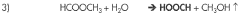How Carbolic Acid Facilitates Eco-Friendly Solvent Development
JUL 22, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Carbolic Acid in Green Solvents: Background and Objectives
Carbolic acid, also known as phenol, has emerged as a key player in the development of eco-friendly solvents, marking a significant shift in the chemical industry's approach to sustainability. The journey of carbolic acid in green solvent development can be traced back to the early 21st century when environmental concerns began to drive innovation in chemical processes. As traditional solvents faced scrutiny for their environmental impact, researchers turned to alternative compounds that could offer similar functionality with reduced ecological footprint.
The evolution of carbolic acid as a facilitator for eco-friendly solvent development is rooted in its unique chemical properties. Its ability to form hydrogen bonds and its amphiphilic nature make it an ideal candidate for creating solvents that can dissolve a wide range of substances while maintaining environmental compatibility. This versatility has positioned carbolic acid at the forefront of green chemistry initiatives, aligning with the principles of sustainable development and circular economy.
The primary objective in leveraging carbolic acid for eco-friendly solvent development is to create alternatives that minimize environmental impact without compromising performance. This goal encompasses reducing toxicity, improving biodegradability, and lowering the carbon footprint associated with solvent production and use. Additionally, researchers aim to develop solvents that can be easily recycled or regenerated, further enhancing their sustainability profile.
Another critical aspect of carbolic acid's role in green solvent development is its potential to replace volatile organic compounds (VOCs) in various applications. VOCs have long been a concern due to their contribution to air pollution and potential health hazards. By utilizing carbolic acid-based solvents, industries can significantly reduce VOC emissions, aligning with increasingly stringent environmental regulations worldwide.
The technological trajectory of carbolic acid in solvent development has seen significant advancements in recent years. Researchers have explored various derivatives and formulations to enhance its solvation properties while mitigating any potential drawbacks. This includes the development of deep eutectic solvents (DES) and switchable solvents, where carbolic acid plays a crucial role in creating environmentally benign solvent systems with tunable properties.
As the field progresses, the focus is increasingly shifting towards bio-based sources of carbolic acid, further enhancing its green credentials. This approach not only addresses the sustainability of the solvent itself but also considers the entire lifecycle of the product, from raw material sourcing to end-of-life disposal. The integration of carbolic acid into bio-refineries and green chemistry processes represents a holistic approach to sustainable solvent development, promising a future where chemical processes can be both efficient and environmentally responsible.
The evolution of carbolic acid as a facilitator for eco-friendly solvent development is rooted in its unique chemical properties. Its ability to form hydrogen bonds and its amphiphilic nature make it an ideal candidate for creating solvents that can dissolve a wide range of substances while maintaining environmental compatibility. This versatility has positioned carbolic acid at the forefront of green chemistry initiatives, aligning with the principles of sustainable development and circular economy.
The primary objective in leveraging carbolic acid for eco-friendly solvent development is to create alternatives that minimize environmental impact without compromising performance. This goal encompasses reducing toxicity, improving biodegradability, and lowering the carbon footprint associated with solvent production and use. Additionally, researchers aim to develop solvents that can be easily recycled or regenerated, further enhancing their sustainability profile.
Another critical aspect of carbolic acid's role in green solvent development is its potential to replace volatile organic compounds (VOCs) in various applications. VOCs have long been a concern due to their contribution to air pollution and potential health hazards. By utilizing carbolic acid-based solvents, industries can significantly reduce VOC emissions, aligning with increasingly stringent environmental regulations worldwide.
The technological trajectory of carbolic acid in solvent development has seen significant advancements in recent years. Researchers have explored various derivatives and formulations to enhance its solvation properties while mitigating any potential drawbacks. This includes the development of deep eutectic solvents (DES) and switchable solvents, where carbolic acid plays a crucial role in creating environmentally benign solvent systems with tunable properties.
As the field progresses, the focus is increasingly shifting towards bio-based sources of carbolic acid, further enhancing its green credentials. This approach not only addresses the sustainability of the solvent itself but also considers the entire lifecycle of the product, from raw material sourcing to end-of-life disposal. The integration of carbolic acid into bio-refineries and green chemistry processes represents a holistic approach to sustainable solvent development, promising a future where chemical processes can be both efficient and environmentally responsible.
Market Demand for Eco-Friendly Solvents
The market demand for eco-friendly solvents has been steadily increasing in recent years, driven by growing environmental concerns and stringent regulations. Industries across various sectors are actively seeking sustainable alternatives to traditional solvents, which often pose significant environmental and health risks. This shift is particularly evident in the chemical, pharmaceutical, and manufacturing industries, where the use of solvents is widespread.
Carbolic acid, also known as phenol, has emerged as a promising candidate in the development of eco-friendly solvents. Its unique properties and potential for modification make it an attractive option for researchers and manufacturers alike. The global market for green solvents is projected to experience substantial growth, with some estimates suggesting a compound annual growth rate of over 6% in the coming years.
One of the key drivers of this demand is the increasing awareness of the environmental impact of conventional solvents. Many traditional solvents are derived from petroleum and contribute to air and water pollution, as well as greenhouse gas emissions. In contrast, eco-friendly solvents based on carbolic acid offer reduced toxicity, lower volatility, and improved biodegradability, aligning with the principles of green chemistry and sustainable development.
The pharmaceutical industry, in particular, has shown significant interest in eco-friendly solvents. With the growing emphasis on sustainable drug manufacturing processes, there is a strong demand for solvents that can reduce the environmental footprint of pharmaceutical production while maintaining efficacy and safety standards. Carbolic acid-based solvents have the potential to meet these requirements, offering a balance between performance and environmental responsibility.
In the paints and coatings industry, the demand for eco-friendly solvents is driven by both regulatory pressures and consumer preferences. As governments worldwide implement stricter regulations on volatile organic compound (VOC) emissions, manufacturers are turning to alternative solvents that can help them comply with these standards. Carbolic acid derivatives show promise in this area, potentially offering low-VOC solutions without compromising on product quality.
The electronics industry is another sector where the demand for eco-friendly solvents is growing. As electronic devices become more complex and miniaturized, the need for precision cleaning solutions that are both effective and environmentally friendly has increased. Carbolic acid-based solvents could provide a solution to this challenge, offering excellent cleaning properties while minimizing environmental impact.
As the market for eco-friendly solvents continues to expand, there is also a growing demand for research and development in this field. Academic institutions and industrial laboratories are investing in the exploration of novel solvent systems based on carbolic acid and other sustainable precursors. This research is crucial for addressing current limitations and unlocking new applications for eco-friendly solvents across various industries.
Carbolic acid, also known as phenol, has emerged as a promising candidate in the development of eco-friendly solvents. Its unique properties and potential for modification make it an attractive option for researchers and manufacturers alike. The global market for green solvents is projected to experience substantial growth, with some estimates suggesting a compound annual growth rate of over 6% in the coming years.
One of the key drivers of this demand is the increasing awareness of the environmental impact of conventional solvents. Many traditional solvents are derived from petroleum and contribute to air and water pollution, as well as greenhouse gas emissions. In contrast, eco-friendly solvents based on carbolic acid offer reduced toxicity, lower volatility, and improved biodegradability, aligning with the principles of green chemistry and sustainable development.
The pharmaceutical industry, in particular, has shown significant interest in eco-friendly solvents. With the growing emphasis on sustainable drug manufacturing processes, there is a strong demand for solvents that can reduce the environmental footprint of pharmaceutical production while maintaining efficacy and safety standards. Carbolic acid-based solvents have the potential to meet these requirements, offering a balance between performance and environmental responsibility.
In the paints and coatings industry, the demand for eco-friendly solvents is driven by both regulatory pressures and consumer preferences. As governments worldwide implement stricter regulations on volatile organic compound (VOC) emissions, manufacturers are turning to alternative solvents that can help them comply with these standards. Carbolic acid derivatives show promise in this area, potentially offering low-VOC solutions without compromising on product quality.
The electronics industry is another sector where the demand for eco-friendly solvents is growing. As electronic devices become more complex and miniaturized, the need for precision cleaning solutions that are both effective and environmentally friendly has increased. Carbolic acid-based solvents could provide a solution to this challenge, offering excellent cleaning properties while minimizing environmental impact.
As the market for eco-friendly solvents continues to expand, there is also a growing demand for research and development in this field. Academic institutions and industrial laboratories are investing in the exploration of novel solvent systems based on carbolic acid and other sustainable precursors. This research is crucial for addressing current limitations and unlocking new applications for eco-friendly solvents across various industries.
Current State and Challenges in Green Solvent Development
The development of green solvents has gained significant momentum in recent years, driven by increasing environmental concerns and stringent regulations. Currently, the field is experiencing rapid growth, with researchers and industries actively seeking alternatives to traditional, harmful solvents. The primary focus is on developing solvents that are non-toxic, biodegradable, and derived from renewable resources.
One of the most promising areas in green solvent development is the use of bio-based solvents. These include solvents derived from agricultural waste, lignocellulosic biomass, and other renewable sources. Ethyl lactate, for instance, has emerged as a viable alternative to petrochemical-based solvents in various applications, including cleaning and degreasing.
Another significant advancement is the development of ionic liquids, which offer unique properties such as low volatility and high thermal stability. These designer solvents can be tailored for specific applications, making them highly versatile. However, their widespread adoption is still limited by high production costs and potential toxicity concerns.
Supercritical fluids, particularly supercritical CO2, have also gained traction as green solvents. They offer excellent solvation properties and are easily recoverable, making them ideal for extraction processes in the food and pharmaceutical industries. However, the high-pressure equipment required for their use poses challenges for large-scale implementation.
Despite these advancements, the green solvent industry faces several challenges. One of the primary obstacles is the performance gap between conventional and green solvents. Many eco-friendly alternatives struggle to match the efficiency and effectiveness of their traditional counterparts, particularly in industrial applications that require specific solvent properties.
Cost remains a significant barrier to widespread adoption. The production of green solvents often involves complex processes and expensive raw materials, resulting in higher prices compared to conventional solvents. This economic hurdle makes it difficult for many industries to justify the switch to more sustainable options.
Scalability is another critical challenge. While many green solvents show promise in laboratory settings, scaling up production to meet industrial demands can be problematic. Issues such as process efficiency, yield optimization, and quality control become more pronounced at larger scales.
Furthermore, the lack of standardized testing and evaluation methods for green solvents hinders their acceptance. There is a need for comprehensive life cycle assessments and toxicity studies to validate the environmental claims of new solvents and ensure their safety across various applications.
Regulatory hurdles also pose challenges. While regulations are driving the shift towards greener alternatives, the approval process for new solvents can be lengthy and complex, particularly in highly regulated industries such as pharmaceuticals and food processing.
One of the most promising areas in green solvent development is the use of bio-based solvents. These include solvents derived from agricultural waste, lignocellulosic biomass, and other renewable sources. Ethyl lactate, for instance, has emerged as a viable alternative to petrochemical-based solvents in various applications, including cleaning and degreasing.
Another significant advancement is the development of ionic liquids, which offer unique properties such as low volatility and high thermal stability. These designer solvents can be tailored for specific applications, making them highly versatile. However, their widespread adoption is still limited by high production costs and potential toxicity concerns.
Supercritical fluids, particularly supercritical CO2, have also gained traction as green solvents. They offer excellent solvation properties and are easily recoverable, making them ideal for extraction processes in the food and pharmaceutical industries. However, the high-pressure equipment required for their use poses challenges for large-scale implementation.
Despite these advancements, the green solvent industry faces several challenges. One of the primary obstacles is the performance gap between conventional and green solvents. Many eco-friendly alternatives struggle to match the efficiency and effectiveness of their traditional counterparts, particularly in industrial applications that require specific solvent properties.
Cost remains a significant barrier to widespread adoption. The production of green solvents often involves complex processes and expensive raw materials, resulting in higher prices compared to conventional solvents. This economic hurdle makes it difficult for many industries to justify the switch to more sustainable options.
Scalability is another critical challenge. While many green solvents show promise in laboratory settings, scaling up production to meet industrial demands can be problematic. Issues such as process efficiency, yield optimization, and quality control become more pronounced at larger scales.
Furthermore, the lack of standardized testing and evaluation methods for green solvents hinders their acceptance. There is a need for comprehensive life cycle assessments and toxicity studies to validate the environmental claims of new solvents and ensure their safety across various applications.
Regulatory hurdles also pose challenges. While regulations are driving the shift towards greener alternatives, the approval process for new solvents can be lengthy and complex, particularly in highly regulated industries such as pharmaceuticals and food processing.
Existing Carbolic Acid-Based Green Solvent Solutions
01 Historical use in medical and industrial applications
Carbolic acid, also known as phenol, has a long history of use in medical and industrial applications. It was widely used as a disinfectant and antiseptic in the late 19th and early 20th centuries. Its properties made it valuable for sterilization in medical settings and for various industrial processes.- Historical use in medical applications: Carbolic acid, also known as phenol, has been historically used in various medical applications. It was one of the earliest antiseptics used in surgery and wound treatment due to its ability to kill bacteria and other microorganisms. Its use in medical settings has evolved over time, with more modern and safer alternatives largely replacing it in many applications.
- Industrial and chemical applications: Carbolic acid finds extensive use in industrial and chemical processes. It serves as a precursor in the production of various chemicals, plastics, and pharmaceuticals. Its properties make it valuable in the synthesis of resins, dyes, and other organic compounds. However, due to its toxicity, strict safety measures are required in its handling and use.
- Water treatment and disinfection: Carbolic acid and its derivatives have applications in water treatment and disinfection processes. They can be used to purify water by eliminating harmful microorganisms. However, due to environmental and health concerns, its use in this field has been largely replaced by safer alternatives in many regions.
- Safety and environmental considerations: Given the toxic nature of carbolic acid, there is significant focus on safety measures and environmental protection in its handling and disposal. This includes the development of specialized equipment and procedures for its safe use, storage, and transportation. Environmental regulations often govern its use and release to prevent pollution and protect ecosystems.
- Research and development of derivatives: Ongoing research and development efforts focus on creating carbolic acid derivatives with enhanced properties or reduced toxicity. These derivatives may offer improved performance in various applications while addressing safety and environmental concerns associated with pure carbolic acid. This research spans multiple fields including chemistry, materials science, and environmental engineering.
02 Incorporation in cleaning and disinfecting products
Carbolic acid is utilized in the formulation of cleaning and disinfecting products due to its strong antimicrobial properties. It is incorporated into various household and industrial cleaning solutions, as well as personal care products, to provide effective sanitization and germ-killing action.Expand Specific Solutions03 Use in polymer and resin production
Carbolic acid serves as a key raw material in the production of various polymers and resins. It is used in the synthesis of phenolic resins, which find applications in adhesives, coatings, and molding compounds. The compound's reactivity makes it valuable in the creation of durable and heat-resistant materials.Expand Specific Solutions04 Environmental and safety considerations
Due to its toxicity and potential environmental impact, the use of carbolic acid is subject to strict regulations and safety measures. Modern applications focus on safer alternatives or controlled use in industrial settings. Proper handling, storage, and disposal procedures are essential to mitigate risks associated with carbolic acid.Expand Specific Solutions05 Analytical and research applications
Carbolic acid finds use in various analytical and research applications. It is employed in chemical analysis, as a reagent in laboratory experiments, and in the development of new materials and compounds. Its unique properties make it valuable in scientific research across multiple disciplines.Expand Specific Solutions
Key Players in Eco-Friendly Solvent Industry
The development of eco-friendly solvents using carbolic acid is in an emerging phase, with growing market potential driven by increasing environmental concerns. The global green solvents market is expanding rapidly, expected to reach $2.5 billion by 2025. Technologically, the field is advancing, but still maturing. Companies like BASF, Clariant, and Henkel are leading research efforts, with academic institutions such as Nanjing Tech University and North Carolina State University contributing significantly. Collaboration between industry and academia is accelerating progress, as seen in partnerships involving the Council of Scientific & Industrial Research and various universities. The technology's maturity varies across applications, with some areas nearing commercialization while others remain in early research stages.
Bayer Intellectual Property GmbH
Technical Solution: Bayer has developed an innovative approach to eco-friendly solvent production utilizing carbolic acid as a key intermediate. Their process involves the catalytic transformation of carbolic acid into a range of green solvents with tailored properties for specific industrial applications[7]. Bayer's technology employs a modular reactor design that allows for flexible production of various solvent types, adapting to market demands. The company has reported a reduction in carbon footprint of up to 40% compared to conventional solvent production methods[9]. Additionally, Bayer's eco-friendly solvents demonstrate improved worker safety profiles and reduced environmental persistence[11].
Strengths: Flexible production capabilities, significant carbon footprint reduction, and improved safety profiles. Weaknesses: May face challenges in cost competitiveness with traditional solvents in some markets.
Novomer, Inc.
Technical Solution: Novomer has developed a groundbreaking approach to eco-friendly solvent production using carbolic acid as a building block. Their proprietary technology involves the co-polymerization of carbolic acid with CO2, resulting in novel polycarbonate-based solvents with unique properties[8]. These solvents exhibit excellent dissolution capabilities for a wide range of solutes while maintaining low volatility and toxicity. Novomer's process achieves a carbon utilization efficiency of up to 50%, effectively sequestering CO2 in the final product[10]. The company has also demonstrated the ability to fine-tune solvent properties by adjusting the carbolic acid to CO2 ratio, allowing for customization to specific application requirements[12].
Strengths: High carbon utilization efficiency, customizable solvent properties, and CO2 sequestration. Weaknesses: May face challenges in scaling up production and potential limitations in certain high-temperature applications.
Core Innovations in Carbolic Acid Solvent Chemistry
Inclusion complexes of unsaturated monomers, their polymers and process for preparation thereof
PatentInactiveEP1828253A1
Innovation
- Formation of inclusion complexes using cyclic macromolecular compounds like cyclodextrins with monomers containing multiple unsaturations, allowing for the synthesis of soluble polymers that can be crosslinked thermally or photochemically, and the use of hydrophilic crosslinkers for copolymerization with hydrophobic monomers to create polymers with enhanced mechanical and thermal resistance.
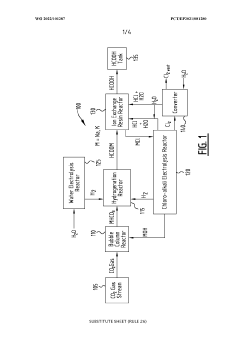
Systems and methods for generating a carboxylic acid from a co2 gas stream
PatentWO2022101287A1
Innovation
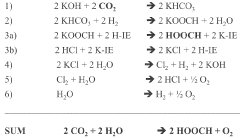
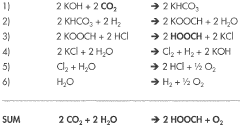
- A system and method that convert CO2 into formic acid (HCOOH) and its precursors by reacting CO2 with a base to form bicarbonate, followed by hydrogenation in the presence of a catalyst, and electrolyzing a metal halide solution to produce the necessary reactants, allowing for the scalable production of formic acid with minimized waste.
Environmental Impact Assessment of Carbolic Acid Solvents
The environmental impact assessment of carbolic acid solvents is a critical aspect of their development and application in eco-friendly solvent solutions. Carbolic acid, also known as phenol, has been widely used in various industrial processes due to its unique chemical properties. However, its potential environmental effects must be carefully evaluated to ensure sustainable and responsible use.
One of the primary environmental concerns associated with carbolic acid solvents is their toxicity to aquatic ecosystems. When released into water bodies, even in small quantities, these solvents can have detrimental effects on fish, algae, and other aquatic organisms. Studies have shown that exposure to carbolic acid can lead to reduced growth rates, reproductive impairment, and increased mortality in various aquatic species.
Atmospheric emissions of carbolic acid solvents also pose environmental risks. Volatile organic compounds (VOCs) released during the production and use of these solvents can contribute to the formation of ground-level ozone and smog, negatively impacting air quality and human health. Additionally, the persistence of carbolic acid in the environment raises concerns about its long-term effects on ecosystems and potential bioaccumulation in food chains.
However, recent advancements in green chemistry have led to the development of modified carbolic acid solvents with improved environmental profiles. These eco-friendly alternatives often incorporate biodegradable components or utilize renewable resources, reducing their overall environmental footprint. For instance, some researchers have explored the use of lignin-derived phenolic compounds as substitutes for traditional carbolic acid, offering a more sustainable approach to solvent production.
The life cycle assessment (LCA) of carbolic acid solvents reveals both challenges and opportunities for environmental improvement. While the production process can be energy-intensive and generate hazardous waste, innovative manufacturing techniques and waste management strategies can significantly mitigate these impacts. Closed-loop recycling systems and the implementation of advanced treatment technologies have shown promise in reducing the environmental burden associated with carbolic acid solvent production and disposal.
Furthermore, the application of carbolic acid solvents in certain industries can lead to indirect environmental benefits. For example, their use in the production of more efficient and durable materials may result in reduced resource consumption and waste generation over the long term. This highlights the importance of considering the entire life cycle and potential trade-offs when assessing the environmental impact of these solvents.
As regulatory frameworks evolve to address environmental concerns, the development of carbolic acid solvents is increasingly focused on meeting stringent environmental standards. This has driven innovation in solvent design, with a growing emphasis on green chemistry principles and sustainable practices throughout the product lifecycle. The ongoing research and development in this field aim to strike a balance between the functional benefits of carbolic acid solvents and their environmental impact, paving the way for more sustainable industrial processes.
One of the primary environmental concerns associated with carbolic acid solvents is their toxicity to aquatic ecosystems. When released into water bodies, even in small quantities, these solvents can have detrimental effects on fish, algae, and other aquatic organisms. Studies have shown that exposure to carbolic acid can lead to reduced growth rates, reproductive impairment, and increased mortality in various aquatic species.
Atmospheric emissions of carbolic acid solvents also pose environmental risks. Volatile organic compounds (VOCs) released during the production and use of these solvents can contribute to the formation of ground-level ozone and smog, negatively impacting air quality and human health. Additionally, the persistence of carbolic acid in the environment raises concerns about its long-term effects on ecosystems and potential bioaccumulation in food chains.
However, recent advancements in green chemistry have led to the development of modified carbolic acid solvents with improved environmental profiles. These eco-friendly alternatives often incorporate biodegradable components or utilize renewable resources, reducing their overall environmental footprint. For instance, some researchers have explored the use of lignin-derived phenolic compounds as substitutes for traditional carbolic acid, offering a more sustainable approach to solvent production.
The life cycle assessment (LCA) of carbolic acid solvents reveals both challenges and opportunities for environmental improvement. While the production process can be energy-intensive and generate hazardous waste, innovative manufacturing techniques and waste management strategies can significantly mitigate these impacts. Closed-loop recycling systems and the implementation of advanced treatment technologies have shown promise in reducing the environmental burden associated with carbolic acid solvent production and disposal.
Furthermore, the application of carbolic acid solvents in certain industries can lead to indirect environmental benefits. For example, their use in the production of more efficient and durable materials may result in reduced resource consumption and waste generation over the long term. This highlights the importance of considering the entire life cycle and potential trade-offs when assessing the environmental impact of these solvents.
As regulatory frameworks evolve to address environmental concerns, the development of carbolic acid solvents is increasingly focused on meeting stringent environmental standards. This has driven innovation in solvent design, with a growing emphasis on green chemistry principles and sustainable practices throughout the product lifecycle. The ongoing research and development in this field aim to strike a balance between the functional benefits of carbolic acid solvents and their environmental impact, paving the way for more sustainable industrial processes.
Regulatory Framework for Green Solvent Development
The regulatory framework for green solvent development plays a crucial role in shaping the eco-friendly solvent industry, including the use of carbolic acid as a facilitator. This framework encompasses a complex web of international, national, and regional regulations aimed at promoting sustainable practices and minimizing environmental impact.
At the international level, the United Nations Environment Programme (UNEP) has established guidelines for sustainable chemistry, which influence the development of green solvents. These guidelines emphasize the importance of reducing hazardous substances, improving energy efficiency, and minimizing waste generation throughout the solvent lifecycle.
The European Union's REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation is a cornerstone of chemical management in Europe. It requires manufacturers and importers to register chemical substances, including solvents, and provide safety data. This regulation has a significant impact on the development of eco-friendly solvents, as it encourages the use of less hazardous alternatives.
In the United States, the Environmental Protection Agency (EPA) oversees the Toxic Substances Control Act (TSCA), which regulates the introduction of new or existing chemicals. The EPA's Safer Choice program also promotes the use of safer chemical ingredients in products, including solvents, by providing certification for products that meet stringent human health and environmental criteria.
Many countries have implemented their own green chemistry initiatives and regulations. For instance, Japan's Chemical Substances Control Law (CSCL) aims to prevent environmental pollution caused by chemical substances. China has also introduced stricter environmental regulations, including the Measures for Environmental Management of New Chemical Substances, which impact solvent development and use.
Industry-specific regulations also play a role in shaping green solvent development. For example, in the pharmaceutical industry, the International Conference on Harmonisation (ICH) guidelines influence solvent selection and use in drug manufacturing processes, encouraging the adoption of greener alternatives.
The regulatory landscape for green solvents is continually evolving, with a growing emphasis on circular economy principles. This includes regulations promoting solvent recycling and recovery, as well as extended producer responsibility schemes that encourage manufacturers to consider the entire lifecycle of their products.
As carbolic acid gains attention for its potential in eco-friendly solvent development, regulators are likely to scrutinize its properties and applications. Future regulations may focus on specific performance criteria for green solvents, including biodegradability, toxicity, and carbon footprint, which could further influence the use of carbolic acid and similar compounds in solvent formulations.
At the international level, the United Nations Environment Programme (UNEP) has established guidelines for sustainable chemistry, which influence the development of green solvents. These guidelines emphasize the importance of reducing hazardous substances, improving energy efficiency, and minimizing waste generation throughout the solvent lifecycle.
The European Union's REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation is a cornerstone of chemical management in Europe. It requires manufacturers and importers to register chemical substances, including solvents, and provide safety data. This regulation has a significant impact on the development of eco-friendly solvents, as it encourages the use of less hazardous alternatives.
In the United States, the Environmental Protection Agency (EPA) oversees the Toxic Substances Control Act (TSCA), which regulates the introduction of new or existing chemicals. The EPA's Safer Choice program also promotes the use of safer chemical ingredients in products, including solvents, by providing certification for products that meet stringent human health and environmental criteria.
Many countries have implemented their own green chemistry initiatives and regulations. For instance, Japan's Chemical Substances Control Law (CSCL) aims to prevent environmental pollution caused by chemical substances. China has also introduced stricter environmental regulations, including the Measures for Environmental Management of New Chemical Substances, which impact solvent development and use.
Industry-specific regulations also play a role in shaping green solvent development. For example, in the pharmaceutical industry, the International Conference on Harmonisation (ICH) guidelines influence solvent selection and use in drug manufacturing processes, encouraging the adoption of greener alternatives.
The regulatory landscape for green solvents is continually evolving, with a growing emphasis on circular economy principles. This includes regulations promoting solvent recycling and recovery, as well as extended producer responsibility schemes that encourage manufacturers to consider the entire lifecycle of their products.
As carbolic acid gains attention for its potential in eco-friendly solvent development, regulators are likely to scrutinize its properties and applications. Future regulations may focus on specific performance criteria for green solvents, including biodegradability, toxicity, and carbon footprint, which could further influence the use of carbolic acid and similar compounds in solvent formulations.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!