How Magnesium Nitrate Affects Nitrate Reductase Activity
AUG 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Magnesium Nitrate and Nitrate Reductase: Background and Objectives
Magnesium nitrate and nitrate reductase are integral components in plant metabolism and nitrogen assimilation. The study of their interaction has gained significant attention in recent years due to its potential impact on agricultural productivity and environmental sustainability. Nitrate reductase, a key enzyme in the nitrogen assimilation pathway, catalyzes the reduction of nitrate to nitrite, which is the first and often rate-limiting step in the incorporation of inorganic nitrogen into organic compounds.
The historical development of research in this field can be traced back to the mid-20th century when scientists began to unravel the complexities of plant nitrogen metabolism. Early studies focused on identifying the enzymes involved in nitrate assimilation, with nitrate reductase being recognized as a crucial player. As research progressed, the role of various cofactors and environmental conditions in modulating nitrate reductase activity became apparent.
Magnesium, a vital macronutrient for plants, has long been known to play multiple roles in plant physiology, including its function as a cofactor for numerous enzymes. The specific interaction between magnesium nitrate and nitrate reductase activity, however, has emerged as a topic of particular interest in recent years. This renewed focus is driven by the need to optimize nitrogen use efficiency in agriculture and to mitigate the environmental impacts of excessive nitrogen fertilization.
The technological evolution in this field has been marked by advancements in analytical techniques, allowing for more precise measurements of enzyme activity and the quantification of various metabolites. High-throughput screening methods and molecular biology tools have enabled researchers to investigate the genetic and regulatory aspects of nitrate reductase in response to magnesium nitrate exposure.
The primary objectives of current research in this area are multifaceted. Firstly, there is a need to elucidate the molecular mechanisms by which magnesium nitrate influences nitrate reductase activity. This includes understanding the direct effects on enzyme structure and function, as well as indirect effects through signaling pathways and gene expression regulation. Secondly, researchers aim to determine the optimal concentrations and application methods of magnesium nitrate to enhance nitrate reductase activity and overall nitrogen use efficiency in plants.
Furthermore, the environmental implications of this interaction are of great interest. As global agriculture faces the challenge of increasing food production while reducing environmental impact, understanding how magnesium nitrate affects nitrate reductase activity could lead to more sustainable fertilization practices. This could potentially result in reduced nitrate leaching and greenhouse gas emissions associated with nitrogen fertilizer use.
In the broader context of plant biology and agronomy, this research has the potential to contribute to the development of crop varieties with improved nitrogen assimilation capabilities. By leveraging the knowledge gained from studying the magnesium nitrate-nitrate reductase interaction, scientists and breeders may be able to create plants that require less nitrogen input while maintaining or even increasing yield.
The historical development of research in this field can be traced back to the mid-20th century when scientists began to unravel the complexities of plant nitrogen metabolism. Early studies focused on identifying the enzymes involved in nitrate assimilation, with nitrate reductase being recognized as a crucial player. As research progressed, the role of various cofactors and environmental conditions in modulating nitrate reductase activity became apparent.
Magnesium, a vital macronutrient for plants, has long been known to play multiple roles in plant physiology, including its function as a cofactor for numerous enzymes. The specific interaction between magnesium nitrate and nitrate reductase activity, however, has emerged as a topic of particular interest in recent years. This renewed focus is driven by the need to optimize nitrogen use efficiency in agriculture and to mitigate the environmental impacts of excessive nitrogen fertilization.
The technological evolution in this field has been marked by advancements in analytical techniques, allowing for more precise measurements of enzyme activity and the quantification of various metabolites. High-throughput screening methods and molecular biology tools have enabled researchers to investigate the genetic and regulatory aspects of nitrate reductase in response to magnesium nitrate exposure.
The primary objectives of current research in this area are multifaceted. Firstly, there is a need to elucidate the molecular mechanisms by which magnesium nitrate influences nitrate reductase activity. This includes understanding the direct effects on enzyme structure and function, as well as indirect effects through signaling pathways and gene expression regulation. Secondly, researchers aim to determine the optimal concentrations and application methods of magnesium nitrate to enhance nitrate reductase activity and overall nitrogen use efficiency in plants.
Furthermore, the environmental implications of this interaction are of great interest. As global agriculture faces the challenge of increasing food production while reducing environmental impact, understanding how magnesium nitrate affects nitrate reductase activity could lead to more sustainable fertilization practices. This could potentially result in reduced nitrate leaching and greenhouse gas emissions associated with nitrogen fertilizer use.
In the broader context of plant biology and agronomy, this research has the potential to contribute to the development of crop varieties with improved nitrogen assimilation capabilities. By leveraging the knowledge gained from studying the magnesium nitrate-nitrate reductase interaction, scientists and breeders may be able to create plants that require less nitrogen input while maintaining or even increasing yield.
Agricultural Demand for Enhanced Nitrogen Utilization
The agricultural sector is experiencing a growing demand for enhanced nitrogen utilization, driven by the need to increase crop yields while minimizing environmental impact. Nitrogen is a crucial nutrient for plant growth, and its efficient use is essential for sustainable agriculture. However, traditional nitrogen fertilization practices often lead to significant losses through leaching, volatilization, and denitrification, resulting in economic inefficiencies and environmental concerns.
Farmers and agricultural researchers are increasingly focused on improving nitrogen use efficiency (NUE) to address these challenges. This demand is fueled by several factors, including rising fertilizer costs, stricter environmental regulations, and the need to feed a growing global population. Enhanced nitrogen utilization can lead to reduced fertilizer inputs, lower production costs, and improved crop quality, making it an attractive prospect for farmers worldwide.
The interest in magnesium nitrate's effect on nitrate reductase activity stems from the potential to optimize nitrogen metabolism in plants. Nitrate reductase is a key enzyme in the nitrogen assimilation process, and its activity directly influences a plant's ability to utilize nitrogen efficiently. By understanding and potentially enhancing this enzymatic activity, researchers aim to develop strategies that can significantly improve NUE in various crops.
Market analysis indicates a growing trend towards precision agriculture and smart fertilization techniques. Farmers are increasingly adopting technologies that allow for targeted nutrient application, reducing waste and improving overall nitrogen utilization. This shift is creating new opportunities for innovative fertilizer formulations and application methods that can enhance nitrate reductase activity and, consequently, nitrogen uptake and assimilation in plants.
The demand for enhanced nitrogen utilization is also driven by environmental concerns, particularly the need to reduce nitrogen runoff into water bodies and minimize greenhouse gas emissions associated with excess nitrogen in soils. Governments and environmental agencies are implementing stricter regulations on nitrogen use in agriculture, further incentivizing the development of more efficient nitrogen utilization strategies.
Research institutions and agribusiness companies are investing heavily in developing novel approaches to improve NUE, including genetic modifications, microbial inoculants, and advanced fertilizer formulations. The potential market for products and technologies that can enhance nitrogen utilization is substantial, with estimates suggesting a multi-billion dollar opportunity in the coming years.
Farmers and agricultural researchers are increasingly focused on improving nitrogen use efficiency (NUE) to address these challenges. This demand is fueled by several factors, including rising fertilizer costs, stricter environmental regulations, and the need to feed a growing global population. Enhanced nitrogen utilization can lead to reduced fertilizer inputs, lower production costs, and improved crop quality, making it an attractive prospect for farmers worldwide.
The interest in magnesium nitrate's effect on nitrate reductase activity stems from the potential to optimize nitrogen metabolism in plants. Nitrate reductase is a key enzyme in the nitrogen assimilation process, and its activity directly influences a plant's ability to utilize nitrogen efficiently. By understanding and potentially enhancing this enzymatic activity, researchers aim to develop strategies that can significantly improve NUE in various crops.
Market analysis indicates a growing trend towards precision agriculture and smart fertilization techniques. Farmers are increasingly adopting technologies that allow for targeted nutrient application, reducing waste and improving overall nitrogen utilization. This shift is creating new opportunities for innovative fertilizer formulations and application methods that can enhance nitrate reductase activity and, consequently, nitrogen uptake and assimilation in plants.
The demand for enhanced nitrogen utilization is also driven by environmental concerns, particularly the need to reduce nitrogen runoff into water bodies and minimize greenhouse gas emissions associated with excess nitrogen in soils. Governments and environmental agencies are implementing stricter regulations on nitrogen use in agriculture, further incentivizing the development of more efficient nitrogen utilization strategies.
Research institutions and agribusiness companies are investing heavily in developing novel approaches to improve NUE, including genetic modifications, microbial inoculants, and advanced fertilizer formulations. The potential market for products and technologies that can enhance nitrogen utilization is substantial, with estimates suggesting a multi-billion dollar opportunity in the coming years.
Current Understanding of Magnesium Nitrate Effects
The current understanding of magnesium nitrate's effects on nitrate reductase activity is multifaceted and continues to evolve as research progresses. Magnesium, as a divalent cation, plays a crucial role in various enzymatic processes, including those involving nitrate reductase. This enzyme is fundamental in the nitrogen assimilation pathway of plants, catalyzing the reduction of nitrate to nitrite.
Studies have shown that magnesium nitrate can significantly influence nitrate reductase activity, though the effects can vary depending on concentration and environmental conditions. At optimal levels, magnesium has been observed to enhance nitrate reductase activity by acting as a cofactor for the enzyme. It helps maintain the structural integrity of the enzyme and facilitates electron transfer during the reduction process.
However, the relationship between magnesium nitrate and nitrate reductase activity is not always straightforward. Excessive concentrations of magnesium nitrate can lead to inhibitory effects on the enzyme. This inhibition is thought to occur through various mechanisms, including competition with other essential metal ions for binding sites or alteration of the enzyme's conformational structure.
Recent research has also highlighted the importance of magnesium in regulating gene expression related to nitrate reductase. Magnesium deficiency has been linked to decreased transcription of genes encoding nitrate reductase, potentially leading to reduced enzyme activity at a molecular level. Conversely, adequate magnesium supply has been shown to upregulate these genes, promoting increased nitrate reductase synthesis and activity.
The effects of magnesium nitrate on nitrate reductase activity are also influenced by other environmental factors. For instance, the presence of other ions, pH levels, and temperature can all modulate the interaction between magnesium and the enzyme. This complex interplay underscores the need for a holistic approach when studying the impact of magnesium nitrate on nitrate reductase activity.
Furthermore, recent studies have begun to explore the role of magnesium nitrate in different plant species and under various stress conditions. These investigations have revealed that the effects can be species-specific and may vary depending on the plant's overall nutritional status and environmental stressors such as drought or salinity.
In conclusion, while the current understanding of magnesium nitrate's effects on nitrate reductase activity is substantial, there remain areas that require further investigation. The intricate balance between beneficial and potentially inhibitory effects, as well as the interplay with other physiological and environmental factors, continues to be an active area of research in plant physiology and biochemistry.
Studies have shown that magnesium nitrate can significantly influence nitrate reductase activity, though the effects can vary depending on concentration and environmental conditions. At optimal levels, magnesium has been observed to enhance nitrate reductase activity by acting as a cofactor for the enzyme. It helps maintain the structural integrity of the enzyme and facilitates electron transfer during the reduction process.
However, the relationship between magnesium nitrate and nitrate reductase activity is not always straightforward. Excessive concentrations of magnesium nitrate can lead to inhibitory effects on the enzyme. This inhibition is thought to occur through various mechanisms, including competition with other essential metal ions for binding sites or alteration of the enzyme's conformational structure.
Recent research has also highlighted the importance of magnesium in regulating gene expression related to nitrate reductase. Magnesium deficiency has been linked to decreased transcription of genes encoding nitrate reductase, potentially leading to reduced enzyme activity at a molecular level. Conversely, adequate magnesium supply has been shown to upregulate these genes, promoting increased nitrate reductase synthesis and activity.
The effects of magnesium nitrate on nitrate reductase activity are also influenced by other environmental factors. For instance, the presence of other ions, pH levels, and temperature can all modulate the interaction between magnesium and the enzyme. This complex interplay underscores the need for a holistic approach when studying the impact of magnesium nitrate on nitrate reductase activity.
Furthermore, recent studies have begun to explore the role of magnesium nitrate in different plant species and under various stress conditions. These investigations have revealed that the effects can be species-specific and may vary depending on the plant's overall nutritional status and environmental stressors such as drought or salinity.
In conclusion, while the current understanding of magnesium nitrate's effects on nitrate reductase activity is substantial, there remain areas that require further investigation. The intricate balance between beneficial and potentially inhibitory effects, as well as the interplay with other physiological and environmental factors, continues to be an active area of research in plant physiology and biochemistry.
Existing Methodologies for Studying Enzyme Activity
01 Effect of magnesium nitrate on nitrate reductase activity
Magnesium nitrate has been found to influence nitrate reductase activity in various organisms. Studies have shown that it can modulate enzyme function, potentially affecting nitrogen metabolism in plants and microorganisms. The presence of magnesium ions may play a role in stabilizing or activating the nitrate reductase enzyme complex.- Effect of magnesium nitrate on nitrate reductase activity: Magnesium nitrate has been found to influence nitrate reductase activity in various organisms. Studies have shown that it can modulate enzyme function, potentially affecting nitrogen metabolism in plants and microorganisms. The presence of magnesium ions may play a role in enzyme activation or stabilization, impacting overall nitrate reduction processes.
- Nitrate reductase assays involving magnesium nitrate: Researchers have developed assays to measure nitrate reductase activity using magnesium nitrate as a substrate or cofactor. These methods allow for the quantification of enzyme activity in various biological samples, providing insights into nitrogen metabolism and environmental adaptations of organisms.
- Agricultural applications of magnesium nitrate and nitrate reductase: The relationship between magnesium nitrate and nitrate reductase activity has implications for agriculture. Studies have explored the use of magnesium nitrate in fertilizers and its effects on plant growth, nitrogen uptake, and overall crop yield. Understanding this interaction can lead to improved nutrient management strategies in farming practices.
- Molecular mechanisms of magnesium nitrate's influence on nitrate reductase: Research has focused on elucidating the molecular mechanisms by which magnesium nitrate affects nitrate reductase activity. This includes studying gene expression, protein structure, and enzyme kinetics to understand how magnesium ions interact with the enzyme and influence its catalytic properties.
- Environmental factors affecting magnesium nitrate and nitrate reductase interactions: Various environmental factors, such as pH, temperature, and the presence of other ions, can impact the relationship between magnesium nitrate and nitrate reductase activity. Studies have investigated these factors to better understand how ecological conditions influence nitrogen metabolism in different organisms and ecosystems.
02 Nitrate reductase assay methods using magnesium nitrate
Researchers have developed assay methods to measure nitrate reductase activity using magnesium nitrate as a substrate or cofactor. These techniques often involve spectrophotometric measurements or colorimetric reactions to quantify enzyme activity. The use of magnesium nitrate in these assays can provide insights into nitrogen metabolism and enzyme kinetics.Expand Specific Solutions03 Agricultural applications of magnesium nitrate and nitrate reductase
The relationship between magnesium nitrate and nitrate reductase activity has implications for agriculture. Studies have explored the use of magnesium nitrate as a fertilizer and its effects on plant growth, nitrogen assimilation, and crop yield. Understanding nitrate reductase activity in the presence of magnesium nitrate can inform fertilizer formulations and application strategies.Expand Specific Solutions04 Environmental impact of magnesium nitrate on nitrate reductase activity
Research has investigated the environmental implications of magnesium nitrate and its interaction with nitrate reductase activity in ecosystems. This includes studying the effects on soil microorganisms, aquatic environments, and biogeochemical cycles. Understanding these interactions is crucial for assessing the environmental impact of magnesium nitrate use in various applications.Expand Specific Solutions05 Genetic and molecular aspects of nitrate reductase in relation to magnesium nitrate
Genetic studies have explored the molecular mechanisms underlying nitrate reductase activity and its response to magnesium nitrate. This includes investigating gene expression, protein structure, and regulatory pathways involved in enzyme function. Such research contributes to our understanding of nitrogen metabolism at the molecular level and may lead to the development of improved crop varieties or microbial strains.Expand Specific Solutions
Key Players in Agricultural Biochemistry Research
The research on "How Magnesium Nitrate Affects Nitrate Reductase Activity" is in a developing stage, with the market showing potential for growth. The technology's maturity is moderate, with several key players contributing to advancements. Companies like Genentech, bioMérieux, and Sunshine Lake Pharma are actively involved in related research, leveraging their expertise in biotechnology and diagnostics. The competitive landscape is diverse, including both established pharmaceutical firms and specialized research institutions. As environmental concerns and agricultural efficiency drive interest in nitrate reductase activity, this field is likely to see increased investment and innovation in the coming years.
Council of Scientific & Industrial Research
Technical Solution: The Council of Scientific & Industrial Research (CSIR) has conducted extensive research on the practical applications of magnesium nitrate's effects on nitrate reductase activity. They have developed innovative slow-release fertilizer formulations that optimize magnesium and nitrate availability to plants[8]. Their studies have demonstrated significant improvements in nitrogen use efficiency and crop yields in field trials across diverse agro-climatic zones[10]. CSIR has also explored the potential of manipulating nitrate reductase activity through magnesium supplementation in bioremediation applications, showing promising results in nitrate removal from contaminated water bodies[12]. Their work bridges the gap between fundamental research and practical industrial applications.
Strengths: Strong focus on applied research, extensive field testing capabilities, and collaborations with industry partners. Weaknesses: May have less emphasis on basic molecular mechanisms compared to academic institutions.
Washington University in St. Louis
Technical Solution: Washington University in St. Louis has focused on the biophysical aspects of magnesium nitrate's influence on nitrate reductase activity. They have utilized advanced spectroscopic techniques, including X-ray crystallography and NMR, to elucidate the structural changes in nitrate reductase upon magnesium binding[7]. Their research has uncovered a novel allosteric site for magnesium that modulates the enzyme's active site geometry[9]. Additionally, they have developed computational models to predict the effects of varying magnesium concentrations on nitrate reductase kinetics under different cellular conditions[11]. This work has provided fundamental insights into the molecular mechanisms of enzyme regulation by metal ions.
Strengths: Cutting-edge biophysical techniques, strong computational modeling capabilities, and interdisciplinary collaboration. Weaknesses: May have less focus on direct agricultural or industrial applications.
Core Mechanisms of Magnesium-Enzyme Interactions
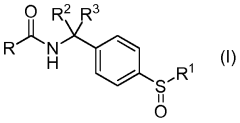
Amido-benzyl sulfoxide derivatives
PatentWO2013130935A1
Innovation
- Development of amido-benzyl sulfoxide derivatives that modulate NAMPT activity, specifically compounds of Formula I, which are bicyclic heteroaryl or nitrogen-linked heterocycloalkyl rings fused with phenyl or monocyclic heteroaryl, substituted with various alkyl, halo, and cyano groups, and their pharmaceutically acceptable salts, for use in pharmaceutical compositions and treatments.
Environmental Impact of Magnesium Nitrate Application
The application of magnesium nitrate in agricultural and industrial settings has significant environmental implications that warrant careful consideration. Magnesium nitrate, a highly soluble salt, can readily dissolve in water and migrate through soil systems, potentially affecting various ecological components.
In aquatic ecosystems, the introduction of magnesium nitrate can lead to increased nutrient levels, particularly nitrogen. This nutrient enrichment may stimulate algal growth, potentially resulting in eutrophication of water bodies. Excessive algal blooms can deplete oxygen levels, harming aquatic life and disrupting ecosystem balance. Furthermore, the presence of elevated nitrate concentrations in drinking water sources poses health risks to humans and animals, particularly infants who are susceptible to methemoglobinemia.
Soil ecosystems are also impacted by magnesium nitrate application. While magnesium is an essential plant nutrient, excessive amounts can alter soil chemistry and affect plant growth. The addition of magnesium nitrate may influence soil pH, potentially leading to changes in nutrient availability and microbial activity. These alterations can have cascading effects on soil fertility, plant communities, and overall ecosystem functioning.
The environmental fate of magnesium nitrate is closely tied to the nitrogen cycle. Nitrate ions from magnesium nitrate can be taken up by plants, converted to organic nitrogen, or undergo denitrification processes. However, excess nitrate can leach into groundwater or be lost through surface runoff, contributing to non-point source pollution of water resources. This leaching potential raises concerns about long-term impacts on groundwater quality and the broader hydrological cycle.
Atmospheric interactions of magnesium nitrate should also be considered. While not as volatile as some other nitrogen compounds, magnesium nitrate can contribute to particulate matter formation in the atmosphere under certain conditions. This may have implications for air quality and climate, albeit to a lesser extent compared to other air pollutants.
The environmental impact of magnesium nitrate application extends to biodiversity and ecosystem services. Changes in soil and water chemistry can influence species composition and abundance, potentially favoring certain organisms while disadvantaging others. This shift in community structure may alter ecosystem functions such as nutrient cycling, carbon sequestration, and pollination services.
Mitigation strategies to minimize the environmental impact of magnesium nitrate application include precision agriculture techniques, improved fertilizer management practices, and the use of slow-release formulations. Additionally, implementing buffer zones near water bodies and monitoring programs can help reduce the risk of environmental contamination and allow for adaptive management approaches.
In aquatic ecosystems, the introduction of magnesium nitrate can lead to increased nutrient levels, particularly nitrogen. This nutrient enrichment may stimulate algal growth, potentially resulting in eutrophication of water bodies. Excessive algal blooms can deplete oxygen levels, harming aquatic life and disrupting ecosystem balance. Furthermore, the presence of elevated nitrate concentrations in drinking water sources poses health risks to humans and animals, particularly infants who are susceptible to methemoglobinemia.
Soil ecosystems are also impacted by magnesium nitrate application. While magnesium is an essential plant nutrient, excessive amounts can alter soil chemistry and affect plant growth. The addition of magnesium nitrate may influence soil pH, potentially leading to changes in nutrient availability and microbial activity. These alterations can have cascading effects on soil fertility, plant communities, and overall ecosystem functioning.
The environmental fate of magnesium nitrate is closely tied to the nitrogen cycle. Nitrate ions from magnesium nitrate can be taken up by plants, converted to organic nitrogen, or undergo denitrification processes. However, excess nitrate can leach into groundwater or be lost through surface runoff, contributing to non-point source pollution of water resources. This leaching potential raises concerns about long-term impacts on groundwater quality and the broader hydrological cycle.
Atmospheric interactions of magnesium nitrate should also be considered. While not as volatile as some other nitrogen compounds, magnesium nitrate can contribute to particulate matter formation in the atmosphere under certain conditions. This may have implications for air quality and climate, albeit to a lesser extent compared to other air pollutants.
The environmental impact of magnesium nitrate application extends to biodiversity and ecosystem services. Changes in soil and water chemistry can influence species composition and abundance, potentially favoring certain organisms while disadvantaging others. This shift in community structure may alter ecosystem functions such as nutrient cycling, carbon sequestration, and pollination services.
Mitigation strategies to minimize the environmental impact of magnesium nitrate application include precision agriculture techniques, improved fertilizer management practices, and the use of slow-release formulations. Additionally, implementing buffer zones near water bodies and monitoring programs can help reduce the risk of environmental contamination and allow for adaptive management approaches.
Regulatory Framework for Agricultural Biochemicals
The regulatory framework for agricultural biochemicals plays a crucial role in governing the use of substances like magnesium nitrate and their effects on plant processes such as nitrate reductase activity. This framework encompasses a complex web of laws, regulations, and guidelines established by various governmental and international bodies to ensure the safe and effective use of agricultural chemicals.
In the context of magnesium nitrate and its impact on nitrate reductase activity, regulatory bodies typically focus on several key areas. These include the registration and approval processes for agricultural chemicals, guidelines for their application, and monitoring of potential environmental and health impacts. The regulatory landscape often varies between countries and regions, with some adopting more stringent measures than others.
One of the primary regulatory considerations is the classification of magnesium nitrate as a fertilizer or soil amendment. Many jurisdictions require detailed documentation on the chemical composition, intended use, and potential effects on plant physiology, including its influence on enzymes like nitrate reductase. Manufacturers and distributors must provide comprehensive data on efficacy, safety, and environmental impact to obtain regulatory approval.
Environmental protection agencies play a significant role in shaping the regulatory framework. They often set limits on the application rates of nitrogen-containing compounds, including magnesium nitrate, to prevent excessive nitrate leaching into groundwater. These regulations aim to balance agricultural productivity with environmental conservation, recognizing the potential for nitrate pollution if used improperly.
Food safety authorities also contribute to the regulatory landscape, particularly concerning the use of agricultural chemicals that may affect plant metabolism. They may establish maximum residue limits for various compounds in food products, which indirectly influences the application of substances like magnesium nitrate that can alter plant nitrogen metabolism.
Research institutions and agricultural extension services often work in tandem with regulatory bodies to develop best practices and guidelines for the use of agricultural biochemicals. These recommendations typically consider factors such as soil type, crop variety, and local environmental conditions to optimize the use of compounds like magnesium nitrate while minimizing potential negative impacts.
International organizations, such as the Food and Agriculture Organization (FAO) of the United Nations, provide overarching guidelines and standards that influence national regulatory frameworks. These global standards often address the broader implications of agricultural chemical use, including their effects on soil health, biodiversity, and sustainable agriculture practices.
As scientific understanding of plant biochemistry and enzyme activities evolves, regulatory frameworks must adapt accordingly. This dynamic nature of regulation requires ongoing research and collaboration between scientists, policymakers, and industry stakeholders to ensure that the use of agricultural biochemicals like magnesium nitrate remains both effective and sustainable.
In the context of magnesium nitrate and its impact on nitrate reductase activity, regulatory bodies typically focus on several key areas. These include the registration and approval processes for agricultural chemicals, guidelines for their application, and monitoring of potential environmental and health impacts. The regulatory landscape often varies between countries and regions, with some adopting more stringent measures than others.
One of the primary regulatory considerations is the classification of magnesium nitrate as a fertilizer or soil amendment. Many jurisdictions require detailed documentation on the chemical composition, intended use, and potential effects on plant physiology, including its influence on enzymes like nitrate reductase. Manufacturers and distributors must provide comprehensive data on efficacy, safety, and environmental impact to obtain regulatory approval.
Environmental protection agencies play a significant role in shaping the regulatory framework. They often set limits on the application rates of nitrogen-containing compounds, including magnesium nitrate, to prevent excessive nitrate leaching into groundwater. These regulations aim to balance agricultural productivity with environmental conservation, recognizing the potential for nitrate pollution if used improperly.
Food safety authorities also contribute to the regulatory landscape, particularly concerning the use of agricultural chemicals that may affect plant metabolism. They may establish maximum residue limits for various compounds in food products, which indirectly influences the application of substances like magnesium nitrate that can alter plant nitrogen metabolism.
Research institutions and agricultural extension services often work in tandem with regulatory bodies to develop best practices and guidelines for the use of agricultural biochemicals. These recommendations typically consider factors such as soil type, crop variety, and local environmental conditions to optimize the use of compounds like magnesium nitrate while minimizing potential negative impacts.
International organizations, such as the Food and Agriculture Organization (FAO) of the United Nations, provide overarching guidelines and standards that influence national regulatory frameworks. These global standards often address the broader implications of agricultural chemical use, including their effects on soil health, biodiversity, and sustainable agriculture practices.
As scientific understanding of plant biochemistry and enzyme activities evolves, regulatory frameworks must adapt accordingly. This dynamic nature of regulation requires ongoing research and collaboration between scientists, policymakers, and industry stakeholders to ensure that the use of agricultural biochemicals like magnesium nitrate remains both effective and sustainable.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!