How Sodium Percarbonate Mitigates Waterborne Pathogens
JUL 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sodium Percarbonate Disinfection Background
Sodium percarbonate, a compound formed by combining sodium carbonate and hydrogen peroxide, has emerged as a powerful disinfectant in water treatment applications. Its effectiveness in mitigating waterborne pathogens stems from its ability to release hydrogen peroxide when dissolved in water, which acts as a potent oxidizing agent against various microorganisms.
The use of sodium percarbonate for water disinfection dates back to the early 20th century, but its widespread adoption has gained momentum in recent decades due to growing concerns about waterborne diseases and the need for safe, eco-friendly disinfection methods. As a solid, stable compound, sodium percarbonate offers advantages in storage and handling compared to liquid hydrogen peroxide, making it particularly suitable for both large-scale water treatment facilities and portable water purification systems.
The mechanism of action for sodium percarbonate involves the generation of reactive oxygen species (ROS) upon dissolution in water. These ROS, primarily hydrogen peroxide and hydroxyl radicals, attack the cellular components of pathogens, including proteins, lipids, and nucleic acids. This broad-spectrum activity allows sodium percarbonate to effectively combat a wide range of waterborne pathogens, including bacteria, viruses, protozoa, and certain parasites.
One of the key advantages of sodium percarbonate as a disinfectant is its environmental friendliness. After disinfection, it decomposes into water, oxygen, and sodium carbonate, leaving no harmful residues. This characteristic makes it an attractive alternative to chlorine-based disinfectants, which can form potentially carcinogenic byproducts.
The efficacy of sodium percarbonate against waterborne pathogens has been demonstrated in numerous studies. Research has shown its effectiveness against common waterborne pathogens such as Escherichia coli, Salmonella, Giardia, and Cryptosporidium. The compound's ability to inactivate both bacterial and viral pathogens makes it a versatile solution for addressing a wide range of water contamination scenarios.
In recent years, the application of sodium percarbonate has expanded beyond traditional water treatment. It has found use in emergency water disinfection kits, swimming pool maintenance, and even in the food industry for sanitizing equipment and surfaces. The growing interest in sustainable and environmentally friendly technologies has further propelled research into optimizing sodium percarbonate-based disinfection methods.
As water scarcity and quality issues continue to be global concerns, the role of sodium percarbonate in ensuring safe drinking water is likely to grow. Ongoing research focuses on enhancing its efficacy, developing novel formulations, and exploring synergistic effects with other disinfection methods to address emerging waterborne pathogens and improve overall water treatment processes.
The use of sodium percarbonate for water disinfection dates back to the early 20th century, but its widespread adoption has gained momentum in recent decades due to growing concerns about waterborne diseases and the need for safe, eco-friendly disinfection methods. As a solid, stable compound, sodium percarbonate offers advantages in storage and handling compared to liquid hydrogen peroxide, making it particularly suitable for both large-scale water treatment facilities and portable water purification systems.
The mechanism of action for sodium percarbonate involves the generation of reactive oxygen species (ROS) upon dissolution in water. These ROS, primarily hydrogen peroxide and hydroxyl radicals, attack the cellular components of pathogens, including proteins, lipids, and nucleic acids. This broad-spectrum activity allows sodium percarbonate to effectively combat a wide range of waterborne pathogens, including bacteria, viruses, protozoa, and certain parasites.
One of the key advantages of sodium percarbonate as a disinfectant is its environmental friendliness. After disinfection, it decomposes into water, oxygen, and sodium carbonate, leaving no harmful residues. This characteristic makes it an attractive alternative to chlorine-based disinfectants, which can form potentially carcinogenic byproducts.
The efficacy of sodium percarbonate against waterborne pathogens has been demonstrated in numerous studies. Research has shown its effectiveness against common waterborne pathogens such as Escherichia coli, Salmonella, Giardia, and Cryptosporidium. The compound's ability to inactivate both bacterial and viral pathogens makes it a versatile solution for addressing a wide range of water contamination scenarios.
In recent years, the application of sodium percarbonate has expanded beyond traditional water treatment. It has found use in emergency water disinfection kits, swimming pool maintenance, and even in the food industry for sanitizing equipment and surfaces. The growing interest in sustainable and environmentally friendly technologies has further propelled research into optimizing sodium percarbonate-based disinfection methods.
As water scarcity and quality issues continue to be global concerns, the role of sodium percarbonate in ensuring safe drinking water is likely to grow. Ongoing research focuses on enhancing its efficacy, developing novel formulations, and exploring synergistic effects with other disinfection methods to address emerging waterborne pathogens and improve overall water treatment processes.
Market Analysis for Water Treatment Solutions
The water treatment solutions market has experienced significant growth in recent years, driven by increasing concerns over water quality and the prevalence of waterborne pathogens. This market encompasses a wide range of products and technologies designed to purify water for various applications, including municipal water treatment, industrial processes, and household use. The global water treatment market was valued at approximately $265 billion in 2020 and is projected to reach $376 billion by 2026, growing at a CAGR of 6.5% during the forecast period.
Sodium percarbonate, a compound formed by sodium carbonate and hydrogen peroxide, has emerged as a promising solution for mitigating waterborne pathogens. Its effectiveness in water treatment has led to increased demand in both developed and developing countries. The market for sodium percarbonate in water treatment applications is expected to grow at a CAGR of 3.8% from 2021 to 2026.
Key factors driving the growth of water treatment solutions, particularly those involving sodium percarbonate, include stringent water quality regulations, growing awareness of waterborne diseases, and the need for sustainable water management practices. Developing countries, especially in Asia-Pacific and Africa, represent significant growth opportunities due to rapid urbanization and industrialization, coupled with inadequate water infrastructure.
The industrial sector, including food and beverage, pharmaceuticals, and power generation, accounts for a substantial portion of the water treatment market. These industries require high-quality water for their processes and are increasingly adopting advanced treatment technologies. The municipal water treatment segment is also experiencing growth, driven by aging infrastructure in developed countries and the expansion of water treatment facilities in emerging economies.
Challenges in the water treatment market include high initial investment costs, especially for advanced technologies, and the need for skilled personnel to operate and maintain treatment systems. However, innovations in treatment processes, such as the use of sodium percarbonate, are helping to address these challenges by offering cost-effective and easy-to-implement solutions.
The competitive landscape of the water treatment market is characterized by the presence of both large multinational corporations and smaller, specialized companies. Key players in the market are focusing on research and development to introduce more efficient and environmentally friendly treatment solutions, with sodium percarbonate-based products gaining traction due to their effectiveness against a wide range of waterborne pathogens.
Sodium percarbonate, a compound formed by sodium carbonate and hydrogen peroxide, has emerged as a promising solution for mitigating waterborne pathogens. Its effectiveness in water treatment has led to increased demand in both developed and developing countries. The market for sodium percarbonate in water treatment applications is expected to grow at a CAGR of 3.8% from 2021 to 2026.
Key factors driving the growth of water treatment solutions, particularly those involving sodium percarbonate, include stringent water quality regulations, growing awareness of waterborne diseases, and the need for sustainable water management practices. Developing countries, especially in Asia-Pacific and Africa, represent significant growth opportunities due to rapid urbanization and industrialization, coupled with inadequate water infrastructure.
The industrial sector, including food and beverage, pharmaceuticals, and power generation, accounts for a substantial portion of the water treatment market. These industries require high-quality water for their processes and are increasingly adopting advanced treatment technologies. The municipal water treatment segment is also experiencing growth, driven by aging infrastructure in developed countries and the expansion of water treatment facilities in emerging economies.
Challenges in the water treatment market include high initial investment costs, especially for advanced technologies, and the need for skilled personnel to operate and maintain treatment systems. However, innovations in treatment processes, such as the use of sodium percarbonate, are helping to address these challenges by offering cost-effective and easy-to-implement solutions.
The competitive landscape of the water treatment market is characterized by the presence of both large multinational corporations and smaller, specialized companies. Key players in the market are focusing on research and development to introduce more efficient and environmentally friendly treatment solutions, with sodium percarbonate-based products gaining traction due to their effectiveness against a wide range of waterborne pathogens.
Current Challenges in Waterborne Pathogen Control
The control of waterborne pathogens remains a significant challenge in water treatment and public health. Despite advancements in water purification technologies, several obstacles persist in effectively mitigating these harmful microorganisms. One of the primary challenges is the diverse nature of waterborne pathogens, including bacteria, viruses, protozoa, and helminths, each requiring different treatment approaches.
The increasing resistance of pathogens to traditional disinfection methods poses a substantial threat. Many microorganisms have developed mechanisms to withstand chlorine-based treatments, UV radiation, and other common disinfection techniques. This resistance necessitates the development of more robust and innovative treatment solutions to ensure water safety.
Another critical challenge is the formation of biofilms in water distribution systems. These complex microbial communities can harbor and protect pathogens from disinfectants, making them particularly difficult to eradicate. Biofilms not only serve as reservoirs for pathogens but also contribute to the degradation of water quality and infrastructure.
The presence of emerging contaminants, such as pharmaceuticals and personal care products, in water sources further complicates pathogen control efforts. These substances can interact with pathogens and disinfectants in unpredictable ways, potentially reducing the effectiveness of treatment processes or creating harmful by-products.
Climate change and environmental factors also present challenges in waterborne pathogen control. Extreme weather events, such as floods and droughts, can overwhelm water treatment systems and introduce new pathogens into water sources. Additionally, rising temperatures may favor the growth and spread of certain waterborne pathogens, altering their distribution and prevalence.
The detection and monitoring of waterborne pathogens remain challenging, particularly in resource-limited settings. Current methods often lack the sensitivity and speed required for real-time monitoring, leading to delays in identifying and responding to contamination events. Developing rapid, accurate, and cost-effective detection technologies is crucial for improving water safety.
Addressing these challenges requires a multifaceted approach, combining advanced treatment technologies, improved monitoring systems, and sustainable water management practices. The exploration of novel disinfection methods, such as the use of sodium percarbonate, offers promising avenues for enhancing waterborne pathogen control and ensuring safer water supplies for communities worldwide.
The increasing resistance of pathogens to traditional disinfection methods poses a substantial threat. Many microorganisms have developed mechanisms to withstand chlorine-based treatments, UV radiation, and other common disinfection techniques. This resistance necessitates the development of more robust and innovative treatment solutions to ensure water safety.
Another critical challenge is the formation of biofilms in water distribution systems. These complex microbial communities can harbor and protect pathogens from disinfectants, making them particularly difficult to eradicate. Biofilms not only serve as reservoirs for pathogens but also contribute to the degradation of water quality and infrastructure.
The presence of emerging contaminants, such as pharmaceuticals and personal care products, in water sources further complicates pathogen control efforts. These substances can interact with pathogens and disinfectants in unpredictable ways, potentially reducing the effectiveness of treatment processes or creating harmful by-products.
Climate change and environmental factors also present challenges in waterborne pathogen control. Extreme weather events, such as floods and droughts, can overwhelm water treatment systems and introduce new pathogens into water sources. Additionally, rising temperatures may favor the growth and spread of certain waterborne pathogens, altering their distribution and prevalence.
The detection and monitoring of waterborne pathogens remain challenging, particularly in resource-limited settings. Current methods often lack the sensitivity and speed required for real-time monitoring, leading to delays in identifying and responding to contamination events. Developing rapid, accurate, and cost-effective detection technologies is crucial for improving water safety.
Addressing these challenges requires a multifaceted approach, combining advanced treatment technologies, improved monitoring systems, and sustainable water management practices. The exploration of novel disinfection methods, such as the use of sodium percarbonate, offers promising avenues for enhancing waterborne pathogen control and ensuring safer water supplies for communities worldwide.
Sodium Percarbonate Disinfection Mechanisms
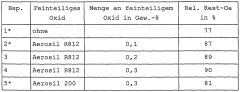
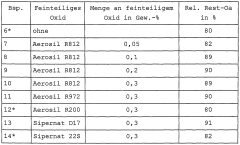
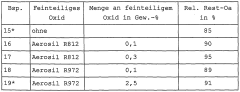
01 Antimicrobial properties of sodium percarbonate
Sodium percarbonate exhibits strong antimicrobial properties, making it effective for pathogen mitigation. When dissolved in water, it releases hydrogen peroxide, which acts as a powerful oxidizing agent capable of destroying various microorganisms, including bacteria, viruses, and fungi. This property makes sodium percarbonate suitable for use in disinfection and sanitization applications.- Antimicrobial properties of sodium percarbonate: Sodium percarbonate exhibits strong antimicrobial properties, making it effective for pathogen mitigation. When dissolved in water, it releases hydrogen peroxide, which acts as a powerful oxidizing agent capable of destroying various microorganisms, including bacteria, viruses, and fungi. This property makes sodium percarbonate useful in disinfection and sanitization applications.
- Formulations for enhanced pathogen mitigation: Combining sodium percarbonate with other active ingredients can enhance its pathogen mitigation efficacy. Formulations may include surfactants, pH adjusters, or other oxidizing agents to create synergistic effects. These combinations can improve the overall antimicrobial performance and broaden the spectrum of pathogens targeted.
- Application methods for pathogen control: Various application methods can be employed to utilize sodium percarbonate for pathogen mitigation. These may include direct application as a powder, dissolution in water for liquid treatments, or incorporation into solid matrices for controlled release. The choice of application method depends on the specific use case and the type of pathogens targeted.
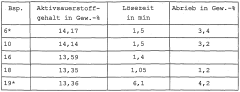
- Stability and storage considerations: Ensuring the stability of sodium percarbonate during storage and use is crucial for maintaining its pathogen mitigation efficacy. Factors such as moisture control, temperature regulation, and appropriate packaging play important roles in preserving the active oxygen content. Proper stabilization techniques can extend the shelf life and effectiveness of sodium percarbonate-based products.
- Environmental and safety aspects: Sodium percarbonate offers advantages in terms of environmental friendliness and safety for pathogen mitigation applications. It breaks down into harmless byproducts (sodium carbonate and water) after use, minimizing environmental impact. Safety considerations include proper handling procedures and potential interactions with other chemicals to ensure effective and safe pathogen control.
02 Formulation of sodium percarbonate-based cleaning products
Sodium percarbonate can be formulated into various cleaning products for enhanced pathogen mitigation. These formulations may include additional components such as surfactants, enzymes, or other oxidizing agents to improve cleaning efficacy and broaden the spectrum of antimicrobial activity. The stability and effectiveness of sodium percarbonate in these formulations can be optimized through careful selection of ingredients and packaging.Expand Specific Solutions03 Use of sodium percarbonate in water treatment
Sodium percarbonate is effective in water treatment applications for pathogen mitigation. It can be used to disinfect drinking water, treat wastewater, and control algae growth in various water systems. The controlled release of hydrogen peroxide from sodium percarbonate provides sustained antimicrobial action, making it suitable for both small-scale and large-scale water treatment processes.Expand Specific Solutions04 Sodium percarbonate in medical and dental applications
Sodium percarbonate finds applications in medical and dental fields for pathogen mitigation. It can be used in disinfectant solutions for medical instruments, dental equipment, and oral care products. The oxidizing properties of sodium percarbonate make it effective against a wide range of pathogens commonly encountered in healthcare settings, contributing to improved hygiene and infection control.Expand Specific Solutions05 Controlled release systems for sodium percarbonate
Controlled release systems can be developed to enhance the efficacy of sodium percarbonate in pathogen mitigation. These systems may involve encapsulation techniques, coating technologies, or incorporation into polymeric matrices. By controlling the release rate of sodium percarbonate, a prolonged antimicrobial effect can be achieved, improving its performance in various applications such as textiles, packaging materials, and surface disinfection.Expand Specific Solutions
Key Players in Water Treatment Industry
The market for sodium percarbonate in mitigating waterborne pathogens is in a growth phase, driven by increasing global water treatment needs. The market size is expanding, with a projected CAGR of 3-5% over the next five years. Technologically, sodium percarbonate is mature but evolving, with companies like Solvay SA, Kemira Oyj, and Evonik Operations GmbH leading innovation. These firms are focusing on enhancing efficacy and developing eco-friendly formulations. Emerging players such as Agri Neo, Inc. and Enviro Tech Chemical Services are introducing novel applications, particularly in food safety and specialized water treatment sectors, indicating a dynamic competitive landscape with opportunities for differentiation and market expansion.
Kemira Oyj
Technical Solution: Kemira Oyj has developed a proprietary sodium percarbonate-based water treatment solution that combines the oxidizing power of hydrogen peroxide with additional synergistic components. Their technology incorporates stabilizers and activators that enhance the efficacy of sodium percarbonate against a broad spectrum of waterborne pathogens. Kemira's approach focuses on optimizing the oxidation potential of sodium percarbonate while minimizing its decomposition rate in water[3]. The company has also implemented advanced particle engineering techniques to improve the dissolution characteristics of their sodium percarbonate products, ensuring rapid and uniform distribution in water treatment systems[4].
Strengths: Enhanced efficacy through synergistic formulations; Optimized oxidation potential; Improved dissolution characteristics. Weaknesses: May be more complex to handle and store compared to pure sodium percarbonate; Potentially higher cost due to additional components.
Solvay SA
Technical Solution: Solvay SA has developed advanced sodium percarbonate formulations specifically designed for water treatment applications. Their technology involves stabilized sodium percarbonate particles that release hydrogen peroxide in a controlled manner when dissolved in water. This controlled release mechanism allows for sustained antimicrobial activity against waterborne pathogens[1]. Solvay's sodium percarbonate products are engineered to maintain efficacy across a wide range of water conditions, including varying pH levels and organic loads. The company has also implemented innovative coating techniques to enhance the stability and shelf-life of their sodium percarbonate, ensuring consistent performance in diverse environmental conditions[2].
Strengths: Controlled release technology for sustained antimicrobial action; Effective across diverse water conditions; Enhanced stability and shelf-life. Weaknesses: May require higher dosages in heavily contaminated water; Potential for rapid decomposition in the presence of certain metal ions.
Innovations in Sodium Percarbonate Applications
Sodium percarbonate particles with improved storage stability
PatentWO2004058932A1
Innovation
- Sodium percarbonate particles coated with 0.01 to 1% by weight of a hydrophobic, finely divided oxide such as hydrophobic silica, aluminum, or titanium oxide, which improves storage stability and handling without dust formation or caking, and ensures easy dispersion in water.
Process to control and kill pathogenic small life forms, particularly insects and worms
PatentInactiveEP0945063A1
Innovation
- A method using an aqueous percarboxylic acid solution with 1 to 6 carbon atoms applied to surfaces and water bodies to control and kill pathogenic insects and worms, particularly effective against mosquito larvae and schistosome larvae, utilizing peracetic acid and optionally surfactants, with concentrations between 1 to 5000 ppm.
Environmental Impact Assessment
The environmental impact assessment of sodium percarbonate's use in mitigating waterborne pathogens reveals both positive and negative effects on aquatic ecosystems and human health. Sodium percarbonate, when dissolved in water, releases hydrogen peroxide and sodium carbonate, both of which have disinfectant properties. This process effectively reduces the presence of harmful pathogens in water bodies, potentially improving overall water quality and reducing the risk of waterborne diseases.
However, the introduction of sodium percarbonate into aquatic environments may also lead to some ecological concerns. The release of hydrogen peroxide can temporarily increase oxygen levels in water, which may benefit aerobic organisms but could potentially stress anaerobic species. Additionally, the increase in pH due to the sodium carbonate component may affect the delicate balance of aquatic ecosystems, particularly in poorly buffered water bodies.
The use of sodium percarbonate as a water treatment method generally results in lower environmental impact compared to traditional chlorine-based disinfectants. Unlike chlorine, sodium percarbonate does not produce harmful byproducts such as trihalomethanes or haloacetic acids, which are known to have negative effects on both human health and aquatic life. This makes it a more environmentally friendly option for water treatment in many scenarios.
Long-term exposure to sodium percarbonate in aquatic environments may lead to changes in microbial communities. While this can be beneficial in reducing pathogenic organisms, it may also affect beneficial microorganisms that play crucial roles in nutrient cycling and ecosystem balance. Further research is needed to fully understand the long-term ecological implications of sustained sodium percarbonate use in natural water bodies.
The environmental fate of sodium percarbonate is generally favorable. Hydrogen peroxide quickly decomposes into water and oxygen, leaving no persistent residues. Sodium carbonate, being a naturally occurring compound, integrates into the environment without significant long-term impacts. However, in cases of large-scale application or accidental spills, temporary localized effects on pH and oxygen levels may occur, potentially affecting sensitive aquatic species.
Human health considerations related to sodium percarbonate use in water treatment are generally positive. The reduction in waterborne pathogens leads to decreased incidence of water-related diseases, improving public health outcomes. However, proper dosing and application methods must be employed to prevent potential irritation to skin and mucous membranes that may occur with direct exposure to concentrated solutions.
In conclusion, while sodium percarbonate offers an effective and relatively environmentally friendly method for mitigating waterborne pathogens, its use should be carefully managed and monitored to minimize potential negative impacts on aquatic ecosystems. Balancing the benefits of pathogen reduction with the need to maintain ecological integrity is crucial for sustainable water management practices.
However, the introduction of sodium percarbonate into aquatic environments may also lead to some ecological concerns. The release of hydrogen peroxide can temporarily increase oxygen levels in water, which may benefit aerobic organisms but could potentially stress anaerobic species. Additionally, the increase in pH due to the sodium carbonate component may affect the delicate balance of aquatic ecosystems, particularly in poorly buffered water bodies.
The use of sodium percarbonate as a water treatment method generally results in lower environmental impact compared to traditional chlorine-based disinfectants. Unlike chlorine, sodium percarbonate does not produce harmful byproducts such as trihalomethanes or haloacetic acids, which are known to have negative effects on both human health and aquatic life. This makes it a more environmentally friendly option for water treatment in many scenarios.
Long-term exposure to sodium percarbonate in aquatic environments may lead to changes in microbial communities. While this can be beneficial in reducing pathogenic organisms, it may also affect beneficial microorganisms that play crucial roles in nutrient cycling and ecosystem balance. Further research is needed to fully understand the long-term ecological implications of sustained sodium percarbonate use in natural water bodies.
The environmental fate of sodium percarbonate is generally favorable. Hydrogen peroxide quickly decomposes into water and oxygen, leaving no persistent residues. Sodium carbonate, being a naturally occurring compound, integrates into the environment without significant long-term impacts. However, in cases of large-scale application or accidental spills, temporary localized effects on pH and oxygen levels may occur, potentially affecting sensitive aquatic species.
Human health considerations related to sodium percarbonate use in water treatment are generally positive. The reduction in waterborne pathogens leads to decreased incidence of water-related diseases, improving public health outcomes. However, proper dosing and application methods must be employed to prevent potential irritation to skin and mucous membranes that may occur with direct exposure to concentrated solutions.
In conclusion, while sodium percarbonate offers an effective and relatively environmentally friendly method for mitigating waterborne pathogens, its use should be carefully managed and monitored to minimize potential negative impacts on aquatic ecosystems. Balancing the benefits of pathogen reduction with the need to maintain ecological integrity is crucial for sustainable water management practices.
Regulatory Framework for Water Disinfectants
The regulatory framework for water disinfectants plays a crucial role in ensuring the safety and efficacy of water treatment processes, including the use of sodium percarbonate to mitigate waterborne pathogens. This framework is typically established and enforced by governmental agencies responsible for public health and environmental protection.
In the United States, the Environmental Protection Agency (EPA) is the primary regulatory body overseeing water disinfection practices. The EPA sets standards for drinking water quality through the Safe Drinking Water Act (SDWA) and regulates the use of disinfectants and their byproducts. The agency maintains a list of approved disinfectants and treatment technologies, which must meet specific criteria for effectiveness against waterborne pathogens.
For sodium percarbonate to be used as a water disinfectant, it must comply with the EPA's registration process under the Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA). This process involves rigorous testing to demonstrate the product's efficacy, safety, and environmental impact. Manufacturers must provide detailed information on the chemical composition, intended use, and potential risks associated with the disinfectant.
Internationally, the World Health Organization (WHO) provides guidelines for drinking water quality, which many countries adopt or adapt for their national regulations. These guidelines include recommendations for disinfection practices and maximum contaminant levels for various pathogens and chemical substances.
The European Union (EU) has its own regulatory framework for water disinfectants, governed by the Biocidal Products Regulation (BPR). This regulation ensures that only authorized biocidal products, including water disinfectants, are made available on the EU market. The European Chemicals Agency (ECHA) oversees the implementation of the BPR and coordinates the evaluation of active substances used in biocidal products.
Regulatory bodies also establish monitoring and reporting requirements for water treatment facilities. These requirements typically include regular testing of water quality parameters, documentation of disinfection processes, and reporting of any deviations from established standards. Compliance with these regulations is essential for water treatment facilities to maintain their operating licenses and ensure public safety.
As new technologies and disinfection methods emerge, regulatory frameworks must adapt to address potential risks and benefits. This may involve updating existing regulations or developing new guidelines specific to novel disinfection approaches. In the case of sodium percarbonate, regulatory agencies would need to consider its unique properties, potential byproducts, and effectiveness against various waterborne pathogens when establishing or revising relevant regulations.
In the United States, the Environmental Protection Agency (EPA) is the primary regulatory body overseeing water disinfection practices. The EPA sets standards for drinking water quality through the Safe Drinking Water Act (SDWA) and regulates the use of disinfectants and their byproducts. The agency maintains a list of approved disinfectants and treatment technologies, which must meet specific criteria for effectiveness against waterborne pathogens.
For sodium percarbonate to be used as a water disinfectant, it must comply with the EPA's registration process under the Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA). This process involves rigorous testing to demonstrate the product's efficacy, safety, and environmental impact. Manufacturers must provide detailed information on the chemical composition, intended use, and potential risks associated with the disinfectant.
Internationally, the World Health Organization (WHO) provides guidelines for drinking water quality, which many countries adopt or adapt for their national regulations. These guidelines include recommendations for disinfection practices and maximum contaminant levels for various pathogens and chemical substances.
The European Union (EU) has its own regulatory framework for water disinfectants, governed by the Biocidal Products Regulation (BPR). This regulation ensures that only authorized biocidal products, including water disinfectants, are made available on the EU market. The European Chemicals Agency (ECHA) oversees the implementation of the BPR and coordinates the evaluation of active substances used in biocidal products.
Regulatory bodies also establish monitoring and reporting requirements for water treatment facilities. These requirements typically include regular testing of water quality parameters, documentation of disinfection processes, and reporting of any deviations from established standards. Compliance with these regulations is essential for water treatment facilities to maintain their operating licenses and ensure public safety.
As new technologies and disinfection methods emerge, regulatory frameworks must adapt to address potential risks and benefits. This may involve updating existing regulations or developing new guidelines specific to novel disinfection approaches. In the case of sodium percarbonate, regulatory agencies would need to consider its unique properties, potential byproducts, and effectiveness against various waterborne pathogens when establishing or revising relevant regulations.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!