How to Combat Cellulitis with Hirudoid?
JUN 23, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Cellulitis and Hirudoid Overview
Cellulitis is a common bacterial skin infection that affects the deeper layers of skin and underlying tissue. It typically occurs when bacteria enter through a break in the skin, causing inflammation, redness, swelling, and pain. The condition can spread rapidly and, if left untreated, may lead to serious complications. Cellulitis most commonly affects the lower legs but can occur on any part of the body.
The primary causative agents of cellulitis are Streptococcus and Staphylococcus bacteria, which are normally present on the skin's surface. Risk factors for developing cellulitis include skin injuries, weakened immune systems, chronic skin conditions, and poor circulation. Prompt diagnosis and treatment are crucial to prevent the infection from spreading and causing more severe health issues.
Hirudoid is a topical medication widely used in the treatment of various skin conditions, including cellulitis. Its active ingredient, mucopolysaccharide polysulfate (MPS), is known for its anti-inflammatory, anti-edematous, and antithrombotic properties. Hirudoid works by improving local blood circulation, reducing swelling, and promoting the healing of damaged tissue.
The use of Hirudoid in combating cellulitis is based on its ability to address several key aspects of the condition. Firstly, it helps reduce inflammation and swelling, which are primary symptoms of cellulitis. By improving blood circulation in the affected area, Hirudoid aids in the delivery of oxygen and nutrients to the infected tissue, supporting the body's natural healing processes.
Furthermore, Hirudoid's antithrombotic properties may help prevent the formation of blood clots, which can be a concern in severe cases of cellulitis. The medication's ability to penetrate the skin and reach deeper tissue layers makes it particularly effective in addressing the infection at its source.
While Hirudoid can be a valuable tool in the management of cellulitis, it is important to note that it is typically used as part of a comprehensive treatment plan. Antibiotics remain the primary treatment for cellulitis, targeting the underlying bacterial infection. Hirudoid is often used as an adjunct therapy to alleviate symptoms and support the healing process.
In conclusion, the combination of understanding cellulitis as a serious bacterial skin infection and recognizing Hirudoid's potential in addressing its symptoms provides a foundation for exploring effective treatment strategies. This overview sets the stage for a more detailed examination of current approaches and potential innovations in combating cellulitis with Hirudoid.
The primary causative agents of cellulitis are Streptococcus and Staphylococcus bacteria, which are normally present on the skin's surface. Risk factors for developing cellulitis include skin injuries, weakened immune systems, chronic skin conditions, and poor circulation. Prompt diagnosis and treatment are crucial to prevent the infection from spreading and causing more severe health issues.
Hirudoid is a topical medication widely used in the treatment of various skin conditions, including cellulitis. Its active ingredient, mucopolysaccharide polysulfate (MPS), is known for its anti-inflammatory, anti-edematous, and antithrombotic properties. Hirudoid works by improving local blood circulation, reducing swelling, and promoting the healing of damaged tissue.
The use of Hirudoid in combating cellulitis is based on its ability to address several key aspects of the condition. Firstly, it helps reduce inflammation and swelling, which are primary symptoms of cellulitis. By improving blood circulation in the affected area, Hirudoid aids in the delivery of oxygen and nutrients to the infected tissue, supporting the body's natural healing processes.
Furthermore, Hirudoid's antithrombotic properties may help prevent the formation of blood clots, which can be a concern in severe cases of cellulitis. The medication's ability to penetrate the skin and reach deeper tissue layers makes it particularly effective in addressing the infection at its source.
While Hirudoid can be a valuable tool in the management of cellulitis, it is important to note that it is typically used as part of a comprehensive treatment plan. Antibiotics remain the primary treatment for cellulitis, targeting the underlying bacterial infection. Hirudoid is often used as an adjunct therapy to alleviate symptoms and support the healing process.
In conclusion, the combination of understanding cellulitis as a serious bacterial skin infection and recognizing Hirudoid's potential in addressing its symptoms provides a foundation for exploring effective treatment strategies. This overview sets the stage for a more detailed examination of current approaches and potential innovations in combating cellulitis with Hirudoid.
Market Analysis for Cellulitis Treatments
The global market for cellulitis treatments has been experiencing steady growth, driven by the increasing prevalence of the condition and the rising demand for effective therapeutic options. Cellulitis, a common bacterial skin infection, affects millions of people worldwide, creating a substantial market opportunity for pharmaceutical companies and healthcare providers.
The cellulitis treatment market is segmented based on drug type, route of administration, and distribution channel. Antibiotics remain the primary treatment option, with both oral and intravenous formulations available. Topical treatments, including Hirudoid, represent a growing segment of the market, offering localized therapy with potentially fewer systemic side effects.
Market research indicates that the global cellulitis treatment market is expected to continue its upward trajectory in the coming years. Factors contributing to this growth include the aging population, which is more susceptible to cellulitis, and the increasing incidence of risk factors such as obesity and diabetes.
North America currently holds the largest share of the cellulitis treatment market, followed by Europe. This dominance is attributed to the well-established healthcare infrastructure, high awareness levels, and the presence of key market players in these regions. However, the Asia-Pacific region is anticipated to witness the fastest growth rate due to improving healthcare access, rising disposable incomes, and a growing patient population.
The competitive landscape of the cellulitis treatment market is characterized by the presence of both established pharmaceutical companies and emerging players. Key market participants are focusing on research and development activities to introduce innovative treatment options and expand their product portfolios.
Hirudoid, as a topical treatment option, occupies a unique position in the cellulitis treatment market. Its active ingredient, mucopolysaccharide polysulfate, has shown promise in managing inflammation and promoting healing in various skin conditions, including cellulitis. The increasing interest in non-antibiotic approaches to combat cellulitis has created opportunities for products like Hirudoid to gain market share.
Consumer trends indicate a growing preference for topical treatments that can be easily applied at home, potentially reducing the need for hospitalization or frequent healthcare visits. This shift in consumer behavior aligns well with the positioning of Hirudoid as a convenient and effective treatment option for cellulitis.
The cellulitis treatment market is segmented based on drug type, route of administration, and distribution channel. Antibiotics remain the primary treatment option, with both oral and intravenous formulations available. Topical treatments, including Hirudoid, represent a growing segment of the market, offering localized therapy with potentially fewer systemic side effects.
Market research indicates that the global cellulitis treatment market is expected to continue its upward trajectory in the coming years. Factors contributing to this growth include the aging population, which is more susceptible to cellulitis, and the increasing incidence of risk factors such as obesity and diabetes.
North America currently holds the largest share of the cellulitis treatment market, followed by Europe. This dominance is attributed to the well-established healthcare infrastructure, high awareness levels, and the presence of key market players in these regions. However, the Asia-Pacific region is anticipated to witness the fastest growth rate due to improving healthcare access, rising disposable incomes, and a growing patient population.
The competitive landscape of the cellulitis treatment market is characterized by the presence of both established pharmaceutical companies and emerging players. Key market participants are focusing on research and development activities to introduce innovative treatment options and expand their product portfolios.
Hirudoid, as a topical treatment option, occupies a unique position in the cellulitis treatment market. Its active ingredient, mucopolysaccharide polysulfate, has shown promise in managing inflammation and promoting healing in various skin conditions, including cellulitis. The increasing interest in non-antibiotic approaches to combat cellulitis has created opportunities for products like Hirudoid to gain market share.
Consumer trends indicate a growing preference for topical treatments that can be easily applied at home, potentially reducing the need for hospitalization or frequent healthcare visits. This shift in consumer behavior aligns well with the positioning of Hirudoid as a convenient and effective treatment option for cellulitis.
Current Challenges in Cellulitis Management
Cellulitis management continues to present significant challenges in modern healthcare, despite advancements in medical treatments. One of the primary obstacles is the difficulty in accurately diagnosing cellulitis, as its symptoms can mimic other conditions such as deep vein thrombosis or stasis dermatitis. This diagnostic uncertainty often leads to overtreatment with antibiotics, contributing to the growing concern of antibiotic resistance.
The increasing prevalence of antibiotic-resistant bacteria further complicates cellulitis treatment. Methicillin-resistant Staphylococcus aureus (MRSA) and other resistant strains have become more common in community-acquired cellulitis cases, limiting the effectiveness of traditional first-line antibiotics. This necessitates the use of broader-spectrum antibiotics, which can have more severe side effects and contribute to the cycle of resistance.
Another significant challenge is the management of recurrent cellulitis. Patients with risk factors such as lymphedema, obesity, or chronic skin conditions are prone to repeated episodes, which can be difficult to prevent and manage effectively. The cumulative impact of recurrent infections can lead to long-term complications, including chronic swelling and skin changes, significantly affecting patients' quality of life.
The optimal duration of antibiotic therapy for cellulitis remains controversial. While shorter courses may reduce the risk of antibiotic resistance and side effects, they may also increase the likelihood of treatment failure or recurrence. Balancing these factors to determine the most appropriate treatment duration for individual patients presents an ongoing challenge for clinicians.
Cellulitis in specific patient populations, such as those with diabetes or immunosuppression, poses additional challenges. These patients often have atypical presentations and are at higher risk for complications, requiring more aggressive and prolonged treatment approaches. Managing cellulitis in these complex cases demands a delicate balance between effective treatment and minimizing potential adverse effects.
The role of adjunctive therapies, including topical treatments like Hirudoid, in cellulitis management remains unclear. While some studies suggest potential benefits in reducing inflammation and promoting healing, the evidence base for their efficacy in cellulitis specifically is limited. Integrating these therapies into standard treatment protocols in a way that maximizes their potential benefits while ensuring patient safety is an ongoing challenge.
Lastly, the lack of standardized guidelines for cellulitis management across different healthcare settings contributes to variability in care. This inconsistency can lead to suboptimal outcomes and inefficient use of healthcare resources. Developing and implementing evidence-based, universally accepted protocols for cellulitis diagnosis, treatment, and prevention remains a critical challenge in improving overall management of this common but potentially serious condition.
The increasing prevalence of antibiotic-resistant bacteria further complicates cellulitis treatment. Methicillin-resistant Staphylococcus aureus (MRSA) and other resistant strains have become more common in community-acquired cellulitis cases, limiting the effectiveness of traditional first-line antibiotics. This necessitates the use of broader-spectrum antibiotics, which can have more severe side effects and contribute to the cycle of resistance.
Another significant challenge is the management of recurrent cellulitis. Patients with risk factors such as lymphedema, obesity, or chronic skin conditions are prone to repeated episodes, which can be difficult to prevent and manage effectively. The cumulative impact of recurrent infections can lead to long-term complications, including chronic swelling and skin changes, significantly affecting patients' quality of life.
The optimal duration of antibiotic therapy for cellulitis remains controversial. While shorter courses may reduce the risk of antibiotic resistance and side effects, they may also increase the likelihood of treatment failure or recurrence. Balancing these factors to determine the most appropriate treatment duration for individual patients presents an ongoing challenge for clinicians.
Cellulitis in specific patient populations, such as those with diabetes or immunosuppression, poses additional challenges. These patients often have atypical presentations and are at higher risk for complications, requiring more aggressive and prolonged treatment approaches. Managing cellulitis in these complex cases demands a delicate balance between effective treatment and minimizing potential adverse effects.
The role of adjunctive therapies, including topical treatments like Hirudoid, in cellulitis management remains unclear. While some studies suggest potential benefits in reducing inflammation and promoting healing, the evidence base for their efficacy in cellulitis specifically is limited. Integrating these therapies into standard treatment protocols in a way that maximizes their potential benefits while ensuring patient safety is an ongoing challenge.
Lastly, the lack of standardized guidelines for cellulitis management across different healthcare settings contributes to variability in care. This inconsistency can lead to suboptimal outcomes and inefficient use of healthcare resources. Developing and implementing evidence-based, universally accepted protocols for cellulitis diagnosis, treatment, and prevention remains a critical challenge in improving overall management of this common but potentially serious condition.
Key Players in Dermatological Pharmaceuticals
The market for combating cellulitis with Hirudoid is in a growth phase, driven by increasing awareness of skin health and rising demand for effective treatments. The global market size for cellulitis treatments is expanding, with a projected CAGR of 6-8% over the next five years. Technologically, the field is moderately mature, with established players like Novartis AG and Eli Lilly & Co. leading research and development efforts. However, there's room for innovation, as evidenced by emerging companies such as Priothera Ltd. and Protagonist Therapeutics, Inc. entering the market with novel approaches. The competitive landscape is diverse, with pharmaceutical giants like Novartis and Merz Pharma GmbH & Co. KGaA competing alongside specialized dermatology firms and research institutions like the Swiss Federal Institute of Technology.
Novartis AG
Technical Solution: Novartis AG has developed a comprehensive approach to combat cellulitis using Hirudoid. Their technology involves a multi-pronged strategy that combines the anti-inflammatory and anti-edema properties of Hirudoid with advanced drug delivery systems. The company has engineered a novel formulation that enhances the penetration of Hirudoid through the skin layers, allowing for more effective targeting of the affected areas. This formulation includes nanoparticle carriers that improve the stability and bioavailability of the active ingredients. Additionally, Novartis has incorporated controlled-release mechanisms to ensure a sustained therapeutic effect over an extended period, reducing the frequency of application and improving patient compliance.
Strengths: Advanced drug delivery system, enhanced penetration, and sustained release mechanism. Weaknesses: Potential high cost of production and possible regulatory hurdles for novel formulations.
Merz Pharma GmbH & Co. KGaA
Technical Solution: Merz Pharma has developed an innovative approach to using Hirudoid for cellulitis treatment. Their technology focuses on combining Hirudoid with other active ingredients to create a synergistic effect. The company has formulated a gel-based product that not only contains Hirudoid but also incorporates antimicrobial agents and skin barrier enhancers. This combination aims to address multiple aspects of cellulitis simultaneously - reducing inflammation, fighting bacterial infection, and improving skin integrity. Merz has also implemented a proprietary emulsion technology that ensures even distribution and prolonged contact of the active ingredients with the affected skin area, maximizing the therapeutic effect.
Strengths: Synergistic formulation addressing multiple aspects of cellulitis, proprietary emulsion technology. Weaknesses: Potential interactions between multiple active ingredients, possible increased risk of side effects.
Hirudoid Mechanism of Action
Cosmetic use of a composition containing at least one oxazoline, serving as an active substance, as a slimming product and/or for preventing and/or treating cellulite
PatentActiveUS7629371B2
Innovation
- A cosmetic composition containing oxazolines as an active substance, which inhibits lipogenesis in human adipocytes, is used topically to prevent and treat cellulitis and combat localized excess weight by reducing triglyceride formation and promoting lipolysis, combined with other slimming and anti-infiltration agents.
Composition for the prevention and treatment of cellulitis
PatentWO2004087191A1
Innovation
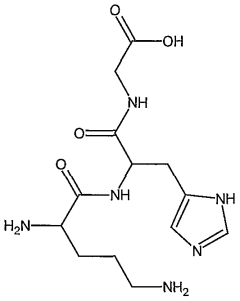
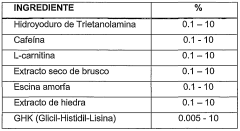
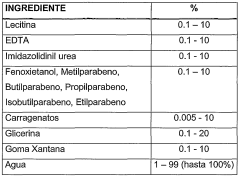
- A topical composition combining classical anti-cellulite extracts with lipolytic and venotonic effects, including caffeine, Ivy extract, amorphous escin, and specifically the tripeptide GHK (glycyl-histidyl-lysine), which acts as a sequestrant for RCS, thereby mitigating cell aging and improving skin appearance.
Regulatory Framework for Topical Medications
The regulatory framework for topical medications, including those used to combat cellulitis such as Hirudoid, is a complex and multifaceted system designed to ensure the safety, efficacy, and quality of these products. In the United States, the Food and Drug Administration (FDA) is the primary regulatory body overseeing topical medications, while in Europe, the European Medicines Agency (EMA) plays a similar role.
For topical medications like Hirudoid, the regulatory process typically begins with preclinical studies to establish safety and potential efficacy. These studies must adhere to Good Laboratory Practice (GLP) guidelines. Once preclinical data is deemed satisfactory, manufacturers can proceed to clinical trials, which are conducted in phases and must follow Good Clinical Practice (GCP) standards.
The FDA's approval process for topical medications involves submitting a New Drug Application (NDA) or an Abbreviated New Drug Application (ANDA) for generic versions. This application must include comprehensive data on the drug's safety, efficacy, and manufacturing processes. The FDA reviews this information and may request additional studies or clarifications before granting approval.
In the European Union, the EMA employs a centralized procedure for novel topical medications, while national regulatory agencies may handle generic versions. The Marketing Authorization Application (MAA) is the European equivalent of the FDA's NDA, requiring similar extensive documentation and data.
Post-approval, regulatory oversight continues through pharmacovigilance programs. Manufacturers are required to monitor and report adverse events, conduct post-marketing studies if mandated, and comply with Good Manufacturing Practice (GMP) standards to ensure consistent product quality.
Labeling and packaging regulations are particularly crucial for topical medications. Clear instructions for use, warnings, and contraindications must be prominently displayed to ensure patient safety and proper application. In the case of Hirudoid and similar products for cellulitis, this includes information on the frequency of application, potential side effects, and when to seek medical attention.
Regulatory bodies also oversee advertising and promotional materials for topical medications, ensuring that claims are substantiated and not misleading. This is especially important for over-the-counter products, which may be marketed directly to consumers.
As the understanding of cellulitis and its treatment evolves, regulatory frameworks must adapt. This may involve updating guidelines for clinical trials, revising labeling requirements, or reassessing the risk-benefit profile of existing medications. The regulatory landscape for topical medications is thus a dynamic field, requiring ongoing collaboration between manufacturers, healthcare professionals, and regulatory agencies to maintain the highest standards of patient care and product integrity.
For topical medications like Hirudoid, the regulatory process typically begins with preclinical studies to establish safety and potential efficacy. These studies must adhere to Good Laboratory Practice (GLP) guidelines. Once preclinical data is deemed satisfactory, manufacturers can proceed to clinical trials, which are conducted in phases and must follow Good Clinical Practice (GCP) standards.
The FDA's approval process for topical medications involves submitting a New Drug Application (NDA) or an Abbreviated New Drug Application (ANDA) for generic versions. This application must include comprehensive data on the drug's safety, efficacy, and manufacturing processes. The FDA reviews this information and may request additional studies or clarifications before granting approval.
In the European Union, the EMA employs a centralized procedure for novel topical medications, while national regulatory agencies may handle generic versions. The Marketing Authorization Application (MAA) is the European equivalent of the FDA's NDA, requiring similar extensive documentation and data.
Post-approval, regulatory oversight continues through pharmacovigilance programs. Manufacturers are required to monitor and report adverse events, conduct post-marketing studies if mandated, and comply with Good Manufacturing Practice (GMP) standards to ensure consistent product quality.
Labeling and packaging regulations are particularly crucial for topical medications. Clear instructions for use, warnings, and contraindications must be prominently displayed to ensure patient safety and proper application. In the case of Hirudoid and similar products for cellulitis, this includes information on the frequency of application, potential side effects, and when to seek medical attention.
Regulatory bodies also oversee advertising and promotional materials for topical medications, ensuring that claims are substantiated and not misleading. This is especially important for over-the-counter products, which may be marketed directly to consumers.
As the understanding of cellulitis and its treatment evolves, regulatory frameworks must adapt. This may involve updating guidelines for clinical trials, revising labeling requirements, or reassessing the risk-benefit profile of existing medications. The regulatory landscape for topical medications is thus a dynamic field, requiring ongoing collaboration between manufacturers, healthcare professionals, and regulatory agencies to maintain the highest standards of patient care and product integrity.
Patient Education and Compliance Strategies
Patient education and compliance strategies play a crucial role in effectively combating cellulitis with Hirudoid. A comprehensive approach to patient education should focus on providing clear, concise information about cellulitis, its causes, symptoms, and the importance of early treatment. Patients should be informed about the role of Hirudoid in managing cellulitis and its potential benefits in reducing inflammation and promoting healing.
To enhance patient understanding, healthcare providers can utilize various educational materials such as brochures, videos, and interactive online resources. These materials should be designed to cater to different learning styles and literacy levels, ensuring that patients from diverse backgrounds can easily grasp the key concepts. Additionally, incorporating visual aids like diagrams and illustrations can help patients better understand the progression of cellulitis and the application of Hirudoid.
Compliance strategies should focus on addressing potential barriers to treatment adherence. This may include simplifying the treatment regimen, providing clear instructions on how to apply Hirudoid, and setting realistic expectations for treatment outcomes. Healthcare providers should emphasize the importance of completing the full course of treatment, even if symptoms improve, to prevent recurrence or complications.
Implementing a patient-centered approach can significantly improve compliance. This involves actively involving patients in their treatment decisions, addressing their concerns, and tailoring the treatment plan to their individual needs and preferences. Regular follow-up appointments or check-ins via telemedicine can help monitor progress, address any side effects, and reinforce the importance of adherence to the treatment plan.
Technology can play a vital role in enhancing patient education and compliance. Mobile applications or text message reminders can be used to provide timely information, track treatment progress, and send medication reminders. These digital tools can also offer a platform for patients to communicate with healthcare providers, ask questions, and report any concerns or side effects promptly.
Support groups or peer-to-peer networks can be valuable resources for patients dealing with cellulitis. These platforms allow patients to share experiences, offer emotional support, and exchange practical tips for managing the condition. Healthcare providers can facilitate these connections and encourage patients to participate in such support networks as part of their overall treatment strategy.
By implementing these comprehensive patient education and compliance strategies, healthcare providers can significantly improve the effectiveness of Hirudoid in combating cellulitis. This approach not only enhances treatment outcomes but also empowers patients to take an active role in their recovery process, leading to better overall health outcomes and reduced risk of recurrence.
To enhance patient understanding, healthcare providers can utilize various educational materials such as brochures, videos, and interactive online resources. These materials should be designed to cater to different learning styles and literacy levels, ensuring that patients from diverse backgrounds can easily grasp the key concepts. Additionally, incorporating visual aids like diagrams and illustrations can help patients better understand the progression of cellulitis and the application of Hirudoid.
Compliance strategies should focus on addressing potential barriers to treatment adherence. This may include simplifying the treatment regimen, providing clear instructions on how to apply Hirudoid, and setting realistic expectations for treatment outcomes. Healthcare providers should emphasize the importance of completing the full course of treatment, even if symptoms improve, to prevent recurrence or complications.
Implementing a patient-centered approach can significantly improve compliance. This involves actively involving patients in their treatment decisions, addressing their concerns, and tailoring the treatment plan to their individual needs and preferences. Regular follow-up appointments or check-ins via telemedicine can help monitor progress, address any side effects, and reinforce the importance of adherence to the treatment plan.
Technology can play a vital role in enhancing patient education and compliance. Mobile applications or text message reminders can be used to provide timely information, track treatment progress, and send medication reminders. These digital tools can also offer a platform for patients to communicate with healthcare providers, ask questions, and report any concerns or side effects promptly.
Support groups or peer-to-peer networks can be valuable resources for patients dealing with cellulitis. These platforms allow patients to share experiences, offer emotional support, and exchange practical tips for managing the condition. Healthcare providers can facilitate these connections and encourage patients to participate in such support networks as part of their overall treatment strategy.
By implementing these comprehensive patient education and compliance strategies, healthcare providers can significantly improve the effectiveness of Hirudoid in combating cellulitis. This approach not only enhances treatment outcomes but also empowers patients to take an active role in their recovery process, leading to better overall health outcomes and reduced risk of recurrence.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!