How Magnesium Nitrate Balances Cation-Exchange Capacity in Soils
AUG 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Mg(NO3)2 and CEC: Background and Objectives
Magnesium nitrate's role in balancing cation-exchange capacity (CEC) in soils has become a critical area of study in agricultural and environmental sciences. The evolution of this technology spans several decades, with significant advancements in understanding soil chemistry and plant nutrition. The primary objective of this research is to elucidate the mechanisms by which Mg(NO3)2 influences CEC and to explore its potential applications in soil management and crop production.
The concept of CEC, first introduced in the early 20th century, has been fundamental in soil science. It represents the soil's ability to hold and exchange positively charged ions, which is crucial for nutrient retention and availability to plants. Magnesium, as a divalent cation, plays a vital role in this exchange process. The use of magnesium nitrate as a soil amendment has gained attention due to its dual function of providing both magnesium and nitrogen, two essential plant nutrients.
Recent technological advancements in soil analysis and molecular modeling have significantly enhanced our understanding of the interactions between Mg(NO3)2 and soil particles. These developments have allowed researchers to investigate the dynamic equilibrium between exchangeable cations and those in soil solution at a microscopic level. The goal is to optimize the balance of cations in the soil, thereby improving soil structure, nutrient availability, and ultimately, crop yield and quality.
The technological trajectory in this field is moving towards precision agriculture, where the application of Mg(NO3)2 can be tailored to specific soil types and crop requirements. This approach aims to maximize the efficiency of nutrient use while minimizing environmental impacts. Researchers are exploring innovative delivery methods, such as controlled-release formulations and site-specific application techniques, to enhance the effectiveness of magnesium nitrate in managing soil CEC.
One of the key objectives of current research is to develop predictive models that can accurately forecast the impact of Mg(NO3)2 application on soil CEC under various environmental conditions. These models integrate data from soil sensors, satellite imagery, and historical crop performance to provide farmers with actionable insights for soil management. The ultimate aim is to create a decision support system that optimizes the use of magnesium nitrate for sustainable agricultural practices.
As we look towards the future, the integration of Mg(NO3)2 technology with other emerging fields such as nanotechnology and biotechnology holds promise for revolutionary advancements in soil science. Researchers are exploring the potential of nano-formulations of magnesium nitrate for enhanced soil penetration and controlled release. Additionally, the development of genetically modified crops that can more efficiently utilize magnesium from the soil is an area of ongoing investigation, which could further optimize the balance between Mg(NO3)2 application and soil CEC.
The concept of CEC, first introduced in the early 20th century, has been fundamental in soil science. It represents the soil's ability to hold and exchange positively charged ions, which is crucial for nutrient retention and availability to plants. Magnesium, as a divalent cation, plays a vital role in this exchange process. The use of magnesium nitrate as a soil amendment has gained attention due to its dual function of providing both magnesium and nitrogen, two essential plant nutrients.
Recent technological advancements in soil analysis and molecular modeling have significantly enhanced our understanding of the interactions between Mg(NO3)2 and soil particles. These developments have allowed researchers to investigate the dynamic equilibrium between exchangeable cations and those in soil solution at a microscopic level. The goal is to optimize the balance of cations in the soil, thereby improving soil structure, nutrient availability, and ultimately, crop yield and quality.
The technological trajectory in this field is moving towards precision agriculture, where the application of Mg(NO3)2 can be tailored to specific soil types and crop requirements. This approach aims to maximize the efficiency of nutrient use while minimizing environmental impacts. Researchers are exploring innovative delivery methods, such as controlled-release formulations and site-specific application techniques, to enhance the effectiveness of magnesium nitrate in managing soil CEC.
One of the key objectives of current research is to develop predictive models that can accurately forecast the impact of Mg(NO3)2 application on soil CEC under various environmental conditions. These models integrate data from soil sensors, satellite imagery, and historical crop performance to provide farmers with actionable insights for soil management. The ultimate aim is to create a decision support system that optimizes the use of magnesium nitrate for sustainable agricultural practices.
As we look towards the future, the integration of Mg(NO3)2 technology with other emerging fields such as nanotechnology and biotechnology holds promise for revolutionary advancements in soil science. Researchers are exploring the potential of nano-formulations of magnesium nitrate for enhanced soil penetration and controlled release. Additionally, the development of genetically modified crops that can more efficiently utilize magnesium from the soil is an area of ongoing investigation, which could further optimize the balance between Mg(NO3)2 application and soil CEC.
Agricultural Market Demand Analysis
The agricultural market for magnesium nitrate and related soil amendments has shown significant growth in recent years, driven by the increasing awareness of soil health and the need for sustainable farming practices. Farmers and agricultural businesses are recognizing the importance of balanced cation-exchange capacity (CEC) in soils for optimal crop yield and quality. This has led to a rising demand for products that can effectively manage soil CEC, with magnesium nitrate emerging as a key solution.
Market analysis indicates that the global soil amendment market, which includes products like magnesium nitrate, is expected to continue its upward trajectory. This growth is particularly pronounced in regions with intensive agriculture and areas facing soil degradation issues. North America and Europe currently lead in market share, but rapid expansion is observed in Asia-Pacific and Latin American countries as they adopt more advanced agricultural practices.
The demand for magnesium nitrate as a soil amendment is closely tied to the broader trends in precision agriculture and sustainable farming. As farmers seek to optimize their input use and maximize crop productivity, there is an increasing focus on tailored soil management solutions. This has created a niche market for specialized fertilizers and soil conditioners that can address specific soil deficiencies, including magnesium deficiency and imbalanced CEC.
In the context of CEC management, magnesium nitrate offers several advantages that align with market needs. It provides a readily available source of magnesium, which is crucial for chlorophyll production and overall plant health. Additionally, its ability to influence soil CEC without significantly altering pH makes it an attractive option for farmers dealing with complex soil chemistry issues.
The market demand is further bolstered by the growing organic farming sector, which requires careful management of soil nutrients without relying on synthetic fertilizers. Magnesium nitrate, when sourced naturally, fits well within organic farming guidelines, opening up additional market opportunities in this rapidly expanding segment of agriculture.
However, the market also faces challenges. Fluctuating raw material prices and the need for education on proper application techniques can impact adoption rates. Additionally, competition from alternative soil amendments and the development of new technologies for soil management create a dynamic market environment that requires continuous innovation and adaptation from suppliers of magnesium nitrate-based products.
Market analysis indicates that the global soil amendment market, which includes products like magnesium nitrate, is expected to continue its upward trajectory. This growth is particularly pronounced in regions with intensive agriculture and areas facing soil degradation issues. North America and Europe currently lead in market share, but rapid expansion is observed in Asia-Pacific and Latin American countries as they adopt more advanced agricultural practices.
The demand for magnesium nitrate as a soil amendment is closely tied to the broader trends in precision agriculture and sustainable farming. As farmers seek to optimize their input use and maximize crop productivity, there is an increasing focus on tailored soil management solutions. This has created a niche market for specialized fertilizers and soil conditioners that can address specific soil deficiencies, including magnesium deficiency and imbalanced CEC.
In the context of CEC management, magnesium nitrate offers several advantages that align with market needs. It provides a readily available source of magnesium, which is crucial for chlorophyll production and overall plant health. Additionally, its ability to influence soil CEC without significantly altering pH makes it an attractive option for farmers dealing with complex soil chemistry issues.
The market demand is further bolstered by the growing organic farming sector, which requires careful management of soil nutrients without relying on synthetic fertilizers. Magnesium nitrate, when sourced naturally, fits well within organic farming guidelines, opening up additional market opportunities in this rapidly expanding segment of agriculture.
However, the market also faces challenges. Fluctuating raw material prices and the need for education on proper application techniques can impact adoption rates. Additionally, competition from alternative soil amendments and the development of new technologies for soil management create a dynamic market environment that requires continuous innovation and adaptation from suppliers of magnesium nitrate-based products.
Current Soil CEC Management Challenges
Cation-Exchange Capacity (CEC) management in soils presents several significant challenges in modern agriculture and environmental science. One of the primary issues is the variability of CEC across different soil types and conditions, making it difficult to implement standardized management practices. Soils with high clay content or organic matter typically have higher CEC, while sandy soils often have lower CEC, requiring tailored approaches for each soil type.
Another challenge is the dynamic nature of CEC, which can change over time due to factors such as pH fluctuations, organic matter decomposition, and mineral weathering. This variability necessitates constant monitoring and adjustment of soil management strategies, adding complexity and cost to agricultural operations.
The competition between different cations for exchange sites poses another significant challenge. Essential plant nutrients like potassium, calcium, and magnesium must compete with potentially harmful elements such as aluminum and heavy metals. Balancing these cations to ensure optimal nutrient availability while minimizing toxicity risks requires precise management and understanding of soil chemistry.
Climate change and its effects on soil properties further complicate CEC management. Increased temperatures and altered precipitation patterns can affect soil organic matter content, pH levels, and mineral weathering rates, all of which influence CEC. Adapting management practices to these changing conditions is an ongoing challenge for soil scientists and agronomists.
The overuse of chemical fertilizers in many agricultural systems has led to imbalances in soil cation ratios, often resulting in nutrient antagonisms and reduced crop productivity. Correcting these imbalances without causing further disruption to soil ecosystems is a delicate and complex task.
Furthermore, the interaction between CEC and soil water management presents additional challenges. Soils with high CEC tend to retain more water, which can be beneficial in drought conditions but problematic in wet seasons. Balancing water retention with adequate drainage and aeration is crucial for maintaining optimal soil conditions for plant growth.
Lastly, the economic aspects of CEC management cannot be overlooked. Implementing sophisticated soil testing and precision agriculture techniques to optimize CEC can be costly, particularly for small-scale farmers. Developing cost-effective methods for CEC management that are accessible to a wide range of agricultural practitioners remains an ongoing challenge in the field.
Another challenge is the dynamic nature of CEC, which can change over time due to factors such as pH fluctuations, organic matter decomposition, and mineral weathering. This variability necessitates constant monitoring and adjustment of soil management strategies, adding complexity and cost to agricultural operations.
The competition between different cations for exchange sites poses another significant challenge. Essential plant nutrients like potassium, calcium, and magnesium must compete with potentially harmful elements such as aluminum and heavy metals. Balancing these cations to ensure optimal nutrient availability while minimizing toxicity risks requires precise management and understanding of soil chemistry.
Climate change and its effects on soil properties further complicate CEC management. Increased temperatures and altered precipitation patterns can affect soil organic matter content, pH levels, and mineral weathering rates, all of which influence CEC. Adapting management practices to these changing conditions is an ongoing challenge for soil scientists and agronomists.
The overuse of chemical fertilizers in many agricultural systems has led to imbalances in soil cation ratios, often resulting in nutrient antagonisms and reduced crop productivity. Correcting these imbalances without causing further disruption to soil ecosystems is a delicate and complex task.
Furthermore, the interaction between CEC and soil water management presents additional challenges. Soils with high CEC tend to retain more water, which can be beneficial in drought conditions but problematic in wet seasons. Balancing water retention with adequate drainage and aeration is crucial for maintaining optimal soil conditions for plant growth.
Lastly, the economic aspects of CEC management cannot be overlooked. Implementing sophisticated soil testing and precision agriculture techniques to optimize CEC can be costly, particularly for small-scale farmers. Developing cost-effective methods for CEC management that are accessible to a wide range of agricultural practitioners remains an ongoing challenge in the field.
Existing Mg(NO3)2 Application Methods
01 Cation-exchange capacity enhancement in soil
Magnesium nitrate can be used to improve the cation-exchange capacity of soil. This enhances nutrient retention and availability for plants, leading to improved soil fertility and crop yields. The application of magnesium nitrate can also help in balancing soil pH and improving soil structure.- Cation-exchange capacity enhancement in soil: Magnesium nitrate can be used to improve the cation-exchange capacity of soil. This enhances nutrient retention and availability for plants, leading to better crop growth and yield. The application of magnesium nitrate can modify soil properties, increasing its ability to hold and exchange positively charged ions.
- Water treatment applications: Magnesium nitrate is utilized in water treatment processes to enhance cation-exchange capacity. It can be employed in ion exchange resins or zeolites to remove unwanted cations from water, improving water quality and purification efficiency. This application is particularly useful in industrial and municipal water treatment systems.
- Catalytic converter performance improvement: The cation-exchange capacity of magnesium nitrate is exploited in catalytic converter technology. It can be used to enhance the performance and longevity of catalytic converters by improving the ion exchange properties of the catalyst support material, leading to more efficient emission control in vehicles.
- Agricultural nutrient delivery systems: Magnesium nitrate is incorporated into advanced nutrient delivery systems for agriculture. Its cation-exchange capacity allows for controlled release of nutrients in fertilizers, ensuring optimal nutrient uptake by plants over time. This approach can lead to more efficient use of fertilizers and reduced environmental impact.
- Industrial separation and purification processes: The cation-exchange capacity of magnesium nitrate is utilized in various industrial separation and purification processes. It can be used in chromatography, metal recovery, and selective ion removal applications. This property makes it valuable in industries such as pharmaceuticals, mining, and chemical manufacturing for efficient separation of ionic species.
02 Water treatment applications
Magnesium nitrate is utilized in water treatment processes to enhance cation-exchange capacity. It can be employed in ion exchange resins or zeolites to remove unwanted cations from water, improving water quality and purification efficiency. This application is particularly useful in industrial and municipal water treatment systems.Expand Specific Solutions03 Catalytic converter technology
Magnesium nitrate is used in the preparation of catalytic converters to enhance cation-exchange capacity. This improves the efficiency of catalytic converters in reducing harmful emissions from vehicles. The increased cation-exchange capacity allows for better adsorption and conversion of pollutants.Expand Specific Solutions04 Agricultural fertilizer formulations
Magnesium nitrate is incorporated into fertilizer formulations to improve nutrient uptake by plants. Its cation-exchange capacity helps in the controlled release of nutrients and enhances the overall effectiveness of fertilizers. This leads to improved crop growth and yield while reducing nutrient leaching.Expand Specific Solutions05 Industrial separation processes
The cation-exchange capacity of magnesium nitrate is utilized in various industrial separation processes. It can be used in chromatography, ion exchange columns, and other separation techniques to selectively remove or isolate specific cations from complex mixtures. This application is valuable in chemical, pharmaceutical, and metallurgical industries.Expand Specific Solutions
Key Players in Soil Amendment Industry
The market for magnesium nitrate in soil cation-exchange capacity balancing is in a growth phase, driven by increasing demand for sustainable agricultural practices. The global market size for specialty fertilizers, including magnesium nitrate, is projected to expand significantly in the coming years. Technologically, the field is moderately mature, with ongoing research and development efforts. Companies like Pioneer Hi-Bred International, Tessenderlo Kerley, and Coromandel International are leading players, focusing on innovative formulations and application methods. Emerging players such as MyLand Co. LLC and Pastoral Robotics Ltd. are introducing novel approaches, combining soil health technologies with precision agriculture, indicating a trend towards more sophisticated and data-driven solutions in this sector.
Pioneer Hi-Bred International, Inc.
Technical Solution: Pioneer Hi-Bred International has developed a precision agriculture approach to balancing CEC using magnesium nitrate in conjunction with their proprietary seed technologies. Their system, "MagOptimize," combines site-specific magnesium nitrate application with genetically optimized crop varieties that efficiently utilize magnesium in various soil conditions[10]. The company's method involves pre-planting soil mapping using advanced spectral imaging to identify areas with suboptimal CEC. Based on this data, variable-rate application equipment precisely delivers magnesium nitrate to targeted zones. Pioneer's crop varieties are designed to have enhanced root systems that more effectively absorb and utilize magnesium, even in soils with lower CEC[11]. This integrated approach has shown to improve magnesium use efficiency by up to 40% compared to standard practices, while also reducing the overall amount of fertilizer required[12].
Strengths: Integration of seed technology with precision fertilizer application, reduced overall fertilizer use, and improved crop performance in varying soil conditions. Weaknesses: Reliance on specific crop varieties may limit flexibility for farmers, and the system may require significant initial investment in technology and equipment.
Tessenderlo Kerley, Inc.
Technical Solution: Tessenderlo Kerley has introduced an innovative liquid magnesium nitrate formulation, "MagniFluid," designed to rapidly balance CEC in a wide range of soil types. Their product utilizes a proprietary surfactant technology that enhances the distribution and penetration of magnesium ions throughout the soil profile[13]. The liquid formulation allows for easy integration with existing irrigation systems, enabling precise and uniform application. Tessenderlo Kerley's approach also includes a complementary soil amendment that temporarily increases soil porosity, facilitating deeper penetration of the magnesium nitrate solution[14]. This combination has been shown to improve CEC balancing in compacted soils and those with high clay content. The company's research indicates that their system can achieve desired CEC levels up to 30% faster than traditional granular magnesium nitrate applications, while also improving water retention in treated soils[15].
Strengths: Rapid CEC balancing, easy integration with irrigation systems, and effectiveness in challenging soil types. Weaknesses: May require more frequent applications compared to slow-release formulations, and the liquid format may be less suitable for dry farming regions.
Core Innovations in CEC Balancing
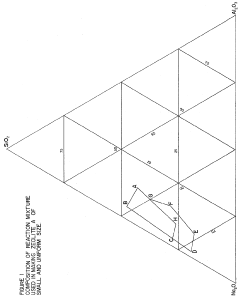
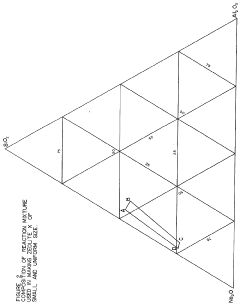
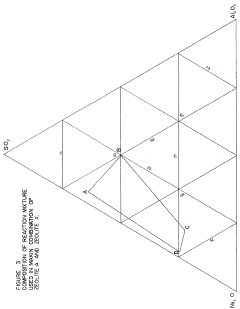
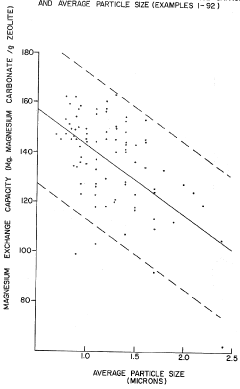
Synthesis of zeolites of small and uniform size having a high magnesium exchange capacity
PatentInactiveUS4416805A
Innovation
- A process involving the formation of a sodium aluminate solution and a sodium silicate solution, combined and heated to form a reaction mixture with specific molar ratios of water to sodium oxide, sodium oxide to silica, and silica to alumina, allowing for the controlled formation of zeolite A or zeolite X with high magnesium exchange capacity and uniform particle size.
Measuring ion exchange capacity
PatentInactiveGB2238488A
Innovation
- A method and apparatus that apply a fluid with substituting ions to the material without physically disrupting it, using a Hassler tube and HPLC pump to measure ion exchange capacity by replacing indigenous cations with ferric ions and then stripping them with sodium ions, allowing for accurate calculation of cation exchange capacity without altering the material's structure.
Environmental Impact Assessment
The use of magnesium nitrate to balance cation-exchange capacity (CEC) in soils has significant environmental implications that warrant careful consideration. This practice affects various aspects of soil ecology and can have both positive and negative consequences for the surrounding ecosystem.
One of the primary environmental impacts is the alteration of soil pH. Magnesium nitrate tends to have a neutral to slightly acidic effect on soil, which can be beneficial in alkaline soils but may exacerbate acidity issues in already acidic environments. This pH change can influence the availability of other nutrients and affect soil microbial communities, potentially altering the soil's overall health and productivity.
The addition of magnesium nitrate also increases the concentration of magnesium and nitrate ions in the soil. While magnesium is an essential plant nutrient, excessive amounts can lead to imbalances in nutrient uptake, particularly affecting the absorption of potassium and calcium. This can impact plant growth and potentially alter the composition of local plant communities.
Nitrate, being highly soluble, poses a risk of leaching into groundwater or surface water bodies. Elevated nitrate levels in water sources can contribute to eutrophication, leading to algal blooms and potential oxygen depletion in aquatic ecosystems. This is particularly concerning in areas with high rainfall or irrigation, where the risk of nitrate runoff is increased.
The use of magnesium nitrate can also influence soil structure and water retention properties. By altering the balance of cations on soil particles, it may affect soil aggregation and porosity. This can impact water infiltration rates, soil aeration, and the soil's capacity to retain moisture, all of which are critical factors in ecosystem health and agricultural productivity.
Furthermore, the production and transportation of magnesium nitrate fertilizers have their own environmental footprint. The manufacturing process requires energy and resources, contributing to greenhouse gas emissions and potentially impacting air and water quality near production facilities. The transportation of these fertilizers also adds to carbon emissions and may pose risks of spills or accidents during handling.
In terms of biodiversity, the changes in soil chemistry induced by magnesium nitrate application can influence the composition and activity of soil microorganisms. This may have cascading effects on the entire soil food web, potentially altering the habitat for various soil-dwelling organisms and affecting above-ground biodiversity.
One of the primary environmental impacts is the alteration of soil pH. Magnesium nitrate tends to have a neutral to slightly acidic effect on soil, which can be beneficial in alkaline soils but may exacerbate acidity issues in already acidic environments. This pH change can influence the availability of other nutrients and affect soil microbial communities, potentially altering the soil's overall health and productivity.
The addition of magnesium nitrate also increases the concentration of magnesium and nitrate ions in the soil. While magnesium is an essential plant nutrient, excessive amounts can lead to imbalances in nutrient uptake, particularly affecting the absorption of potassium and calcium. This can impact plant growth and potentially alter the composition of local plant communities.
Nitrate, being highly soluble, poses a risk of leaching into groundwater or surface water bodies. Elevated nitrate levels in water sources can contribute to eutrophication, leading to algal blooms and potential oxygen depletion in aquatic ecosystems. This is particularly concerning in areas with high rainfall or irrigation, where the risk of nitrate runoff is increased.
The use of magnesium nitrate can also influence soil structure and water retention properties. By altering the balance of cations on soil particles, it may affect soil aggregation and porosity. This can impact water infiltration rates, soil aeration, and the soil's capacity to retain moisture, all of which are critical factors in ecosystem health and agricultural productivity.
Furthermore, the production and transportation of magnesium nitrate fertilizers have their own environmental footprint. The manufacturing process requires energy and resources, contributing to greenhouse gas emissions and potentially impacting air and water quality near production facilities. The transportation of these fertilizers also adds to carbon emissions and may pose risks of spills or accidents during handling.
In terms of biodiversity, the changes in soil chemistry induced by magnesium nitrate application can influence the composition and activity of soil microorganisms. This may have cascading effects on the entire soil food web, potentially altering the habitat for various soil-dwelling organisms and affecting above-ground biodiversity.
Regulatory Framework for Fertilizers
The regulatory framework for fertilizers plays a crucial role in ensuring the safe and effective use of magnesium nitrate and other soil amendments. In many countries, fertilizers are subject to strict regulations that govern their production, distribution, and application. These regulations aim to protect human health, environmental integrity, and agricultural productivity.
At the federal level, agencies such as the Environmental Protection Agency (EPA) and the Food and Drug Administration (FDA) in the United States oversee the registration and regulation of fertilizers. The EPA, for instance, regulates fertilizers under the Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA) if they contain pesticides or plant growth regulators. The FDA regulates fertilizers that may come into contact with food crops.
State-level regulations often complement federal guidelines, with some states imposing additional requirements on fertilizer manufacturers and distributors. These may include specific labeling requirements, quality control measures, and restrictions on certain ingredients. For magnesium nitrate and other fertilizers that affect soil cation-exchange capacity (CEC), regulations may focus on application rates, timing, and methods to prevent nutrient runoff and groundwater contamination.
In the European Union, the Fertilising Products Regulation (FPR) sets harmonized rules for the marketing of EU fertilizing products. This regulation covers various types of fertilizers, including those containing magnesium nitrate, and establishes criteria for safety, quality, and labeling. The FPR also introduces limits on contaminants and defines product function categories to ensure appropriate use.
Many countries have implemented nutrient management regulations to address concerns about water pollution from agricultural runoff. These regulations often require farmers to develop and follow nutrient management plans, which may include guidelines for the use of magnesium nitrate and other fertilizers that influence soil CEC. Such plans typically consider factors like soil type, crop requirements, and environmental conditions to optimize fertilizer use and minimize environmental impact.
Regulatory frameworks also often include provisions for organic farming and sustainable agriculture practices. These may set specific standards for fertilizers used in organic production systems, potentially limiting or prohibiting the use of synthetic fertilizers like magnesium nitrate in favor of organic alternatives that can also influence soil CEC.
As research continues to reveal the complex interactions between fertilizers, soil properties, and environmental outcomes, regulatory frameworks are evolving to incorporate new scientific understanding. This includes an increasing focus on precision agriculture techniques and site-specific management practices that can optimize the use of fertilizers like magnesium nitrate to balance soil CEC while minimizing potential negative impacts.
At the federal level, agencies such as the Environmental Protection Agency (EPA) and the Food and Drug Administration (FDA) in the United States oversee the registration and regulation of fertilizers. The EPA, for instance, regulates fertilizers under the Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA) if they contain pesticides or plant growth regulators. The FDA regulates fertilizers that may come into contact with food crops.
State-level regulations often complement federal guidelines, with some states imposing additional requirements on fertilizer manufacturers and distributors. These may include specific labeling requirements, quality control measures, and restrictions on certain ingredients. For magnesium nitrate and other fertilizers that affect soil cation-exchange capacity (CEC), regulations may focus on application rates, timing, and methods to prevent nutrient runoff and groundwater contamination.
In the European Union, the Fertilising Products Regulation (FPR) sets harmonized rules for the marketing of EU fertilizing products. This regulation covers various types of fertilizers, including those containing magnesium nitrate, and establishes criteria for safety, quality, and labeling. The FPR also introduces limits on contaminants and defines product function categories to ensure appropriate use.
Many countries have implemented nutrient management regulations to address concerns about water pollution from agricultural runoff. These regulations often require farmers to develop and follow nutrient management plans, which may include guidelines for the use of magnesium nitrate and other fertilizers that influence soil CEC. Such plans typically consider factors like soil type, crop requirements, and environmental conditions to optimize fertilizer use and minimize environmental impact.
Regulatory frameworks also often include provisions for organic farming and sustainable agriculture practices. These may set specific standards for fertilizers used in organic production systems, potentially limiting or prohibiting the use of synthetic fertilizers like magnesium nitrate in favor of organic alternatives that can also influence soil CEC.
As research continues to reveal the complex interactions between fertilizers, soil properties, and environmental outcomes, regulatory frameworks are evolving to incorporate new scientific understanding. This includes an increasing focus on precision agriculture techniques and site-specific management practices that can optimize the use of fertilizers like magnesium nitrate to balance soil CEC while minimizing potential negative impacts.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!