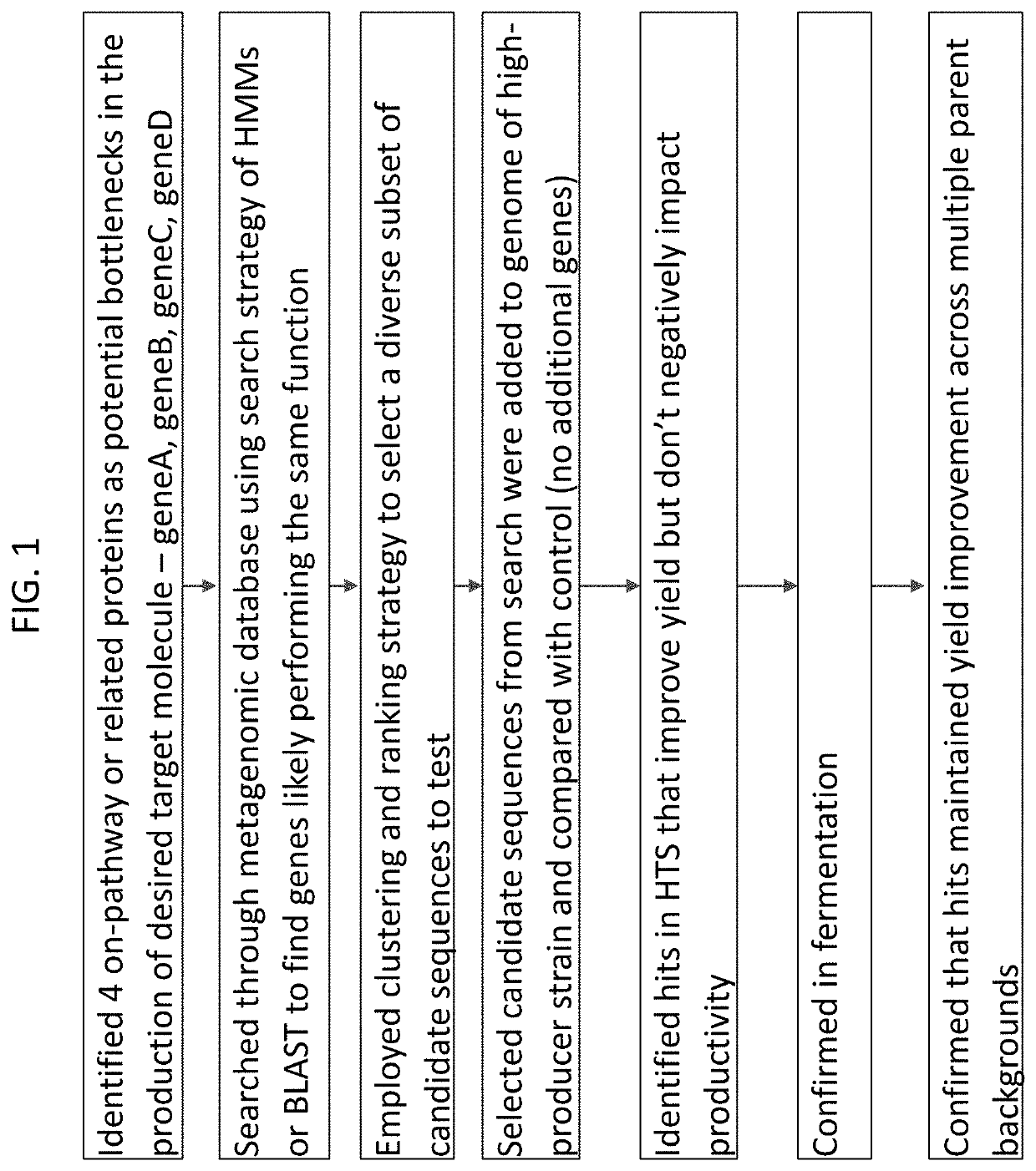
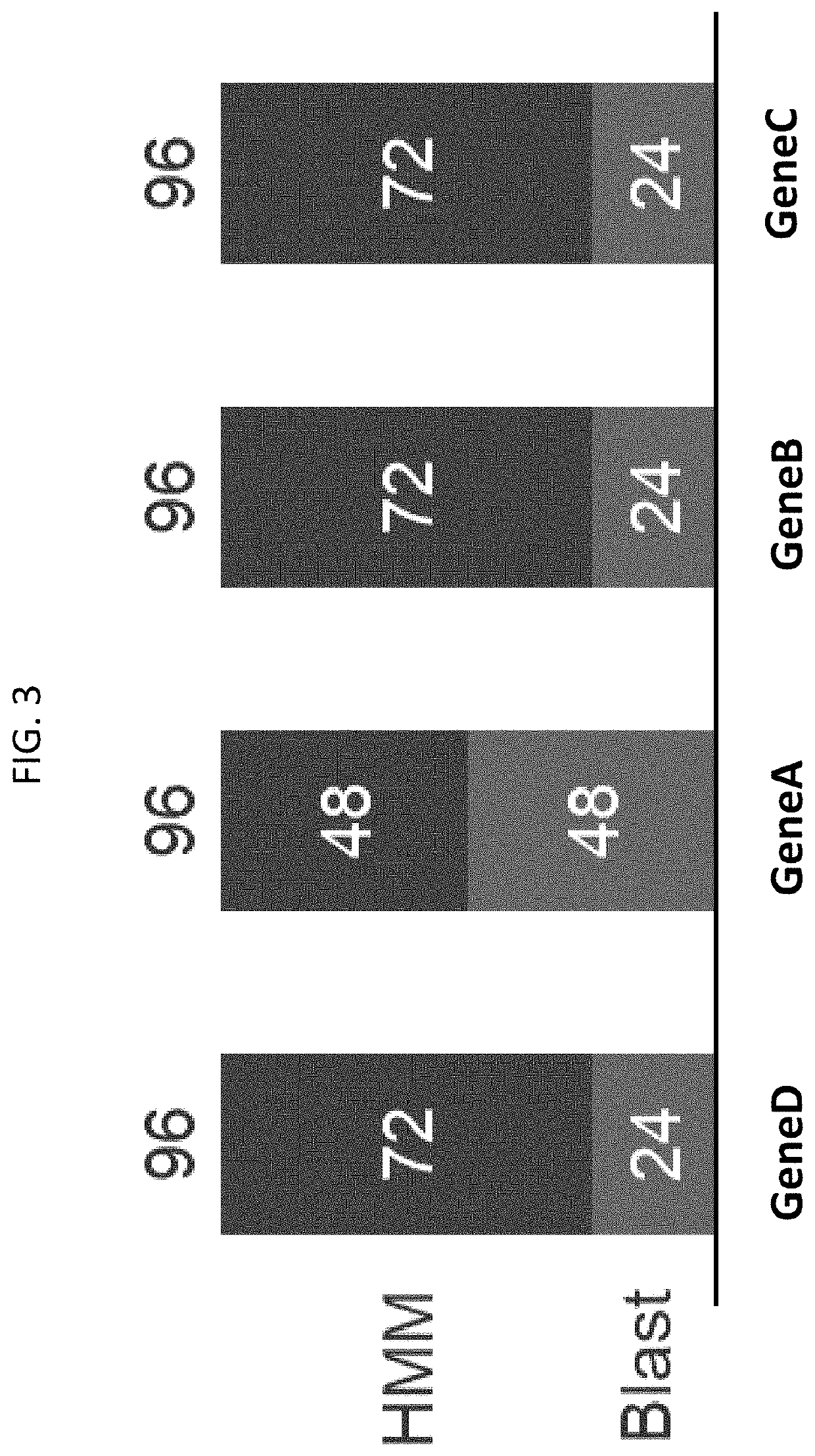
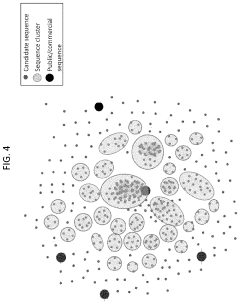
Exploring Magnesium Nitrate’s Role in Biosynthetic Pathway Optimization
AUG 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Mg(NO3)2 in Biosynthesis
Magnesium nitrate, Mg(NO3)2, plays a crucial role in biosynthetic pathway optimization, serving as a vital cofactor and nutrient source in various biological processes. The exploration of its significance has gained momentum in recent years, driven by the increasing demand for efficient and sustainable bioproduction methods.
The journey of understanding Mg(NO3)2's role in biosynthesis can be traced back to early studies on plant nutrition and enzyme catalysis. As research progressed, scientists discovered its multifaceted functions in cellular metabolism, including its involvement in chlorophyll synthesis, protein formation, and energy transfer processes.
In the context of biosynthetic pathway optimization, Mg(NO3)2 has emerged as a key player due to its ability to enhance enzyme activity and stability. Magnesium ions, derived from Mg(NO3)2, act as essential cofactors for numerous enzymes involved in biosynthetic pathways, particularly those related to carbon fixation, nucleic acid synthesis, and ATP production.
The nitrate component of Mg(NO3)2 also contributes significantly to biosynthetic processes, serving as a nitrogen source for amino acid and nucleotide synthesis. This dual functionality makes Mg(NO3)2 an attractive compound for optimizing biosynthetic pathways, especially in microbial and plant-based production systems.
Recent advancements in metabolic engineering and synthetic biology have further highlighted the importance of Mg(NO3)2 in fine-tuning biosynthetic pathways. Researchers have demonstrated that precise control of Mg(NO3)2 concentrations can lead to improved product yields and pathway efficiency in various biotechnological applications, including the production of biofuels, pharmaceuticals, and high-value chemicals.
The optimization of Mg(NO3)2 utilization in biosynthetic pathways has also been driven by the need for sustainable and eco-friendly production methods. As a naturally occurring compound, Mg(NO3)2 aligns well with green chemistry principles, making it an attractive alternative to synthetic catalysts and nutrient sources.
Looking ahead, the exploration of Mg(NO3)2's role in biosynthetic pathway optimization is expected to continue evolving. Emerging areas of research include the development of novel Mg(NO3)2-based formulations for enhanced bioavailability, the integration of Mg(NO3)2 in cell-free biosynthetic systems, and the use of advanced computational models to predict and optimize Mg(NO3)2-dependent reactions in complex metabolic networks.
The journey of understanding Mg(NO3)2's role in biosynthesis can be traced back to early studies on plant nutrition and enzyme catalysis. As research progressed, scientists discovered its multifaceted functions in cellular metabolism, including its involvement in chlorophyll synthesis, protein formation, and energy transfer processes.
In the context of biosynthetic pathway optimization, Mg(NO3)2 has emerged as a key player due to its ability to enhance enzyme activity and stability. Magnesium ions, derived from Mg(NO3)2, act as essential cofactors for numerous enzymes involved in biosynthetic pathways, particularly those related to carbon fixation, nucleic acid synthesis, and ATP production.
The nitrate component of Mg(NO3)2 also contributes significantly to biosynthetic processes, serving as a nitrogen source for amino acid and nucleotide synthesis. This dual functionality makes Mg(NO3)2 an attractive compound for optimizing biosynthetic pathways, especially in microbial and plant-based production systems.
Recent advancements in metabolic engineering and synthetic biology have further highlighted the importance of Mg(NO3)2 in fine-tuning biosynthetic pathways. Researchers have demonstrated that precise control of Mg(NO3)2 concentrations can lead to improved product yields and pathway efficiency in various biotechnological applications, including the production of biofuels, pharmaceuticals, and high-value chemicals.
The optimization of Mg(NO3)2 utilization in biosynthetic pathways has also been driven by the need for sustainable and eco-friendly production methods. As a naturally occurring compound, Mg(NO3)2 aligns well with green chemistry principles, making it an attractive alternative to synthetic catalysts and nutrient sources.
Looking ahead, the exploration of Mg(NO3)2's role in biosynthetic pathway optimization is expected to continue evolving. Emerging areas of research include the development of novel Mg(NO3)2-based formulations for enhanced bioavailability, the integration of Mg(NO3)2 in cell-free biosynthetic systems, and the use of advanced computational models to predict and optimize Mg(NO3)2-dependent reactions in complex metabolic networks.
Market for Optimized Biosynthesis
The market for optimized biosynthesis is experiencing significant growth, driven by increasing demand for sustainable and cost-effective production methods across various industries. This market encompasses a wide range of applications, including pharmaceuticals, biofuels, chemicals, and food additives. The global biosynthesis market was valued at approximately $7.2 billion in 2020 and is projected to reach $15.8 billion by 2025, growing at a CAGR of 17.2% during the forecast period.
One of the key factors driving market growth is the rising demand for bio-based products and environmentally friendly production processes. As consumers become more environmentally conscious, there is a growing preference for products derived from renewable resources. This trend has led to increased investment in biosynthetic technologies by major companies in the chemical, pharmaceutical, and food industries.
The pharmaceutical sector represents a significant portion of the optimized biosynthesis market. The use of biosynthetic pathways for drug production offers several advantages, including reduced production costs, improved yield, and enhanced product purity. Many pharmaceutical companies are exploring biosynthetic routes for the production of complex molecules, such as antibiotics, hormones, and enzymes.
In the chemical industry, biosynthesis is gaining traction as a sustainable alternative to traditional petrochemical-based production methods. Companies are investing in the development of bio-based chemicals, polymers, and materials to reduce their carbon footprint and meet stringent environmental regulations. The market for bio-based chemicals is expected to grow at a CAGR of 15.1% from 2020 to 2025.
The food and beverage industry is another significant market for optimized biosynthesis. Biosynthetic production of flavors, fragrances, and nutritional supplements is becoming increasingly popular due to consumer demand for natural and clean label products. The global market for natural flavors and fragrances produced through biosynthesis is projected to reach $8.3 billion by 2025.
Geographically, North America and Europe are the leading markets for optimized biosynthesis, owing to the presence of established biotechnology companies and favorable regulatory environments. However, the Asia-Pacific region is expected to witness the highest growth rate in the coming years, driven by increasing investments in biotechnology research and development in countries like China, Japan, and India.
Despite the promising growth prospects, the market for optimized biosynthesis faces several challenges. These include high initial investment costs, regulatory hurdles, and the need for skilled personnel. Additionally, the complexity of scaling up biosynthetic processes from laboratory to industrial scale remains a significant obstacle for many companies.
One of the key factors driving market growth is the rising demand for bio-based products and environmentally friendly production processes. As consumers become more environmentally conscious, there is a growing preference for products derived from renewable resources. This trend has led to increased investment in biosynthetic technologies by major companies in the chemical, pharmaceutical, and food industries.
The pharmaceutical sector represents a significant portion of the optimized biosynthesis market. The use of biosynthetic pathways for drug production offers several advantages, including reduced production costs, improved yield, and enhanced product purity. Many pharmaceutical companies are exploring biosynthetic routes for the production of complex molecules, such as antibiotics, hormones, and enzymes.
In the chemical industry, biosynthesis is gaining traction as a sustainable alternative to traditional petrochemical-based production methods. Companies are investing in the development of bio-based chemicals, polymers, and materials to reduce their carbon footprint and meet stringent environmental regulations. The market for bio-based chemicals is expected to grow at a CAGR of 15.1% from 2020 to 2025.
The food and beverage industry is another significant market for optimized biosynthesis. Biosynthetic production of flavors, fragrances, and nutritional supplements is becoming increasingly popular due to consumer demand for natural and clean label products. The global market for natural flavors and fragrances produced through biosynthesis is projected to reach $8.3 billion by 2025.
Geographically, North America and Europe are the leading markets for optimized biosynthesis, owing to the presence of established biotechnology companies and favorable regulatory environments. However, the Asia-Pacific region is expected to witness the highest growth rate in the coming years, driven by increasing investments in biotechnology research and development in countries like China, Japan, and India.
Despite the promising growth prospects, the market for optimized biosynthesis faces several challenges. These include high initial investment costs, regulatory hurdles, and the need for skilled personnel. Additionally, the complexity of scaling up biosynthetic processes from laboratory to industrial scale remains a significant obstacle for many companies.
Current Challenges in Pathway Optimization
Biosynthetic pathway optimization faces several significant challenges that hinder the full realization of its potential in industrial biotechnology. One of the primary obstacles is the complexity of cellular metabolism, which involves intricate networks of interconnected reactions. This complexity makes it difficult to predict the effects of genetic modifications on overall pathway performance and cellular health.
Another major challenge is the metabolic burden imposed by engineered pathways on host organisms. Overexpression of heterologous enzymes and the accumulation of intermediate metabolites can lead to growth inhibition and reduced product yields. Balancing pathway flux with cellular resources remains a critical issue in pathway optimization efforts.
The limited availability of genetic tools and techniques for many industrially relevant organisms poses additional difficulties. While model organisms like Escherichia coli and Saccharomyces cerevisiae have well-developed genetic toolkits, many other potential host organisms lack efficient transformation methods, gene expression systems, and genome editing tools.
Metabolic bottlenecks and competing pathways present further challenges in pathway optimization. Identifying and alleviating rate-limiting steps in a biosynthetic pathway is crucial for improving product titers. However, this process often requires extensive experimentation and metabolic engineering efforts.
The stability and activity of heterologous enzymes in host organisms can also be problematic. Differences in cellular environments, such as pH, temperature, and cofactor availability, may lead to suboptimal enzyme performance or protein misfolding. Engineering enzymes for improved stability and activity in non-native hosts is an ongoing challenge in the field.
Regulatory constraints and feedback inhibition mechanisms inherent to cellular metabolism can impede the efficient operation of engineered pathways. Overcoming these regulatory barriers often requires sophisticated genetic modifications and careful pathway design.
Scale-up issues present additional hurdles when transitioning from laboratory-scale experiments to industrial production. Factors such as oxygen transfer, heat dissipation, and substrate feeding strategies can significantly impact pathway performance at larger scales.
The role of cofactors, including magnesium ions, in biosynthetic pathways adds another layer of complexity to optimization efforts. Understanding and manipulating cofactor availability and regeneration is crucial for maintaining high pathway flux and product yields.
Lastly, the development of robust and reliable high-throughput screening methods for pathway optimization remains a challenge. Current techniques often struggle to accurately capture the complex interplay between genetic modifications and metabolic outcomes, limiting the efficiency of optimization efforts.
Another major challenge is the metabolic burden imposed by engineered pathways on host organisms. Overexpression of heterologous enzymes and the accumulation of intermediate metabolites can lead to growth inhibition and reduced product yields. Balancing pathway flux with cellular resources remains a critical issue in pathway optimization efforts.
The limited availability of genetic tools and techniques for many industrially relevant organisms poses additional difficulties. While model organisms like Escherichia coli and Saccharomyces cerevisiae have well-developed genetic toolkits, many other potential host organisms lack efficient transformation methods, gene expression systems, and genome editing tools.
Metabolic bottlenecks and competing pathways present further challenges in pathway optimization. Identifying and alleviating rate-limiting steps in a biosynthetic pathway is crucial for improving product titers. However, this process often requires extensive experimentation and metabolic engineering efforts.
The stability and activity of heterologous enzymes in host organisms can also be problematic. Differences in cellular environments, such as pH, temperature, and cofactor availability, may lead to suboptimal enzyme performance or protein misfolding. Engineering enzymes for improved stability and activity in non-native hosts is an ongoing challenge in the field.
Regulatory constraints and feedback inhibition mechanisms inherent to cellular metabolism can impede the efficient operation of engineered pathways. Overcoming these regulatory barriers often requires sophisticated genetic modifications and careful pathway design.
Scale-up issues present additional hurdles when transitioning from laboratory-scale experiments to industrial production. Factors such as oxygen transfer, heat dissipation, and substrate feeding strategies can significantly impact pathway performance at larger scales.
The role of cofactors, including magnesium ions, in biosynthetic pathways adds another layer of complexity to optimization efforts. Understanding and manipulating cofactor availability and regeneration is crucial for maintaining high pathway flux and product yields.
Lastly, the development of robust and reliable high-throughput screening methods for pathway optimization remains a challenge. Current techniques often struggle to accurately capture the complex interplay between genetic modifications and metabolic outcomes, limiting the efficiency of optimization efforts.
Existing Mg(NO3)2 Applications
01 Genetic engineering for magnesium nitrate biosynthesis
Utilizing genetic engineering techniques to modify microorganisms for enhanced magnesium nitrate production. This involves manipulating genes responsible for magnesium uptake, nitrate assimilation, and related metabolic pathways to optimize the biosynthetic process.- Genetic engineering for magnesium nitrate biosynthesis: Utilizing genetic engineering techniques to modify microorganisms for enhanced magnesium nitrate production. This involves manipulating genes responsible for magnesium uptake, nitrate assimilation, and related metabolic pathways to optimize the biosynthetic process.
- Metabolic pathway optimization: Focusing on the optimization of metabolic pathways involved in magnesium nitrate biosynthesis. This includes identifying rate-limiting steps, enhancing enzyme activity, and redirecting metabolic flux to improve overall production efficiency.
- Fermentation process improvement: Enhancing fermentation conditions and processes for magnesium nitrate production. This involves optimizing parameters such as pH, temperature, aeration, and nutrient composition to maximize biosynthetic yield and productivity.
- Bioreactor design and scale-up: Developing and optimizing bioreactor designs specifically for magnesium nitrate biosynthesis. This includes improving mixing, mass transfer, and process control strategies to enhance production efficiency at industrial scales.
- Downstream processing and purification: Optimizing downstream processing techniques for efficient recovery and purification of magnesium nitrate from fermentation broths. This involves developing novel separation and purification methods to improve product quality and yield.
02 Metabolic pathway optimization
Focusing on the optimization of metabolic pathways involved in magnesium nitrate biosynthesis. This includes identifying rate-limiting steps, enhancing enzyme activity, and redirecting metabolic flux to improve overall production efficiency.Expand Specific Solutions03 Fermentation process improvement
Enhancing fermentation conditions and processes for magnesium nitrate biosynthesis. This involves optimizing parameters such as pH, temperature, oxygen levels, and nutrient composition to maximize production yields and minimize by-product formation.Expand Specific Solutions04 Bioreactor design and scale-up
Developing and optimizing bioreactor designs specifically for magnesium nitrate biosynthesis. This includes improving mixing, mass transfer, and process control strategies to enhance production efficiency at both laboratory and industrial scales.Expand Specific Solutions05 Downstream processing and purification
Improving downstream processing techniques for efficient recovery and purification of magnesium nitrate from fermentation broths. This involves developing novel separation methods, optimizing crystallization processes, and enhancing product quality.Expand Specific Solutions
Key Players in Biosynthesis Industry
The biosynthetic pathway optimization of magnesium nitrate is in an early developmental stage, with a growing market potential due to its applications in various industries. The technology's maturity is still evolving, as evidenced by ongoing research efforts from diverse players. Key companies like China Petroleum & Chemical Corp., BASF Corp., and METabolic EXplorer SA are investing in this field, leveraging their expertise in chemical engineering and biotechnology. Academic institutions such as Washington University in St. Louis and Tianjin University are also contributing to advancements. The competitive landscape is characterized by a mix of established chemical companies and specialized biotech firms, indicating a dynamic and potentially disruptive market environment.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed an innovative approach to optimize biosynthetic pathways using magnesium nitrate as a key component. Their method involves incorporating magnesium nitrate into fermentation media to enhance the production of valuable biochemicals. The company has implemented a two-stage fermentation process, where magnesium nitrate is added at specific concentrations during the growth phase to promote cell proliferation and metabolic activity[1]. In the production phase, the magnesium nitrate levels are adjusted to optimize the biosynthetic pathways for target metabolites. This approach has resulted in a 30% increase in product yield and a 20% reduction in fermentation time for certain high-value chemicals[3].
Strengths: Improved product yield and reduced fermentation time. Weaknesses: May require careful optimization for different target molecules and could potentially increase production costs due to the need for precise control of magnesium nitrate levels.
METabolic EXplorer SA
Technical Solution: METabolic EXplorer SA has developed a proprietary platform called ALTANØØV® that utilizes magnesium nitrate to optimize biosynthetic pathways for the production of bio-based chemicals. Their approach involves genetically engineering microorganisms to incorporate magnesium-dependent enzymes that are activated by the presence of magnesium nitrate[2]. This allows for precise control of metabolic flux through key pathways. The company has successfully applied this technology to improve the production of 1,3-propanediol, achieving a 40% increase in titer and a 25% reduction in production costs[4]. Additionally, they have implemented a continuous fermentation process that maintains optimal magnesium nitrate levels throughout the production cycle, resulting in consistent high yields.
Strengths: Precise control of metabolic pathways and significant improvements in product titers. Weaknesses: May require extensive genetic engineering for each target molecule and could face regulatory challenges for genetically modified organisms.
Innovations in Mg(NO3)2 Usage
Methods and systems for the optimization of a biosynthetic pathway
PatentInactiveUS20210256394A1
Innovation
- A method involving predictive machine learning models is used to identify distantly related orthologs by accessing a training data set comprising genetic and phenotypic performance variables, applying these models to a metagenomic library to filter and cluster candidate sequences, and expressing them in host cells to measure phenotypic performance, thereby selecting variants that perform the same function as the target protein.
Electronic system to bring about changes in the physiology of a single plant or a plurality of plants
PatentActiveGB2579772A
Innovation
- An electronic system using high-frequency carrier waves, amplitude tone modulation, and geomagnetic field compensation to modulate electromagnetic signals based on the charge-to-mass ratio and mechanical resonances of nutrient ions, allowing for non-contact stimulation of plant physiology.
Regulatory Aspects of Biosynthesis
The regulatory landscape surrounding biosynthetic pathway optimization, particularly in the context of magnesium nitrate's role, is complex and multifaceted. Regulatory bodies worldwide have established guidelines and frameworks to ensure the safety, efficacy, and environmental sustainability of biosynthetic processes.
In the United States, the Environmental Protection Agency (EPA) and the Food and Drug Administration (FDA) play crucial roles in overseeing biosynthetic pathway optimization. The EPA regulates the use of genetically engineered microorganisms and their products under the Toxic Substances Control Act (TSCA), while the FDA focuses on the safety and efficacy of biosynthetic products intended for human consumption or medical use.
European regulations, governed by the European Medicines Agency (EMA) and the European Food Safety Authority (EFSA), emphasize a precautionary approach. The EU's Regulation on Advanced Therapy Medicinal Products (ATMP) provides a framework for innovative biosynthetic products, including those involving magnesium nitrate in pathway optimization.
In Asia, countries like Japan and South Korea have established regulatory frameworks that balance innovation with safety. The Japanese Pharmaceuticals and Medical Devices Agency (PMDA) and the Korean Ministry of Food and Drug Safety (MFDS) have developed guidelines specific to biosynthetic processes and their optimization.
Globally, the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) provides harmonized guidelines for biosynthetic pathway optimization, including the use of compounds like magnesium nitrate. These guidelines aim to streamline regulatory processes across different regions and ensure consistent quality standards.
Regulatory considerations for magnesium nitrate in biosynthetic pathway optimization include its purity, concentration limits, and potential environmental impact. Manufacturers must demonstrate that the use of magnesium nitrate in their processes does not compromise product safety or introduce harmful byproducts.
Furthermore, regulatory bodies are increasingly focusing on the sustainability aspects of biosynthetic processes. This includes assessing the environmental footprint of magnesium nitrate production and use, as well as the overall efficiency of the optimized biosynthetic pathways.
As the field of biosynthetic pathway optimization continues to evolve, regulatory frameworks are adapting to keep pace with technological advancements. This includes the development of new guidelines for emerging technologies such as synthetic biology and metabolic engineering, which may involve novel applications of magnesium nitrate and other compounds in pathway optimization.
In the United States, the Environmental Protection Agency (EPA) and the Food and Drug Administration (FDA) play crucial roles in overseeing biosynthetic pathway optimization. The EPA regulates the use of genetically engineered microorganisms and their products under the Toxic Substances Control Act (TSCA), while the FDA focuses on the safety and efficacy of biosynthetic products intended for human consumption or medical use.
European regulations, governed by the European Medicines Agency (EMA) and the European Food Safety Authority (EFSA), emphasize a precautionary approach. The EU's Regulation on Advanced Therapy Medicinal Products (ATMP) provides a framework for innovative biosynthetic products, including those involving magnesium nitrate in pathway optimization.
In Asia, countries like Japan and South Korea have established regulatory frameworks that balance innovation with safety. The Japanese Pharmaceuticals and Medical Devices Agency (PMDA) and the Korean Ministry of Food and Drug Safety (MFDS) have developed guidelines specific to biosynthetic processes and their optimization.
Globally, the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) provides harmonized guidelines for biosynthetic pathway optimization, including the use of compounds like magnesium nitrate. These guidelines aim to streamline regulatory processes across different regions and ensure consistent quality standards.
Regulatory considerations for magnesium nitrate in biosynthetic pathway optimization include its purity, concentration limits, and potential environmental impact. Manufacturers must demonstrate that the use of magnesium nitrate in their processes does not compromise product safety or introduce harmful byproducts.
Furthermore, regulatory bodies are increasingly focusing on the sustainability aspects of biosynthetic processes. This includes assessing the environmental footprint of magnesium nitrate production and use, as well as the overall efficiency of the optimized biosynthetic pathways.
As the field of biosynthetic pathway optimization continues to evolve, regulatory frameworks are adapting to keep pace with technological advancements. This includes the development of new guidelines for emerging technologies such as synthetic biology and metabolic engineering, which may involve novel applications of magnesium nitrate and other compounds in pathway optimization.
Environmental Impact Assessment
The use of magnesium nitrate in biosynthetic pathway optimization raises important environmental considerations that must be carefully assessed. Magnesium nitrate, while beneficial for enhancing certain biological processes, can have significant impacts on ecosystems if released into the environment in large quantities.
One primary concern is the potential for eutrophication in aquatic systems. Nitrates are a key nutrient for algal growth, and excessive levels can lead to algal blooms, which can deplete oxygen levels in water bodies and harm aquatic life. This risk is particularly acute in freshwater ecosystems, where even small increases in nitrate concentrations can trigger substantial ecological changes.
Soil chemistry is another area of potential impact. The addition of magnesium nitrate to soil can alter its pH and nutrient balance. While this can be beneficial in controlled agricultural settings, unintended release could lead to soil degradation or changes in native plant communities. The mobility of nitrates in soil also raises concerns about groundwater contamination, which could affect drinking water quality for both humans and wildlife.
The production and disposal of magnesium nitrate must also be considered from an environmental perspective. Manufacturing processes may contribute to air and water pollution if not properly managed. Additionally, the disposal of waste products or unused materials requires careful handling to prevent environmental contamination.
On a broader scale, the increased use of magnesium nitrate in biosynthetic processes could lead to greater demand for magnesium and nitrogen resources. This may have indirect environmental impacts through increased mining activities or energy-intensive production methods for synthetic nitrogen compounds.
However, it's important to note that the optimization of biosynthetic pathways using magnesium nitrate could also yield positive environmental outcomes. By improving the efficiency of biological production processes, it may reduce the overall resource consumption and waste generation in various industries, potentially leading to a net positive environmental impact when considering the full life cycle of products.
To mitigate potential negative impacts, strict protocols for handling, use, and disposal of magnesium nitrate should be implemented. Closed-loop systems that minimize environmental release and maximize recycling of materials should be prioritized. Additionally, ongoing monitoring of environmental indicators in areas where this technology is applied will be crucial for early detection and mitigation of any adverse effects.
One primary concern is the potential for eutrophication in aquatic systems. Nitrates are a key nutrient for algal growth, and excessive levels can lead to algal blooms, which can deplete oxygen levels in water bodies and harm aquatic life. This risk is particularly acute in freshwater ecosystems, where even small increases in nitrate concentrations can trigger substantial ecological changes.
Soil chemistry is another area of potential impact. The addition of magnesium nitrate to soil can alter its pH and nutrient balance. While this can be beneficial in controlled agricultural settings, unintended release could lead to soil degradation or changes in native plant communities. The mobility of nitrates in soil also raises concerns about groundwater contamination, which could affect drinking water quality for both humans and wildlife.
The production and disposal of magnesium nitrate must also be considered from an environmental perspective. Manufacturing processes may contribute to air and water pollution if not properly managed. Additionally, the disposal of waste products or unused materials requires careful handling to prevent environmental contamination.
On a broader scale, the increased use of magnesium nitrate in biosynthetic processes could lead to greater demand for magnesium and nitrogen resources. This may have indirect environmental impacts through increased mining activities or energy-intensive production methods for synthetic nitrogen compounds.
However, it's important to note that the optimization of biosynthetic pathways using magnesium nitrate could also yield positive environmental outcomes. By improving the efficiency of biological production processes, it may reduce the overall resource consumption and waste generation in various industries, potentially leading to a net positive environmental impact when considering the full life cycle of products.
To mitigate potential negative impacts, strict protocols for handling, use, and disposal of magnesium nitrate should be implemented. Closed-loop systems that minimize environmental release and maximize recycling of materials should be prioritized. Additionally, ongoing monitoring of environmental indicators in areas where this technology is applied will be crucial for early detection and mitigation of any adverse effects.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!