostics has garnered significant attention from researchers and medical professionals alike. This growing interest stems from the unique chemical properties of carboxylic acids and their ubiquitous presence in living organisms.
The evolution of carboxylic acid-based diagnostics can be traced back to the early 20th century when simple pH indicators were used to detect the presence of organic acids in biological samples. However, it wasn't until the advent of more sophisticated analytical techniques, such as gas chromatography and mass spectrometry, that the full diagnostic potential of carboxylic acids began to be realized.
Today, the field of carboxylic acid diagnostics is rapidly expanding, driven by advancements in technology and a deeper understanding of the role these compounds play in health and disease. The primary objective of this research is to explore and develop novel diagnostic tools and methodologies that leverage the unique properties of carboxylic acids to detect and monitor various medical conditions with greater accuracy and efficiency.
One of the key areas of focus is the development of biosensors that can detect specific carboxylic acid profiles associated with certain diseases. These biosensors hold promise for early disease detection, personalized medicine, and real-time monitoring of treatment efficacy. Additionally, researchers are investigating the use of carboxylic acids as biomarkers for a wide range of conditions, from metabolic disorders to cancer.
The potential applications of carboxylic acid diagnostics extend beyond traditional medical settings. Environmental monitoring, food safety, and industrial quality control are just a few areas where these techniques could have a significant impact. As such, the development of portable, user-friendly diagnostic devices based on carboxylic acid detection is a major goal for many research teams.
As we delve deeper into this field, it is crucial to consider the challenges that lie ahead. These include improving the sensitivity and specificity of detection methods, developing standardized protocols for sample collection and analysis, and addressing potential confounding factors that may affect carboxylic acid profiles in biological samples.
In conclusion, the exploration of carboxylic acids' role in advanced diagnostics represents a promising frontier in medical technology. By harnessing the power of these ubiquitous molecules, we aim to develop innovative diagnostic tools that can revolutionize healthcare delivery, improve patient outcomes, and contribute to our understanding of human biology and disease processes.
The evolution of carboxylic acid-based diagnostics can be traced back to the early 20th century when simple pH indicators were used to detect the presence of organic acids in biological samples. However, it wasn't until the advent of more sophisticated analytical techniques, such as gas chromatography and mass spectrometry, that the full diagnostic potential of carboxylic acids began to be realized.
Today, the field of carboxylic acid diagnostics is rapidly expanding, driven by advancements in technology and a deeper understanding of the role these compounds play in health and disease. The primary objective of this research is to explore and develop novel diagnostic tools and methodologies that leverage the unique properties of carboxylic acids to detect and monitor various medical conditions with greater accuracy and efficiency.
One of the key areas of focus is the development of biosensors that can detect specific carboxylic acid profiles associated with certain diseases. These biosensors hold promise for early disease detection, personalized medicine, and real-time monitoring of treatment efficacy. Additionally, researchers are investigating the use of carboxylic acids as biomarkers for a wide range of conditions, from metabolic disorders to cancer.
The potential applications of carboxylic acid diagnostics extend beyond traditional medical settings. Environmental monitoring, food safety, and industrial quality control are just a few areas where these techniques could have a significant impact. As such, the development of portable, user-friendly diagnostic devices based on carboxylic acid detection is a major goal for many research teams.
As we delve deeper into this field, it is crucial to consider the challenges that lie ahead. These include improving the sensitivity and specificity of detection methods, developing standardized protocols for sample collection and analysis, and addressing potential confounding factors that may affect carboxylic acid profiles in biological samples.
In conclusion, the exploration of carboxylic acids' role in advanced diagnostics represents a promising frontier in medical technology. By harnessing the power of these ubiquitous molecules, we aim to develop innovative diagnostic tools that can revolutionize healthcare delivery, improve patient outcomes, and contribute to our understanding of human biology and disease processes.
Market Analysis for Advanced Diagnostic Technologies
The advanced diagnostics market has experienced significant growth in recent years, driven by increasing demand for early disease detection, personalized medicine, and improved patient outcomes. The global market for advanced diagnostic technologies is projected to reach substantial value in the coming years, with a compound annual growth rate outpacing many other healthcare sectors.
Carboxylic acids, as versatile organic compounds, are emerging as key players in this expanding market. Their unique chemical properties and potential applications in diagnostic tools have garnered attention from both researchers and industry stakeholders. The integration of carboxylic acids in advanced diagnostic technologies is opening new avenues for more accurate, sensitive, and rapid diagnostic methods.
One of the primary drivers for the adoption of carboxylic acid-based diagnostics is the growing prevalence of chronic diseases worldwide. As healthcare systems grapple with the increasing burden of conditions such as cancer, cardiovascular diseases, and neurological disorders, there is a pressing need for more sophisticated diagnostic tools. Carboxylic acids offer promising solutions in areas such as biomarker detection, imaging contrast agents, and molecular probes.
The pharmaceutical and biotechnology industries are major contributors to the demand for advanced diagnostic technologies incorporating carboxylic acids. These sectors are investing heavily in research and development to leverage the potential of carboxylic acids in drug discovery, development, and personalized medicine approaches. The ability of carboxylic acids to form diverse chemical bonds and interact with biological systems makes them valuable tools in diagnostic applications.
In the clinical diagnostics segment, there is a growing trend towards point-of-care testing and rapid diagnostic kits. Carboxylic acid-based sensors and assays are being developed to meet this demand, offering the potential for faster, more accessible, and cost-effective diagnostic solutions. This trend is particularly relevant in resource-limited settings and for managing infectious diseases, where rapid and accurate diagnosis is crucial.
The market for advanced diagnostic technologies is also being shaped by technological advancements in fields such as nanotechnology, microfluidics, and artificial intelligence. These innovations are enabling the development of more sophisticated diagnostic platforms that can harness the properties of carboxylic acids for enhanced sensitivity and specificity. As a result, there is growing interest in integrating carboxylic acid-based diagnostics with these cutting-edge technologies to create next-generation diagnostic tools.
Geographically, North America and Europe currently dominate the advanced diagnostics market, with the highest adoption rates of innovative technologies. However, emerging economies in Asia-Pacific and Latin America are expected to witness rapid growth in the coming years, driven by improving healthcare infrastructure, rising healthcare expenditure, and increasing awareness of the benefits of early diagnosis.
Carboxylic acids, as versatile organic compounds, are emerging as key players in this expanding market. Their unique chemical properties and potential applications in diagnostic tools have garnered attention from both researchers and industry stakeholders. The integration of carboxylic acids in advanced diagnostic technologies is opening new avenues for more accurate, sensitive, and rapid diagnostic methods.
One of the primary drivers for the adoption of carboxylic acid-based diagnostics is the growing prevalence of chronic diseases worldwide. As healthcare systems grapple with the increasing burden of conditions such as cancer, cardiovascular diseases, and neurological disorders, there is a pressing need for more sophisticated diagnostic tools. Carboxylic acids offer promising solutions in areas such as biomarker detection, imaging contrast agents, and molecular probes.
The pharmaceutical and biotechnology industries are major contributors to the demand for advanced diagnostic technologies incorporating carboxylic acids. These sectors are investing heavily in research and development to leverage the potential of carboxylic acids in drug discovery, development, and personalized medicine approaches. The ability of carboxylic acids to form diverse chemical bonds and interact with biological systems makes them valuable tools in diagnostic applications.
In the clinical diagnostics segment, there is a growing trend towards point-of-care testing and rapid diagnostic kits. Carboxylic acid-based sensors and assays are being developed to meet this demand, offering the potential for faster, more accessible, and cost-effective diagnostic solutions. This trend is particularly relevant in resource-limited settings and for managing infectious diseases, where rapid and accurate diagnosis is crucial.
The market for advanced diagnostic technologies is also being shaped by technological advancements in fields such as nanotechnology, microfluidics, and artificial intelligence. These innovations are enabling the development of more sophisticated diagnostic platforms that can harness the properties of carboxylic acids for enhanced sensitivity and specificity. As a result, there is growing interest in integrating carboxylic acid-based diagnostics with these cutting-edge technologies to create next-generation diagnostic tools.
Geographically, North America and Europe currently dominate the advanced diagnostics market, with the highest adoption rates of innovative technologies. However, emerging economies in Asia-Pacific and Latin America are expected to witness rapid growth in the coming years, driven by improving healthcare infrastructure, rising healthcare expenditure, and increasing awareness of the benefits of early diagnosis.
Current Challenges in Carboxylic Acid-Based Diagnostics
Despite the promising potential of carboxylic acid-based diagnostics, several significant challenges currently hinder their widespread adoption and effectiveness in advanced diagnostic applications. One of the primary obstacles is the inherent instability of carboxylic acid compounds in various physiological environments. The pH-sensitive nature of these molecules can lead to rapid degradation or alteration of their chemical structure, potentially compromising the accuracy and reliability of diagnostic results.
Another critical challenge lies in the development of highly specific and sensitive detection methods for carboxylic acids in complex biological matrices. The presence of numerous interfering substances in blood, urine, or other bodily fluids can mask or mimic the signals of target carboxylic acids, leading to false positives or negatives. This necessitates the creation of more sophisticated and robust analytical techniques capable of distinguishing between structurally similar compounds.
The limited solubility of certain carboxylic acids in aqueous solutions poses additional difficulties in their application as diagnostic markers. This property can restrict the concentration range at which these compounds can be effectively detected, potentially limiting their utility in identifying early-stage diseases or subtle metabolic changes. Researchers are actively exploring various solubilization strategies and delivery systems to overcome this limitation.
Standardization of carboxylic acid-based diagnostic protocols across different laboratories and clinical settings remains a significant hurdle. The lack of universally accepted reference materials and calibration standards can lead to inconsistencies in test results, making it challenging to establish reliable diagnostic thresholds and compare data from different sources. This issue is particularly pronounced in the case of complex carboxylic acid profiles used for metabolomic analyses.
The development of point-of-care devices for rapid carboxylic acid detection presents another set of challenges. Miniaturization of analytical instruments while maintaining high sensitivity and specificity is a complex task. Additionally, ensuring the stability of reagents and calibration standards in portable devices exposed to varying environmental conditions is crucial for obtaining accurate results in field settings.
Regulatory hurdles and the need for extensive clinical validation studies further complicate the integration of carboxylic acid-based diagnostics into routine clinical practice. The process of demonstrating the clinical utility and cost-effectiveness of these novel diagnostic approaches can be time-consuming and resource-intensive, potentially slowing down their adoption in healthcare systems.
Addressing these challenges requires a multidisciplinary approach, combining advances in analytical chemistry, materials science, and bioengineering. Overcoming these obstacles will be crucial for realizing the full potential of carboxylic acid-based diagnostics in improving disease detection, monitoring, and personalized medicine strategies.
Another critical challenge lies in the development of highly specific and sensitive detection methods for carboxylic acids in complex biological matrices. The presence of numerous interfering substances in blood, urine, or other bodily fluids can mask or mimic the signals of target carboxylic acids, leading to false positives or negatives. This necessitates the creation of more sophisticated and robust analytical techniques capable of distinguishing between structurally similar compounds.
The limited solubility of certain carboxylic acids in aqueous solutions poses additional difficulties in their application as diagnostic markers. This property can restrict the concentration range at which these compounds can be effectively detected, potentially limiting their utility in identifying early-stage diseases or subtle metabolic changes. Researchers are actively exploring various solubilization strategies and delivery systems to overcome this limitation.
Standardization of carboxylic acid-based diagnostic protocols across different laboratories and clinical settings remains a significant hurdle. The lack of universally accepted reference materials and calibration standards can lead to inconsistencies in test results, making it challenging to establish reliable diagnostic thresholds and compare data from different sources. This issue is particularly pronounced in the case of complex carboxylic acid profiles used for metabolomic analyses.
The development of point-of-care devices for rapid carboxylic acid detection presents another set of challenges. Miniaturization of analytical instruments while maintaining high sensitivity and specificity is a complex task. Additionally, ensuring the stability of reagents and calibration standards in portable devices exposed to varying environmental conditions is crucial for obtaining accurate results in field settings.
Regulatory hurdles and the need for extensive clinical validation studies further complicate the integration of carboxylic acid-based diagnostics into routine clinical practice. The process of demonstrating the clinical utility and cost-effectiveness of these novel diagnostic approaches can be time-consuming and resource-intensive, potentially slowing down their adoption in healthcare systems.
Addressing these challenges requires a multidisciplinary approach, combining advances in analytical chemistry, materials science, and bioengineering. Overcoming these obstacles will be crucial for realizing the full potential of carboxylic acid-based diagnostics in improving disease detection, monitoring, and personalized medicine strategies.
Existing Carboxylic Acid Diagnostic Methodologies
01 Synthesis of carboxylic acids
Various methods for synthesizing carboxylic acids are described, including oxidation of primary alcohols or aldehydes, hydrolysis of nitriles, and carbonylation reactions. These processes often involve catalysts and specific reaction conditions to achieve high yields and selectivity.- Synthesis of carboxylic acids: Various methods for synthesizing carboxylic acids are described, including oxidation of primary alcohols or aldehydes, hydrolysis of nitriles, and carbonylation reactions. These processes often involve catalysts and specific reaction conditions to achieve high yields and selectivity.
- Derivatives and applications of carboxylic acids: Carboxylic acids serve as precursors for various derivatives such as esters, amides, and anhydrides. These compounds find applications in pharmaceuticals, polymers, and industrial processes. The synthesis and properties of these derivatives are explored in several patents.
- Purification and separation techniques: Methods for purifying and separating carboxylic acids from reaction mixtures or natural sources are described. These techniques include crystallization, distillation, extraction, and chromatography, aimed at obtaining high-purity carboxylic acids for various applications.
- Carboxylic acids in polymer chemistry: Carboxylic acids play a crucial role in polymer chemistry, serving as monomers or modifiers in the production of various polymers and copolymers. Patents describe their use in polyesters, polyamides, and other specialty polymers with unique properties.
- Industrial production and scale-up: Patents focus on the industrial-scale production of carboxylic acids, addressing challenges in process efficiency, cost-effectiveness, and environmental impact. Continuous flow processes, reactor designs, and catalytic systems for large-scale manufacturing are described.
02 Carboxylic acid derivatives and applications
Carboxylic acids can be converted into various derivatives such as esters, amides, and anhydrides. These derivatives have wide-ranging applications in industries including pharmaceuticals, polymers, and fine chemicals. The synthesis and properties of these derivatives are explored in several patents.Expand Specific Solutions03 Purification and separation of carboxylic acids
Methods for purifying and separating carboxylic acids from reaction mixtures or natural sources are described. These include techniques such as crystallization, distillation, extraction, and chromatography. The focus is on achieving high purity and efficient separation of desired carboxylic acids from impurities or isomers.Expand Specific Solutions04 Carboxylic acids in polymer chemistry
Carboxylic acids play a crucial role in polymer chemistry, serving as monomers or modifiers in various polymerization processes. Patents describe the use of carboxylic acids in the synthesis of polyesters, polyamides, and other functional polymers with specific properties for diverse applications.Expand Specific Solutions05 Industrial production of carboxylic acids
Large-scale industrial processes for the production of commercially important carboxylic acids are detailed. These include fermentation methods, catalytic oxidation of hydrocarbons, and other chemical routes. The focus is on improving efficiency, reducing costs, and enhancing sustainability in the production of carboxylic acids.Expand Specific Solutions
Key Players in Advanced Diagnostic Industry
The field of advanced diagnostics utilizing carboxylic acid is in a growth phase, with increasing market size and technological advancements. The global market for such diagnostics is expanding rapidly, driven by the rising demand for precise and early disease detection. Companies like Gen-Probe, Celera, and Novartis are at the forefront, leveraging their expertise in nucleic acid probe-based products and molecular diagnostics. The technology's maturity is progressing, with firms like Astellas Pharma and Kyowa Kirin investing heavily in R&D to enhance the sensitivity and specificity of carboxylic acid-based diagnostic tools. Emerging players such as FutureChem and PreTect are also contributing to the field's innovation, indicating a competitive and dynamic landscape.
Celera Corp.
Technical Solution: Celera has focused on developing genomic and proteomic approaches to identify novel carboxylic acid-based biomarkers for various diseases[10]. Their research has led to the discovery of several carboxylic acid metabolites that serve as indicators for cardiovascular diseases, cancer, and metabolic disorders[11]. Celera's diagnostic platform integrates high-throughput genomic sequencing with advanced metabolomic profiling to provide comprehensive disease risk assessment and early detection capabilities. The company has also developed proprietary algorithms for interpreting complex metabolomic data, enhancing the clinical utility of their carboxylic acid-based diagnostic tests[12].
Strengths: Comprehensive approach combining genomics and metabolomics, strong focus on biomarker discovery. Weaknesses: May require extensive clinical validation for newly identified biomarkers.
Human Metabolome Technologies, Inc.
Technical Solution: Human Metabolome Technologies (HMT) specializes in metabolome analysis, with a strong focus on carboxylic acid profiling for diagnostic applications. Their CE-MS (Capillary Electrophoresis-Mass Spectrometry) technology allows for highly sensitive and comprehensive analysis of ionic metabolites, including various carboxylic acids[13]. HMT has developed standardized protocols for sample preparation and analysis, ensuring reproducibility across different laboratories. Their metabolome database, which includes extensive information on carboxylic acid metabolites, aids in the interpretation of complex metabolomic data for diagnostic purposes[14]. HMT also offers custom assay development services for specific carboxylic acid biomarkers of interest to researchers and clinicians.
Strengths: Comprehensive metabolome analysis, extensive database for data interpretation. Weaknesses: May be more focused on research applications rather than clinical diagnostics.
Innovative Carboxylic Acid Detection Techniques
Altering high-density lipoprotein levels through carboxylesterase 1 modulation
PatentWO2009077479A2
Innovation
- Inhibiting carboxylesterase 1 (CES1) activity using specific antibodies or functional fragments to raise plasma HDL cholesterol levels and potentially reduce LDL cholesterol levels, thereby treating atherosclerotic diseases.
Carboxylic acid derivatives and drugs containing the same as the active ingredient
PatentInactiveUS7179817B2
Innovation
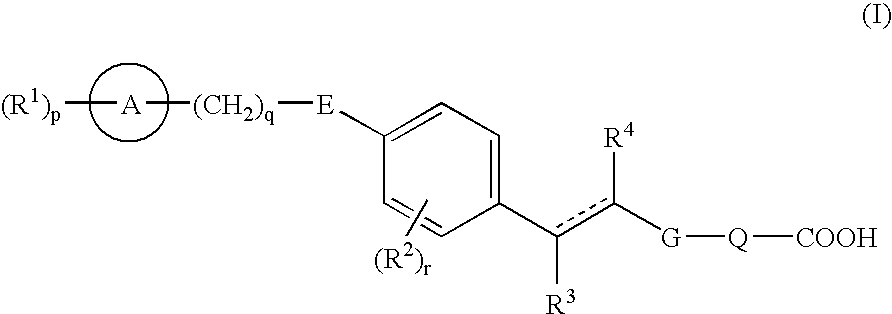
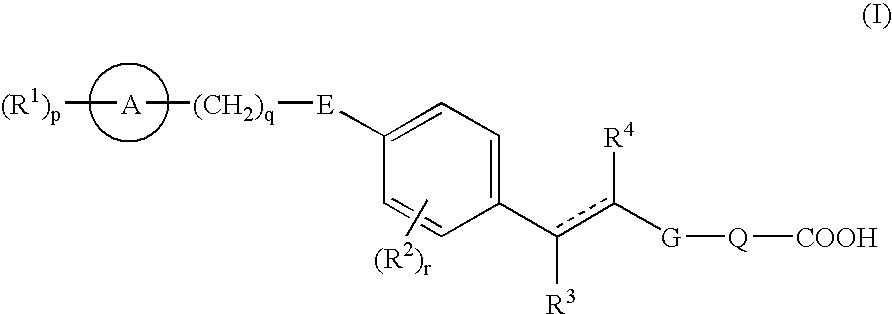
- Development of carboxylic acid derivatives represented by formula (I) and their prodrugs or non-toxic salts, which act as EDG-1 agonists, providing superior agonistic action while ensuring safety.
Regulatory Framework for Diagnostic Technologies
The regulatory framework for diagnostic technologies plays a crucial role in ensuring the safety, efficacy, and ethical use of advanced diagnostic tools, including those involving carboxylic acids. As these technologies continue to evolve, regulatory bodies worldwide are adapting their guidelines to address the unique challenges posed by innovative diagnostic methods.
In the United States, the Food and Drug Administration (FDA) oversees the regulation of diagnostic technologies through its Center for Devices and Radiological Health (CDRH). The FDA classifies diagnostic devices into three categories based on their risk level and intended use. Class I devices are subject to general controls, while Class II and III devices require more stringent premarket approval processes.
For diagnostic technologies utilizing carboxylic acids, the regulatory pathway often depends on the specific application and the level of risk associated with the test. Novel biomarkers or diagnostic methods may require extensive clinical validation studies to demonstrate their accuracy, precision, and clinical utility. The FDA's guidance on Bioanalytical Method Validation provides a framework for evaluating the performance of diagnostic assays, including those based on carboxylic acid detection.
In Europe, the In Vitro Diagnostic Regulation (IVDR) has replaced the previous In Vitro Diagnostic Directive (IVDD), introducing more stringent requirements for diagnostic devices. The IVDR emphasizes a risk-based approach, with four risk classes (A, B, C, and D) determining the level of scrutiny applied to each device. Manufacturers of carboxylic acid-based diagnostics must comply with these regulations, which include requirements for clinical evidence, post-market surveillance, and unique device identification.
Globally, the International Medical Device Regulators Forum (IMDRF) works to harmonize regulatory approaches across different countries. Their guidelines on Software as a Medical Device (SaMD) are particularly relevant for advanced diagnostic technologies that incorporate artificial intelligence or machine learning algorithms for data analysis.
As the field of advanced diagnostics continues to evolve, regulatory bodies are increasingly focusing on the ethical implications of these technologies. Issues such as data privacy, informed consent, and the potential for genetic discrimination are becoming central to regulatory discussions. For carboxylic acid-based diagnostics that may reveal sensitive health information, regulators are developing frameworks to ensure patient confidentiality and prevent misuse of diagnostic results.
The regulatory landscape for diagnostic technologies is dynamic, with ongoing efforts to balance innovation with patient safety. Manufacturers and researchers working on carboxylic acid-based diagnostics must stay informed about these evolving regulations and engage proactively with regulatory bodies to ensure compliance and facilitate the timely introduction of new diagnostic tools to the market.
In the United States, the Food and Drug Administration (FDA) oversees the regulation of diagnostic technologies through its Center for Devices and Radiological Health (CDRH). The FDA classifies diagnostic devices into three categories based on their risk level and intended use. Class I devices are subject to general controls, while Class II and III devices require more stringent premarket approval processes.
For diagnostic technologies utilizing carboxylic acids, the regulatory pathway often depends on the specific application and the level of risk associated with the test. Novel biomarkers or diagnostic methods may require extensive clinical validation studies to demonstrate their accuracy, precision, and clinical utility. The FDA's guidance on Bioanalytical Method Validation provides a framework for evaluating the performance of diagnostic assays, including those based on carboxylic acid detection.
In Europe, the In Vitro Diagnostic Regulation (IVDR) has replaced the previous In Vitro Diagnostic Directive (IVDD), introducing more stringent requirements for diagnostic devices. The IVDR emphasizes a risk-based approach, with four risk classes (A, B, C, and D) determining the level of scrutiny applied to each device. Manufacturers of carboxylic acid-based diagnostics must comply with these regulations, which include requirements for clinical evidence, post-market surveillance, and unique device identification.
Globally, the International Medical Device Regulators Forum (IMDRF) works to harmonize regulatory approaches across different countries. Their guidelines on Software as a Medical Device (SaMD) are particularly relevant for advanced diagnostic technologies that incorporate artificial intelligence or machine learning algorithms for data analysis.
As the field of advanced diagnostics continues to evolve, regulatory bodies are increasingly focusing on the ethical implications of these technologies. Issues such as data privacy, informed consent, and the potential for genetic discrimination are becoming central to regulatory discussions. For carboxylic acid-based diagnostics that may reveal sensitive health information, regulators are developing frameworks to ensure patient confidentiality and prevent misuse of diagnostic results.
The regulatory landscape for diagnostic technologies is dynamic, with ongoing efforts to balance innovation with patient safety. Manufacturers and researchers working on carboxylic acid-based diagnostics must stay informed about these evolving regulations and engage proactively with regulatory bodies to ensure compliance and facilitate the timely introduction of new diagnostic tools to the market.
Bioethical Implications of Advanced Diagnostics
The rapid advancement of carboxylic acid-based diagnostic technologies raises significant bioethical concerns that warrant careful consideration. These advanced diagnostic tools, while promising improved health outcomes, also present potential risks to patient privacy, autonomy, and social equity.
One primary ethical issue is the protection of genetic information obtained through these diagnostic methods. As carboxylic acid-based tests become more sophisticated, they may reveal increasingly detailed genetic profiles. This raises questions about data ownership, storage security, and potential misuse by employers, insurers, or other third parties. Robust safeguards and clear regulations are necessary to ensure that sensitive genetic information remains confidential and is used solely for medical purposes.
The increased predictive power of these diagnostics also introduces complex ethical dilemmas regarding patient autonomy and the "right not to know." Some individuals may prefer not to learn about their genetic predispositions to certain diseases, especially if effective treatments are not available. Balancing the potential benefits of early detection with respect for personal choice becomes a critical consideration in the implementation of these advanced diagnostic tools.
Furthermore, the accessibility and equitable distribution of carboxylic acid-based diagnostics raise concerns about healthcare disparities. As these technologies become more sophisticated and potentially more expensive, there is a risk of exacerbating existing inequalities in healthcare access. Ensuring that advanced diagnostic tools are available to all segments of society, regardless of socioeconomic status, is crucial to prevent the widening of health gaps.
The potential for these diagnostics to influence reproductive decisions also presents ethical challenges. As the ability to detect genetic abnormalities in fetuses or embryos improves, questions arise about the ethical implications of selective reproduction and the societal impact of such choices. This intersects with broader debates about disability rights, genetic diversity, and the definition of a "healthy" individual.
Lastly, the rapid pace of innovation in this field outstrips the development of ethical frameworks and regulations. There is an urgent need for interdisciplinary collaboration between scientists, ethicists, policymakers, and healthcare providers to establish guidelines that address these complex issues. These guidelines must be flexible enough to adapt to evolving technologies while maintaining core ethical principles.
In conclusion, while carboxylic acid-based advanced diagnostics offer tremendous potential for improving healthcare, their development and implementation must be guided by careful ethical considerations. Balancing the benefits of these technologies with the protection of individual rights and societal values is essential for their responsible and equitable integration into medical practice.
One primary ethical issue is the protection of genetic information obtained through these diagnostic methods. As carboxylic acid-based tests become more sophisticated, they may reveal increasingly detailed genetic profiles. This raises questions about data ownership, storage security, and potential misuse by employers, insurers, or other third parties. Robust safeguards and clear regulations are necessary to ensure that sensitive genetic information remains confidential and is used solely for medical purposes.
The increased predictive power of these diagnostics also introduces complex ethical dilemmas regarding patient autonomy and the "right not to know." Some individuals may prefer not to learn about their genetic predispositions to certain diseases, especially if effective treatments are not available. Balancing the potential benefits of early detection with respect for personal choice becomes a critical consideration in the implementation of these advanced diagnostic tools.
Furthermore, the accessibility and equitable distribution of carboxylic acid-based diagnostics raise concerns about healthcare disparities. As these technologies become more sophisticated and potentially more expensive, there is a risk of exacerbating existing inequalities in healthcare access. Ensuring that advanced diagnostic tools are available to all segments of society, regardless of socioeconomic status, is crucial to prevent the widening of health gaps.
The potential for these diagnostics to influence reproductive decisions also presents ethical challenges. As the ability to detect genetic abnormalities in fetuses or embryos improves, questions arise about the ethical implications of selective reproduction and the societal impact of such choices. This intersects with broader debates about disability rights, genetic diversity, and the definition of a "healthy" individual.
Lastly, the rapid pace of innovation in this field outstrips the development of ethical frameworks and regulations. There is an urgent need for interdisciplinary collaboration between scientists, ethicists, policymakers, and healthcare providers to establish guidelines that address these complex issues. These guidelines must be flexible enough to adapt to evolving technologies while maintaining core ethical principles.
In conclusion, while carboxylic acid-based advanced diagnostics offer tremendous potential for improving healthcare, their development and implementation must be guided by careful ethical considerations. Balancing the benefits of these technologies with the protection of individual rights and societal values is essential for their responsible and equitable integration into medical practice.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!