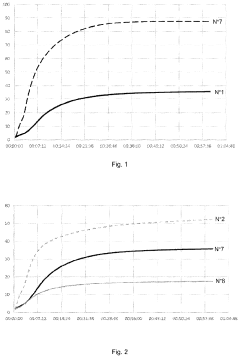
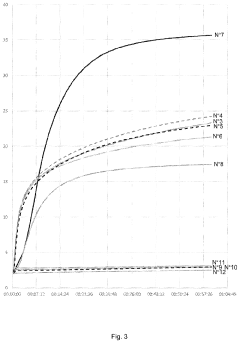
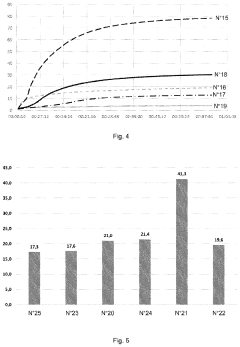
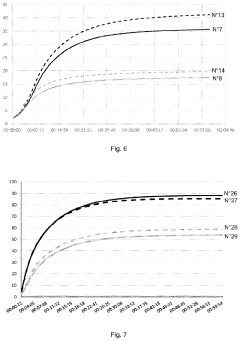
How Magnesium Nitrate Facilitates Cross-Linking in Polymers
AUG 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Magnesium Nitrate Polymer Cross-Linking Overview
Magnesium nitrate has emerged as a significant facilitator in polymer cross-linking processes, revolutionizing the field of polymer science and engineering. This inorganic compound plays a crucial role in enhancing the mechanical and thermal properties of various polymers, making them suitable for a wide range of applications across industries.
The use of magnesium nitrate in polymer cross-linking can be traced back to the early 2000s when researchers began exploring alternative cross-linking agents to improve polymer performance. Since then, significant advancements have been made in understanding the mechanisms by which magnesium nitrate promotes cross-linking and its effects on polymer structures.
One of the key advantages of using magnesium nitrate as a cross-linking facilitator is its ability to form ionic bonds with polymer chains. This ionic interaction creates strong, three-dimensional networks within the polymer matrix, resulting in improved mechanical strength, thermal stability, and chemical resistance. The process is particularly effective in polymers containing functional groups such as carboxyl, hydroxyl, or amine groups.
The cross-linking mechanism facilitated by magnesium nitrate typically involves the formation of coordination complexes between the magnesium ions and the polymer chains. These complexes act as junction points, effectively linking different polymer chains together. The nitrate ions, on the other hand, can participate in oxidation reactions, further promoting the cross-linking process.
Recent studies have shown that the concentration of magnesium nitrate and the processing conditions significantly influence the degree of cross-linking and the resulting polymer properties. Researchers have been working on optimizing these parameters to achieve desired material characteristics for specific applications.
The use of magnesium nitrate in polymer cross-linking has found applications in various industries, including automotive, aerospace, and biomedical fields. For instance, in the automotive industry, magnesium nitrate-facilitated cross-linked polymers are used to produce lightweight, high-strength components, contributing to improved fuel efficiency and vehicle performance.
As the demand for advanced materials continues to grow, the role of magnesium nitrate in polymer cross-linking is expected to expand further. Ongoing research is focused on developing novel polymer systems that can leverage the unique properties of magnesium nitrate to create materials with enhanced functionality and performance.
The use of magnesium nitrate in polymer cross-linking can be traced back to the early 2000s when researchers began exploring alternative cross-linking agents to improve polymer performance. Since then, significant advancements have been made in understanding the mechanisms by which magnesium nitrate promotes cross-linking and its effects on polymer structures.
One of the key advantages of using magnesium nitrate as a cross-linking facilitator is its ability to form ionic bonds with polymer chains. This ionic interaction creates strong, three-dimensional networks within the polymer matrix, resulting in improved mechanical strength, thermal stability, and chemical resistance. The process is particularly effective in polymers containing functional groups such as carboxyl, hydroxyl, or amine groups.
The cross-linking mechanism facilitated by magnesium nitrate typically involves the formation of coordination complexes between the magnesium ions and the polymer chains. These complexes act as junction points, effectively linking different polymer chains together. The nitrate ions, on the other hand, can participate in oxidation reactions, further promoting the cross-linking process.
Recent studies have shown that the concentration of magnesium nitrate and the processing conditions significantly influence the degree of cross-linking and the resulting polymer properties. Researchers have been working on optimizing these parameters to achieve desired material characteristics for specific applications.
The use of magnesium nitrate in polymer cross-linking has found applications in various industries, including automotive, aerospace, and biomedical fields. For instance, in the automotive industry, magnesium nitrate-facilitated cross-linked polymers are used to produce lightweight, high-strength components, contributing to improved fuel efficiency and vehicle performance.
As the demand for advanced materials continues to grow, the role of magnesium nitrate in polymer cross-linking is expected to expand further. Ongoing research is focused on developing novel polymer systems that can leverage the unique properties of magnesium nitrate to create materials with enhanced functionality and performance.
Market Demand for Enhanced Polymer Properties
The market demand for enhanced polymer properties has been steadily increasing across various industries, driven by the need for materials with superior performance characteristics. Magnesium nitrate's role in facilitating cross-linking in polymers has garnered significant attention due to its potential to improve mechanical strength, thermal stability, and chemical resistance of polymer-based products.
In the automotive sector, there is a growing demand for lightweight yet durable materials to improve fuel efficiency and reduce emissions. Cross-linked polymers enhanced by magnesium nitrate offer the potential to replace heavier metal components while maintaining structural integrity. This has led to increased interest from automotive manufacturers seeking to meet stringent environmental regulations and consumer preferences for eco-friendly vehicles.
The construction industry has also shown a keen interest in advanced polymer materials. With the rise of sustainable building practices, there is a demand for polymers with enhanced durability and weather resistance. Magnesium nitrate-facilitated cross-linking can potentially extend the lifespan of construction materials, reducing maintenance costs and improving overall building performance.
In the electronics sector, the miniaturization trend has created a need for polymers with improved heat resistance and dimensional stability. Cross-linked polymers offer these properties, making them ideal for use in compact electronic devices and advanced circuit boards. The ability of magnesium nitrate to enhance these characteristics has attracted attention from electronics manufacturers looking to develop more reliable and long-lasting products.
The packaging industry is another significant market for enhanced polymers. With increasing concerns about food safety and shelf life, there is a demand for packaging materials with improved barrier properties and chemical resistance. Cross-linked polymers facilitated by magnesium nitrate can potentially meet these requirements, offering opportunities for innovation in food packaging and preservation.
The medical device industry has also shown interest in advanced polymer materials. The need for biocompatible, sterilizable, and durable materials for implants and medical equipment has driven research into cross-linked polymers. Magnesium nitrate's role in enhancing these properties could lead to the development of more effective and longer-lasting medical devices.
As environmental concerns continue to grow, there is an increasing demand for recyclable and biodegradable polymers. Research into how magnesium nitrate affects the recyclability and biodegradability of cross-linked polymers could open up new markets in the sustainable materials sector.
In the automotive sector, there is a growing demand for lightweight yet durable materials to improve fuel efficiency and reduce emissions. Cross-linked polymers enhanced by magnesium nitrate offer the potential to replace heavier metal components while maintaining structural integrity. This has led to increased interest from automotive manufacturers seeking to meet stringent environmental regulations and consumer preferences for eco-friendly vehicles.
The construction industry has also shown a keen interest in advanced polymer materials. With the rise of sustainable building practices, there is a demand for polymers with enhanced durability and weather resistance. Magnesium nitrate-facilitated cross-linking can potentially extend the lifespan of construction materials, reducing maintenance costs and improving overall building performance.
In the electronics sector, the miniaturization trend has created a need for polymers with improved heat resistance and dimensional stability. Cross-linked polymers offer these properties, making them ideal for use in compact electronic devices and advanced circuit boards. The ability of magnesium nitrate to enhance these characteristics has attracted attention from electronics manufacturers looking to develop more reliable and long-lasting products.
The packaging industry is another significant market for enhanced polymers. With increasing concerns about food safety and shelf life, there is a demand for packaging materials with improved barrier properties and chemical resistance. Cross-linked polymers facilitated by magnesium nitrate can potentially meet these requirements, offering opportunities for innovation in food packaging and preservation.
The medical device industry has also shown interest in advanced polymer materials. The need for biocompatible, sterilizable, and durable materials for implants and medical equipment has driven research into cross-linked polymers. Magnesium nitrate's role in enhancing these properties could lead to the development of more effective and longer-lasting medical devices.
As environmental concerns continue to grow, there is an increasing demand for recyclable and biodegradable polymers. Research into how magnesium nitrate affects the recyclability and biodegradability of cross-linked polymers could open up new markets in the sustainable materials sector.
Current Challenges in Polymer Cross-Linking
Polymer cross-linking is a critical process in materials science, offering enhanced mechanical properties, thermal stability, and chemical resistance. However, several challenges persist in achieving optimal cross-linking outcomes. One of the primary obstacles is controlling the cross-linking density and distribution. Achieving a uniform cross-link density throughout the polymer matrix remains difficult, often resulting in heterogeneous material properties.
The kinetics of cross-linking reactions pose another significant challenge. Many cross-linking processes are either too slow for practical industrial applications or occur too rapidly, making it challenging to manipulate the material during processing. This balance between reaction speed and workability is crucial for producing high-quality cross-linked polymers.
Temperature sensitivity is a persistent issue in polymer cross-linking. Many cross-linking agents and catalysts are highly temperature-dependent, leading to inconsistent results when temperature fluctuations occur during the manufacturing process. This sensitivity can result in incomplete cross-linking or over-cross-linking, both of which can compromise the final product's performance.
The environmental impact of traditional cross-linking agents is becoming increasingly concerning. Many conventional cross-linking chemicals are toxic or produce harmful byproducts, necessitating the development of more environmentally friendly alternatives. This shift towards green chemistry in polymer cross-linking is challenging but essential for sustainable manufacturing practices.
Compatibility between cross-linking agents and polymer matrices presents another hurdle. Not all cross-linking agents are universally applicable across different polymer types, limiting their versatility. Finding cross-linking agents that are effective across a broad spectrum of polymers without compromising other material properties is an ongoing challenge.
The reversibility of cross-linking is a double-edged sword. While permanent cross-links provide stability, they also make the material difficult to recycle or reprocess. Developing reversible cross-linking mechanisms that maintain material integrity during use but allow for easy recycling is a complex but important area of research.
Lastly, the scalability of cross-linking processes from laboratory to industrial scale remains challenging. Methods that work well in small-scale experiments often face difficulties when scaled up for mass production, necessitating significant process engineering and optimization.
The kinetics of cross-linking reactions pose another significant challenge. Many cross-linking processes are either too slow for practical industrial applications or occur too rapidly, making it challenging to manipulate the material during processing. This balance between reaction speed and workability is crucial for producing high-quality cross-linked polymers.
Temperature sensitivity is a persistent issue in polymer cross-linking. Many cross-linking agents and catalysts are highly temperature-dependent, leading to inconsistent results when temperature fluctuations occur during the manufacturing process. This sensitivity can result in incomplete cross-linking or over-cross-linking, both of which can compromise the final product's performance.
The environmental impact of traditional cross-linking agents is becoming increasingly concerning. Many conventional cross-linking chemicals are toxic or produce harmful byproducts, necessitating the development of more environmentally friendly alternatives. This shift towards green chemistry in polymer cross-linking is challenging but essential for sustainable manufacturing practices.
Compatibility between cross-linking agents and polymer matrices presents another hurdle. Not all cross-linking agents are universally applicable across different polymer types, limiting their versatility. Finding cross-linking agents that are effective across a broad spectrum of polymers without compromising other material properties is an ongoing challenge.
The reversibility of cross-linking is a double-edged sword. While permanent cross-links provide stability, they also make the material difficult to recycle or reprocess. Developing reversible cross-linking mechanisms that maintain material integrity during use but allow for easy recycling is a complex but important area of research.
Lastly, the scalability of cross-linking processes from laboratory to industrial scale remains challenging. Methods that work well in small-scale experiments often face difficulties when scaled up for mass production, necessitating significant process engineering and optimization.
Existing Magnesium Nitrate Cross-Linking Methods
01 Cross-linking of polymers using magnesium nitrate
Magnesium nitrate is used as a cross-linking agent for various polymers, enhancing their mechanical properties and thermal stability. This process is particularly useful in the production of hydrogels and other advanced materials with improved characteristics.- Cross-linking of polymers using magnesium nitrate: Magnesium nitrate is used as a cross-linking agent for various polymers, enhancing their mechanical properties and thermal stability. This process is particularly useful in the production of hydrogels and other advanced materials with improved characteristics.
- Magnesium nitrate in flame retardant compositions: Magnesium nitrate is incorporated into flame retardant formulations, often in combination with other compounds, to improve the fire resistance of materials. This application is particularly relevant in the textile and construction industries.
- Use of magnesium nitrate in agricultural applications: Magnesium nitrate is utilized in fertilizers and soil amendments, providing both magnesium and nitrogen to plants. It can also be used in cross-linking processes for controlled-release fertilizer formulations, improving nutrient efficiency.
- Magnesium nitrate in catalytic processes: Magnesium nitrate serves as a precursor or component in various catalytic systems, particularly in the preparation of mixed metal oxide catalysts. These catalysts find applications in chemical synthesis and environmental remediation processes.
- Magnesium nitrate in energy storage applications: Magnesium nitrate is explored for use in thermal energy storage systems and as a component in advanced battery technologies. Its unique properties contribute to improved energy density and cycle stability in these applications.
02 Magnesium nitrate in biomedical applications
Magnesium nitrate cross-linking is employed in biomedical applications, such as tissue engineering and drug delivery systems. The cross-linked materials exhibit improved biocompatibility and controlled degradation rates, making them suitable for various medical purposes.Expand Specific Solutions03 Environmental and agricultural uses of magnesium nitrate cross-linking
Cross-linking with magnesium nitrate is utilized in environmental remediation and agricultural applications. This includes the development of slow-release fertilizers, soil conditioners, and materials for water treatment and pollutant removal.Expand Specific Solutions04 Industrial applications of magnesium nitrate cross-linking
Magnesium nitrate cross-linking finds applications in various industrial processes, including the production of flame-retardant materials, corrosion-resistant coatings, and high-performance adhesives. The cross-linked materials exhibit enhanced durability and resistance to harsh environments.Expand Specific Solutions05 Novel cross-linking techniques involving magnesium nitrate
Innovative cross-linking methods using magnesium nitrate are being developed, including combination with other cross-linking agents, in-situ cross-linking processes, and stimuli-responsive cross-linking systems. These techniques aim to improve the efficiency and versatility of the cross-linking process.Expand Specific Solutions
Key Players in Polymer Industry
The market for magnesium nitrate's role in polymer cross-linking is in a growth phase, driven by increasing demand for advanced materials in various industries. The global market size is expanding, with a projected CAGR of 4-5% over the next five years. Technologically, the field is moderately mature, with ongoing research focused on optimizing cross-linking processes and enhancing polymer properties. Key players like Shanxi Jiaocheng Hongxing Chemicals, Vive Crop Protection, and Coloplast A/S are investing in R&D to improve product performance and expand applications. Companies such as ISP Investments LLC and Arkema France SA are also contributing to the competitive landscape, driving innovation in polymer cross-linking technologies.
Arkema France SA
Technical Solution: Arkema France SA has developed a novel approach to facilitate cross-linking in polymers using magnesium nitrate. Their method involves incorporating magnesium nitrate into polymer matrices as a cross-linking agent. The magnesium ions act as coordination centers, forming ionic bonds with functional groups on the polymer chains[1]. This process enhances the mechanical properties and thermal stability of the resulting polymer network. Arkema's technique is particularly effective in improving the performance of thermoplastic elastomers and adhesives[3]. The company has also explored the use of magnesium nitrate in combination with other metal salts to create synergistic cross-linking effects, leading to polymers with tailored properties for specific applications[5].
Strengths: Improved mechanical properties and thermal stability of polymers. Versatile application in various polymer systems. Weaknesses: Potential for moisture sensitivity in some formulations. May require careful control of cross-linking density to avoid brittleness.
NIPPON STEEL Chemical & Material Co., Ltd.
Technical Solution: NIPPON STEEL Chemical & Material Co., Ltd. has pioneered a unique approach to using magnesium nitrate for cross-linking in high-performance polymers. Their method involves the in-situ generation of magnesium oxide nanoparticles from magnesium nitrate within the polymer matrix[2]. These nanoparticles serve as cross-linking points, creating a three-dimensional network structure. The company has successfully applied this technique to enhance the heat resistance and mechanical strength of engineering plastics[4]. Additionally, they have developed a controlled release mechanism for the magnesium ions, allowing for gradual cross-linking over time, which is particularly useful in self-healing polymer systems[6]. NIPPON STEEL's research has also shown that the magnesium nitrate-based cross-linking can improve the flame retardancy of certain polymers without compromising their other properties[8].
Strengths: Enhanced heat resistance and mechanical properties. Potential for self-healing polymer systems. Improved flame retardancy. Weaknesses: Complexity in controlling nanoparticle formation and distribution. Possible limitations in compatibility with all polymer types.
Core Mechanisms of Mg(NO3)2 in Cross-Linking
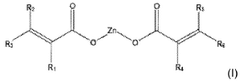
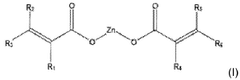
Use of magnesium oxide for crosslinking polymers
PatentActiveUS20210002454A1
Innovation
- The use of magnesium oxide in conjunction with crosslinkable polymers, specifically diene elastomers, and organic peroxides, along with zinc salts as crosslinking co-agents, to enhance crosslinking density and rate, while avoiding halogen and carboxylic acid functional groups.
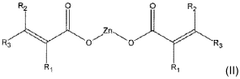
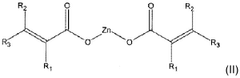
Use of magnesium oxide for crosslinking polymers
PatentWO2019166737A1
Innovation
- The use of magnesium oxide in conjunction with a co-crosslinking agent, such as a zinc salt, and an organic peroxide to enhance the crosslinking process of crosslinkable polymers, particularly diene elastomers, significantly increasing crosslinking density and speed.
Environmental Impact of Cross-Linking Agents
The use of cross-linking agents in polymer manufacturing has significant environmental implications that warrant careful consideration. Magnesium nitrate, as a facilitator of cross-linking in polymers, contributes to these environmental concerns alongside other cross-linking agents commonly used in the industry.
One of the primary environmental impacts of cross-linking agents is their potential for water pollution. When these chemicals are not fully consumed in the cross-linking process or are improperly disposed of, they can leach into groundwater and surface water systems. This contamination can have detrimental effects on aquatic ecosystems and potentially enter the human water supply, posing risks to public health.
Air pollution is another environmental concern associated with cross-linking agents. During the manufacturing process, volatile organic compounds (VOCs) may be released into the atmosphere. These emissions can contribute to the formation of ground-level ozone and smog, which have negative impacts on air quality and human respiratory health.
The production of cross-linking agents, including magnesium nitrate, often involves energy-intensive processes that contribute to greenhouse gas emissions. The carbon footprint of these chemicals extends from their manufacture to their transportation and eventual disposal, adding to the overall environmental burden of polymer production.
Waste management is a critical issue in the use of cross-linking agents. Unused or expired chemicals require proper disposal to prevent environmental contamination. Additionally, cross-linked polymers can be more challenging to recycle or biodegrade, potentially increasing the volume of plastic waste in landfills or the environment.
The persistence of cross-linked polymers in the environment is a growing concern. While cross-linking can enhance the durability and longevity of materials, it also means that these products may take significantly longer to break down naturally. This persistence can lead to long-term accumulation of plastic waste in ecosystems, particularly in marine environments.
Efforts to mitigate the environmental impact of cross-linking agents include the development of more environmentally friendly alternatives, such as bio-based cross-linkers. Research is also focused on improving the efficiency of cross-linking processes to reduce the amount of chemicals required and minimize waste. Additionally, advancements in recycling technologies are being pursued to better handle cross-linked polymers at the end of their lifecycle.
As regulations around environmental protection become more stringent, manufacturers are increasingly required to assess and disclose the environmental impacts of their cross-linking processes. This has led to a growing emphasis on life cycle assessments and the adoption of more sustainable practices in polymer production.
One of the primary environmental impacts of cross-linking agents is their potential for water pollution. When these chemicals are not fully consumed in the cross-linking process or are improperly disposed of, they can leach into groundwater and surface water systems. This contamination can have detrimental effects on aquatic ecosystems and potentially enter the human water supply, posing risks to public health.
Air pollution is another environmental concern associated with cross-linking agents. During the manufacturing process, volatile organic compounds (VOCs) may be released into the atmosphere. These emissions can contribute to the formation of ground-level ozone and smog, which have negative impacts on air quality and human respiratory health.
The production of cross-linking agents, including magnesium nitrate, often involves energy-intensive processes that contribute to greenhouse gas emissions. The carbon footprint of these chemicals extends from their manufacture to their transportation and eventual disposal, adding to the overall environmental burden of polymer production.
Waste management is a critical issue in the use of cross-linking agents. Unused or expired chemicals require proper disposal to prevent environmental contamination. Additionally, cross-linked polymers can be more challenging to recycle or biodegrade, potentially increasing the volume of plastic waste in landfills or the environment.
The persistence of cross-linked polymers in the environment is a growing concern. While cross-linking can enhance the durability and longevity of materials, it also means that these products may take significantly longer to break down naturally. This persistence can lead to long-term accumulation of plastic waste in ecosystems, particularly in marine environments.
Efforts to mitigate the environmental impact of cross-linking agents include the development of more environmentally friendly alternatives, such as bio-based cross-linkers. Research is also focused on improving the efficiency of cross-linking processes to reduce the amount of chemicals required and minimize waste. Additionally, advancements in recycling technologies are being pursued to better handle cross-linked polymers at the end of their lifecycle.
As regulations around environmental protection become more stringent, manufacturers are increasingly required to assess and disclose the environmental impacts of their cross-linking processes. This has led to a growing emphasis on life cycle assessments and the adoption of more sustainable practices in polymer production.
Scalability of Mg(NO3)2 Cross-Linking Processes
The scalability of Mg(NO3)2 cross-linking processes is a critical factor in determining the feasibility of large-scale production and industrial applications of magnesium nitrate-facilitated polymer cross-linking. As the demand for advanced materials with enhanced properties continues to grow, the ability to scale up these processes becomes increasingly important.
One of the key advantages of using magnesium nitrate as a cross-linking agent is its relatively low cost and wide availability. This makes it an attractive option for large-scale production, as the raw material costs can be kept manageable even at higher volumes. Additionally, magnesium nitrate is generally stable and easy to handle, which simplifies the scaling process from a safety and logistics perspective.
The cross-linking reaction itself is typically rapid and can occur at room temperature or with mild heating, which is beneficial for scaling up production. This reduces the need for complex and energy-intensive equipment, making it easier to design and implement larger-scale reactors. However, careful consideration must be given to heat management in larger batches, as the exothermic nature of the cross-linking reaction can lead to temperature control challenges.
Another aspect that supports scalability is the versatility of magnesium nitrate in cross-linking various types of polymers. This allows for the development of standardized processes that can be adapted to different polymer systems with minimal modifications, enhancing the overall efficiency of production facilities.
The solubility of magnesium nitrate in water and some organic solvents provides flexibility in process design, allowing for both solution-based and melt-processing techniques to be scaled up. This versatility can be particularly advantageous when adapting the cross-linking process to different polymer formulations or end-product requirements.
However, there are challenges to consider when scaling up Mg(NO3)2 cross-linking processes. One potential issue is the uniformity of cross-linking in larger volumes. Ensuring consistent distribution of the cross-linking agent throughout the polymer matrix becomes more difficult as batch sizes increase. This may require the development of specialized mixing technologies or the implementation of continuous processing methods to maintain product quality at scale.
Another consideration is the potential for side reactions or unwanted byproducts at larger scales, which could affect the final product properties or process efficiency. Careful optimization of reaction conditions and the implementation of robust quality control measures will be necessary to mitigate these risks.
In conclusion, while the scalability of Mg(NO3)2 cross-linking processes shows promise due to the favorable characteristics of magnesium nitrate and the relatively straightforward reaction conditions, successful large-scale implementation will require careful engineering and process optimization to overcome the challenges associated with increased production volumes.
One of the key advantages of using magnesium nitrate as a cross-linking agent is its relatively low cost and wide availability. This makes it an attractive option for large-scale production, as the raw material costs can be kept manageable even at higher volumes. Additionally, magnesium nitrate is generally stable and easy to handle, which simplifies the scaling process from a safety and logistics perspective.
The cross-linking reaction itself is typically rapid and can occur at room temperature or with mild heating, which is beneficial for scaling up production. This reduces the need for complex and energy-intensive equipment, making it easier to design and implement larger-scale reactors. However, careful consideration must be given to heat management in larger batches, as the exothermic nature of the cross-linking reaction can lead to temperature control challenges.
Another aspect that supports scalability is the versatility of magnesium nitrate in cross-linking various types of polymers. This allows for the development of standardized processes that can be adapted to different polymer systems with minimal modifications, enhancing the overall efficiency of production facilities.
The solubility of magnesium nitrate in water and some organic solvents provides flexibility in process design, allowing for both solution-based and melt-processing techniques to be scaled up. This versatility can be particularly advantageous when adapting the cross-linking process to different polymer formulations or end-product requirements.
However, there are challenges to consider when scaling up Mg(NO3)2 cross-linking processes. One potential issue is the uniformity of cross-linking in larger volumes. Ensuring consistent distribution of the cross-linking agent throughout the polymer matrix becomes more difficult as batch sizes increase. This may require the development of specialized mixing technologies or the implementation of continuous processing methods to maintain product quality at scale.
Another consideration is the potential for side reactions or unwanted byproducts at larger scales, which could affect the final product properties or process efficiency. Careful optimization of reaction conditions and the implementation of robust quality control measures will be necessary to mitigate these risks.
In conclusion, while the scalability of Mg(NO3)2 cross-linking processes shows promise due to the favorable characteristics of magnesium nitrate and the relatively straightforward reaction conditions, successful large-scale implementation will require careful engineering and process optimization to overcome the challenges associated with increased production volumes.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!