Innovations in Wearable Piezoelectric Therapeutic Devices
JUL 17, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Piezoelectric Therapy Evolution and Objectives
Piezoelectric therapy has evolved significantly over the past few decades, driven by advancements in materials science and biomedical engineering. The field began with the discovery of the piezoelectric effect in certain materials, which could generate electrical charges when subjected to mechanical stress. This phenomenon laid the foundation for developing therapeutic devices that could harness this property for medical applications.
Initially, piezoelectric therapy was primarily used in bone healing and tissue regeneration. Early devices were bulky and limited in their applications. However, as technology progressed, researchers began exploring ways to miniaturize these devices and expand their potential uses. The advent of nanotechnology and flexible electronics in the early 2000s marked a turning point in the field, enabling the development of more sophisticated and versatile piezoelectric therapeutic devices.
The evolution of wearable technology in the past decade has further accelerated innovations in piezoelectric therapy. Researchers and engineers have been working on integrating piezoelectric materials into flexible, conformable substrates that can be worn comfortably on the body. This has opened up new possibilities for continuous, non-invasive therapeutic interventions for a wide range of medical conditions.
Current objectives in the field of wearable piezoelectric therapeutic devices focus on several key areas. One primary goal is to enhance the efficiency and output of these devices, maximizing the therapeutic effect while minimizing power consumption. This involves developing new piezoelectric materials with improved properties and optimizing device designs to capture and utilize mechanical energy more effectively.
Another important objective is to improve the biocompatibility and long-term stability of these devices. Researchers are exploring novel materials and encapsulation techniques to ensure that wearable piezoelectric devices can function reliably in diverse physiological environments without causing adverse reactions or degrading over time.
Expanding the range of applications for wearable piezoelectric therapy is also a key focus. Scientists are investigating the potential of these devices in areas such as pain management, wound healing, muscle stimulation, and even neurological disorders. The goal is to develop targeted therapies that can address specific medical needs with precision and minimal side effects.
Furthermore, there is a growing emphasis on creating smart, adaptive piezoelectric therapeutic devices. By incorporating sensors and advanced control systems, researchers aim to develop devices that can dynamically adjust their output based on real-time physiological feedback. This could lead to more personalized and effective treatments tailored to individual patient needs.
Initially, piezoelectric therapy was primarily used in bone healing and tissue regeneration. Early devices were bulky and limited in their applications. However, as technology progressed, researchers began exploring ways to miniaturize these devices and expand their potential uses. The advent of nanotechnology and flexible electronics in the early 2000s marked a turning point in the field, enabling the development of more sophisticated and versatile piezoelectric therapeutic devices.
The evolution of wearable technology in the past decade has further accelerated innovations in piezoelectric therapy. Researchers and engineers have been working on integrating piezoelectric materials into flexible, conformable substrates that can be worn comfortably on the body. This has opened up new possibilities for continuous, non-invasive therapeutic interventions for a wide range of medical conditions.
Current objectives in the field of wearable piezoelectric therapeutic devices focus on several key areas. One primary goal is to enhance the efficiency and output of these devices, maximizing the therapeutic effect while minimizing power consumption. This involves developing new piezoelectric materials with improved properties and optimizing device designs to capture and utilize mechanical energy more effectively.
Another important objective is to improve the biocompatibility and long-term stability of these devices. Researchers are exploring novel materials and encapsulation techniques to ensure that wearable piezoelectric devices can function reliably in diverse physiological environments without causing adverse reactions or degrading over time.
Expanding the range of applications for wearable piezoelectric therapy is also a key focus. Scientists are investigating the potential of these devices in areas such as pain management, wound healing, muscle stimulation, and even neurological disorders. The goal is to develop targeted therapies that can address specific medical needs with precision and minimal side effects.
Furthermore, there is a growing emphasis on creating smart, adaptive piezoelectric therapeutic devices. By incorporating sensors and advanced control systems, researchers aim to develop devices that can dynamically adjust their output based on real-time physiological feedback. This could lead to more personalized and effective treatments tailored to individual patient needs.
Market Analysis for Wearable Therapeutic Devices
The wearable therapeutic devices market has been experiencing significant growth in recent years, driven by increasing health consciousness, technological advancements, and the rising prevalence of chronic diseases. This market segment encompasses a wide range of devices, including those utilizing piezoelectric technology for therapeutic purposes.
The global wearable medical devices market, which includes therapeutic devices, was valued at $16.6 billion in 2020 and is projected to reach $67.2 billion by 2030, growing at a CAGR of 15.1% during the forecast period. Within this broader market, wearable therapeutic devices are gaining traction due to their non-invasive nature and potential for continuous, personalized treatment.
Piezoelectric therapeutic devices, in particular, are emerging as a promising subset of wearable therapeutics. These devices harness the piezoelectric effect to convert mechanical stress into electrical energy, which can be used for various therapeutic applications, including pain management, tissue regeneration, and physical therapy.
The market for wearable piezoelectric therapeutic devices is driven by several factors. Firstly, the growing aging population and the associated increase in chronic conditions such as arthritis, back pain, and musculoskeletal disorders create a substantial demand for non-pharmacological pain management solutions. Secondly, the shift towards personalized medicine and home-based care is fueling the adoption of wearable therapeutic devices that can provide continuous monitoring and treatment.
Consumer preferences are also shaping the market landscape. There is a growing demand for discreet, comfortable, and user-friendly devices that can be seamlessly integrated into daily life. This has led to innovations in device design, focusing on miniaturization, flexibility, and improved battery life.
Geographically, North America currently dominates the wearable therapeutic devices market, followed by Europe. However, the Asia-Pacific region is expected to witness the highest growth rate in the coming years, driven by improving healthcare infrastructure, rising disposable incomes, and increasing awareness about preventive healthcare.
Key market players in the wearable therapeutic devices sector include Medtronic, Abbott Laboratories, Johnson & Johnson, and Philips Healthcare. These companies are investing heavily in research and development to create innovative piezoelectric-based wearable solutions. Additionally, several startups and smaller companies are entering the market with novel technologies and applications, contributing to the overall market dynamism.
Despite the positive outlook, the market faces challenges such as regulatory hurdles, data privacy concerns, and the need for clinical validation of new technologies. Overcoming these obstacles will be crucial for the widespread adoption of wearable piezoelectric therapeutic devices and the realization of their full market potential.
The global wearable medical devices market, which includes therapeutic devices, was valued at $16.6 billion in 2020 and is projected to reach $67.2 billion by 2030, growing at a CAGR of 15.1% during the forecast period. Within this broader market, wearable therapeutic devices are gaining traction due to their non-invasive nature and potential for continuous, personalized treatment.
Piezoelectric therapeutic devices, in particular, are emerging as a promising subset of wearable therapeutics. These devices harness the piezoelectric effect to convert mechanical stress into electrical energy, which can be used for various therapeutic applications, including pain management, tissue regeneration, and physical therapy.
The market for wearable piezoelectric therapeutic devices is driven by several factors. Firstly, the growing aging population and the associated increase in chronic conditions such as arthritis, back pain, and musculoskeletal disorders create a substantial demand for non-pharmacological pain management solutions. Secondly, the shift towards personalized medicine and home-based care is fueling the adoption of wearable therapeutic devices that can provide continuous monitoring and treatment.
Consumer preferences are also shaping the market landscape. There is a growing demand for discreet, comfortable, and user-friendly devices that can be seamlessly integrated into daily life. This has led to innovations in device design, focusing on miniaturization, flexibility, and improved battery life.
Geographically, North America currently dominates the wearable therapeutic devices market, followed by Europe. However, the Asia-Pacific region is expected to witness the highest growth rate in the coming years, driven by improving healthcare infrastructure, rising disposable incomes, and increasing awareness about preventive healthcare.
Key market players in the wearable therapeutic devices sector include Medtronic, Abbott Laboratories, Johnson & Johnson, and Philips Healthcare. These companies are investing heavily in research and development to create innovative piezoelectric-based wearable solutions. Additionally, several startups and smaller companies are entering the market with novel technologies and applications, contributing to the overall market dynamism.
Despite the positive outlook, the market faces challenges such as regulatory hurdles, data privacy concerns, and the need for clinical validation of new technologies. Overcoming these obstacles will be crucial for the widespread adoption of wearable piezoelectric therapeutic devices and the realization of their full market potential.
Piezoelectric Technology: Current State and Challenges
Piezoelectric technology has made significant strides in recent years, particularly in the realm of wearable therapeutic devices. However, the current state of this technology faces several challenges that need to be addressed for further advancement and widespread adoption.
One of the primary challenges is the limited power output of piezoelectric materials in wearable applications. While these materials can generate electricity from mechanical stress or vibration, the amount of power produced is often insufficient for continuous operation of therapeutic devices. This limitation restricts the functionality and duration of use for wearable piezoelectric devices, necessitating frequent recharging or replacement of power sources.
Another significant challenge lies in the integration of piezoelectric materials into flexible and comfortable wearable form factors. Traditional piezoelectric materials are often rigid and brittle, making them unsuitable for conforming to the human body's contours. Researchers are exploring novel materials and fabrication techniques to create more flexible and durable piezoelectric elements, but achieving the right balance between flexibility and performance remains a complex task.
The biocompatibility and long-term safety of piezoelectric materials in direct contact with the skin pose additional challenges. While many piezoelectric materials are considered safe for short-term use, their long-term effects on the human body are not fully understood. This uncertainty raises concerns about potential allergic reactions, skin irritation, or other adverse effects that may arise from prolonged exposure to these materials in wearable therapeutic devices.
Miniaturization of piezoelectric components presents another hurdle in the development of wearable devices. As the demand for smaller, less obtrusive wearables grows, engineers must find ways to reduce the size of piezoelectric elements without compromising their performance. This challenge extends to the associated circuitry and power management systems, which must also be miniaturized to create compact, user-friendly devices.
The variability in piezoelectric performance across different users and usage scenarios is a significant challenge for therapeutic applications. Factors such as body temperature, movement patterns, and applied pressure can all affect the consistency and reliability of piezoelectric energy generation. Developing adaptive systems that can maintain therapeutic efficacy across a wide range of conditions is crucial for the widespread adoption of these devices.
Lastly, the cost-effectiveness of piezoelectric materials and manufacturing processes remains a challenge for mass production of wearable therapeutic devices. While the technology shows promise, the current production costs are often prohibitive for large-scale commercialization. Innovations in materials science and manufacturing techniques are needed to reduce costs and make piezoelectric wearables more accessible to a broader market.
One of the primary challenges is the limited power output of piezoelectric materials in wearable applications. While these materials can generate electricity from mechanical stress or vibration, the amount of power produced is often insufficient for continuous operation of therapeutic devices. This limitation restricts the functionality and duration of use for wearable piezoelectric devices, necessitating frequent recharging or replacement of power sources.
Another significant challenge lies in the integration of piezoelectric materials into flexible and comfortable wearable form factors. Traditional piezoelectric materials are often rigid and brittle, making them unsuitable for conforming to the human body's contours. Researchers are exploring novel materials and fabrication techniques to create more flexible and durable piezoelectric elements, but achieving the right balance between flexibility and performance remains a complex task.
The biocompatibility and long-term safety of piezoelectric materials in direct contact with the skin pose additional challenges. While many piezoelectric materials are considered safe for short-term use, their long-term effects on the human body are not fully understood. This uncertainty raises concerns about potential allergic reactions, skin irritation, or other adverse effects that may arise from prolonged exposure to these materials in wearable therapeutic devices.
Miniaturization of piezoelectric components presents another hurdle in the development of wearable devices. As the demand for smaller, less obtrusive wearables grows, engineers must find ways to reduce the size of piezoelectric elements without compromising their performance. This challenge extends to the associated circuitry and power management systems, which must also be miniaturized to create compact, user-friendly devices.
The variability in piezoelectric performance across different users and usage scenarios is a significant challenge for therapeutic applications. Factors such as body temperature, movement patterns, and applied pressure can all affect the consistency and reliability of piezoelectric energy generation. Developing adaptive systems that can maintain therapeutic efficacy across a wide range of conditions is crucial for the widespread adoption of these devices.
Lastly, the cost-effectiveness of piezoelectric materials and manufacturing processes remains a challenge for mass production of wearable therapeutic devices. While the technology shows promise, the current production costs are often prohibitive for large-scale commercialization. Innovations in materials science and manufacturing techniques are needed to reduce costs and make piezoelectric wearables more accessible to a broader market.
Existing Wearable Piezoelectric Therapy Solutions
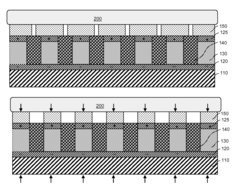
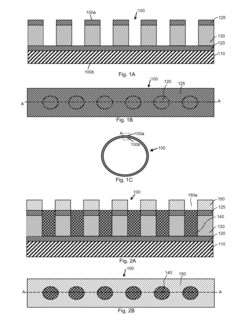
01 Wearable piezoelectric devices for therapeutic applications
These devices utilize piezoelectric materials to convert mechanical stress or vibrations into electrical energy for therapeutic purposes. They can be worn on various parts of the body and are designed to provide targeted treatment for different medical conditions.- Wearable piezoelectric devices for therapeutic applications: These devices incorporate piezoelectric materials into wearable form factors, allowing for therapeutic effects through the conversion of mechanical stress or movement into electrical energy. The devices can be used for various health applications, including pain management, tissue healing, and physical therapy.
- Integration of piezoelectric elements with flexible substrates: This approach involves embedding piezoelectric components into flexible or stretchable materials, enabling the device to conform to the body's contours. This integration enhances user comfort and allows for more effective energy harvesting from body movements.
- Combination of piezoelectric technology with other therapeutic modalities: These devices combine piezoelectric elements with other therapeutic technologies such as heat therapy, electrical stimulation, or light therapy. This multi-modal approach aims to enhance overall therapeutic efficacy and provide more comprehensive treatment options.
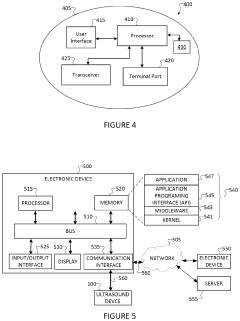
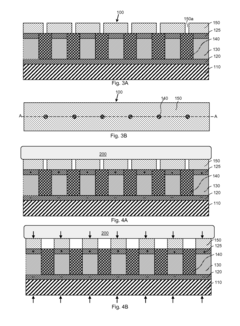
- Smart wearable piezoelectric devices with integrated sensors and feedback systems: Advanced wearable piezoelectric devices incorporate sensors and feedback mechanisms to monitor therapeutic progress and adjust treatment parameters in real-time. These smart devices may also include connectivity features for data tracking and remote monitoring.
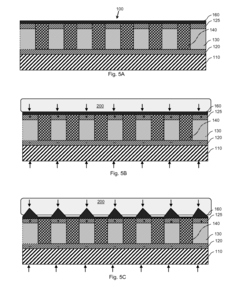
- Miniaturization and power optimization of piezoelectric therapeutic devices: This focuses on developing compact, energy-efficient piezoelectric therapeutic devices. Techniques include optimizing piezoelectric material properties, improving energy harvesting efficiency, and implementing low-power electronic components to extend device operation time and enhance portability.
02 Integration of sensors and feedback mechanisms
Advanced wearable piezoelectric therapeutic devices incorporate sensors to monitor physiological parameters and provide real-time feedback. This allows for personalized treatment adjustments and improved efficacy of the therapy.Expand Specific Solutions03 Energy harvesting and self-powered operation
Some wearable piezoelectric therapeutic devices are designed to harvest energy from body movements or ambient vibrations. This enables self-powered operation, reducing the need for external power sources and enhancing portability and convenience.Expand Specific Solutions04 Flexible and conformable piezoelectric materials
Development of flexible and conformable piezoelectric materials allows for better adaptation to body contours and improved comfort during wear. These materials enable the creation of thin, lightweight, and unobtrusive therapeutic devices.Expand Specific Solutions05 Multi-modal therapeutic applications
Wearable piezoelectric devices are being designed for various therapeutic applications, including pain management, wound healing, muscle stimulation, and neurological disorders. These devices can often provide multiple treatment modalities in a single wearable unit.Expand Specific Solutions
Key Players in Piezoelectric Therapeutic Wearables
The wearable piezoelectric therapeutic devices market is in an early growth stage, characterized by increasing research and development activities. The market size is expanding as more companies recognize the potential of this technology for non-invasive treatments. While still evolving, the technology is showing promise in various medical applications. Key players like Boston Scientific and the University of Connecticut are driving innovation, with research institutions such as the Agency for Science, Technology & Research contributing to technological advancements. The involvement of diverse organizations, from established medical device companies to academic institutions, indicates a growing interest in commercializing these devices, suggesting a trajectory towards increased market maturity and adoption in the coming years.
Boston Scientific Scimed, Inc.
Technical Solution: Boston Scientific Scimed has developed innovative wearable piezoelectric therapeutic devices that utilize advanced materials and miniaturization techniques. Their devices incorporate flexible piezoelectric polymers that can conform to body contours, enhancing patient comfort and treatment efficacy. The company's technology employs multi-layer piezoelectric structures to generate precise mechanical vibrations, which can be tailored for various therapeutic applications such as pain management, muscle stimulation, and wound healing[1][3]. Their devices also feature wireless connectivity for remote monitoring and adjustment of treatment parameters, allowing for personalized therapy regimens[5].
Strengths: Advanced materials technology, miniaturization expertise, and integration of wireless connectivity. Weaknesses: Potential high cost of production and limited battery life in wearable form factors.
Agency for Science, Technology & Research
Technical Solution: The Agency for Science, Technology & Research (A*STAR) has made significant strides in wearable piezoelectric therapeutic devices through their interdisciplinary approach. They have developed ultra-thin, flexible piezoelectric nanogenerators that can harvest energy from body movements to power the devices[2]. A*STAR's innovation lies in their use of biocompatible piezoelectric materials and nanostructured surfaces that enhance the energy conversion efficiency. Their devices incorporate microfluidic channels for targeted drug delivery, activated by piezoelectric stimulation[4]. Additionally, A*STAR has pioneered the integration of AI algorithms to optimize therapeutic protocols based on real-time biofeedback from the wearable sensors[6].
Strengths: Cutting-edge nanomaterial research, energy harvesting capabilities, and AI integration. Weaknesses: Potential challenges in scaling up production and regulatory hurdles for novel materials.
Breakthrough Piezoelectric Materials and Designs
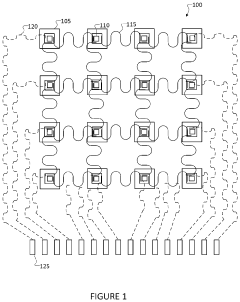
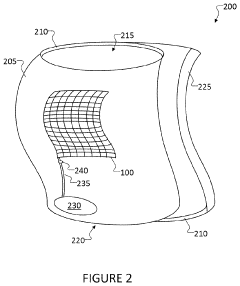
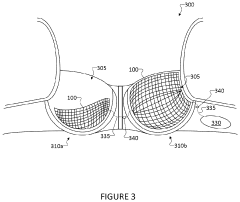
Wearable ultrasonic treatment device
PatentPendingUS20220331613A1
Innovation
- A portable, wearable ultrasound therapy system featuring a flexible ultrasound device with piezoelectric transducers integrated into a garment, allowing for safe and comfortable at-home treatment of scar tissue by emitting ultrasonic energy directly to affected areas.
Medical devices employing piezoelectric materials for delivery of therapeutic agents
PatentInactiveUS8702682B2
Innovation
- Incorporating piezoelectric materials that generate a voltage in response to mechanical stress, allowing for controlled therapeutic agent release through aperture creation or electrical migration, enabling precise and on-demand delivery.
Regulatory Framework for Therapeutic Wearables
The regulatory framework for therapeutic wearables is a complex and evolving landscape that plays a crucial role in ensuring the safety, efficacy, and quality of these innovative devices. As wearable piezoelectric therapeutic devices continue to advance, regulatory bodies worldwide are adapting their guidelines to address the unique challenges posed by these technologies.
In the United States, the Food and Drug Administration (FDA) is the primary regulatory authority for therapeutic wearables. The FDA classifies these devices based on their intended use and risk level, with most falling under Class II (moderate risk) or Class III (high risk) categories. Manufacturers must navigate the premarket notification (510(k)) process or the more rigorous premarket approval (PMA) pathway, depending on the device classification.
The European Union has implemented the Medical Device Regulation (MDR), which came into full effect in May 2021. This regulation introduces stricter requirements for clinical evidence, post-market surveillance, and traceability of medical devices, including therapeutic wearables. Manufacturers must obtain CE marking to demonstrate compliance with the MDR before marketing their devices in the EU.
In Asia, countries like Japan and China have their own regulatory frameworks. Japan's Pharmaceuticals and Medical Devices Agency (PMDA) oversees the approval process for therapeutic wearables, while China's National Medical Products Administration (NMPA) regulates these devices through its medical device registration system.
Regulatory bodies are increasingly focusing on cybersecurity and data privacy concerns associated with connected therapeutic wearables. The FDA has issued guidance on cybersecurity for medical devices, emphasizing the importance of secure design, risk management, and ongoing monitoring throughout the product lifecycle.
Interoperability and standardization are emerging as key regulatory considerations. Regulatory agencies are encouraging the development of standards that facilitate seamless integration of wearable devices with healthcare systems and other medical technologies.
As the field of wearable piezoelectric therapeutic devices continues to evolve, regulatory frameworks are likely to adapt further. Regulators are exploring more agile approaches to keep pace with rapid technological advancements while maintaining rigorous safety and efficacy standards. This may include the development of specialized guidelines for novel technologies like piezoelectric-based therapies and the implementation of real-world evidence in regulatory decision-making processes.
In the United States, the Food and Drug Administration (FDA) is the primary regulatory authority for therapeutic wearables. The FDA classifies these devices based on their intended use and risk level, with most falling under Class II (moderate risk) or Class III (high risk) categories. Manufacturers must navigate the premarket notification (510(k)) process or the more rigorous premarket approval (PMA) pathway, depending on the device classification.
The European Union has implemented the Medical Device Regulation (MDR), which came into full effect in May 2021. This regulation introduces stricter requirements for clinical evidence, post-market surveillance, and traceability of medical devices, including therapeutic wearables. Manufacturers must obtain CE marking to demonstrate compliance with the MDR before marketing their devices in the EU.
In Asia, countries like Japan and China have their own regulatory frameworks. Japan's Pharmaceuticals and Medical Devices Agency (PMDA) oversees the approval process for therapeutic wearables, while China's National Medical Products Administration (NMPA) regulates these devices through its medical device registration system.
Regulatory bodies are increasingly focusing on cybersecurity and data privacy concerns associated with connected therapeutic wearables. The FDA has issued guidance on cybersecurity for medical devices, emphasizing the importance of secure design, risk management, and ongoing monitoring throughout the product lifecycle.
Interoperability and standardization are emerging as key regulatory considerations. Regulatory agencies are encouraging the development of standards that facilitate seamless integration of wearable devices with healthcare systems and other medical technologies.
As the field of wearable piezoelectric therapeutic devices continues to evolve, regulatory frameworks are likely to adapt further. Regulators are exploring more agile approaches to keep pace with rapid technological advancements while maintaining rigorous safety and efficacy standards. This may include the development of specialized guidelines for novel technologies like piezoelectric-based therapies and the implementation of real-world evidence in regulatory decision-making processes.
User Experience and Adoption Factors
The user experience and adoption factors of wearable piezoelectric therapeutic devices play a crucial role in their success and widespread implementation. These devices, which harness the power of piezoelectric materials to deliver therapeutic benefits, must be designed with the end-user in mind to ensure optimal acceptance and effectiveness.
Comfort and wearability are paramount considerations in the design of these devices. Users expect seamless integration into their daily lives, with minimal disruption to their activities. This necessitates lightweight, flexible, and ergonomic designs that can be worn for extended periods without causing discomfort or irritation. The form factor of the device should be carefully considered to ensure it does not interfere with the user's range of motion or daily activities.
Ease of use is another critical factor influencing adoption. The device's interface should be intuitive and user-friendly, allowing for simple operation even by individuals with limited technological proficiency. Clear instructions and feedback mechanisms are essential to guide users through the therapeutic process and provide reassurance about the device's functionality.
Aesthetics also play a significant role in user adoption. As wearable devices become increasingly visible in everyday life, users seek products that align with their personal style and preferences. Offering customization options in terms of colors, materials, and designs can enhance the appeal of these therapeutic devices to a broader audience.
Battery life and charging convenience are crucial aspects of the user experience. Long-lasting battery performance reduces the frequency of charging, enhancing the device's usability. Wireless charging capabilities or easily accessible charging ports can further improve the user experience by minimizing downtime and simplifying the maintenance process.
Data visualization and feedback mechanisms are essential for user engagement and motivation. Providing clear, actionable insights into the therapeutic progress through companion mobile apps or integrated displays can help users track their progress and understand the benefits of the treatment. This data-driven approach can increase user compliance and long-term adoption of the technology.
Privacy and data security concerns must be addressed to build trust and encourage adoption. Users need assurance that their personal health data is protected and used responsibly. Implementing robust encryption methods and transparent data handling policies can alleviate these concerns and promote user confidence in the technology.
Lastly, the perceived value and effectiveness of the device significantly impact adoption rates. Clinical studies demonstrating the efficacy of piezoelectric therapeutic devices in treating specific conditions can bolster user confidence. Additionally, testimonials from healthcare professionals and early adopters can help overcome skepticism and encourage wider acceptance of this innovative technology in the healthcare and wellness sectors.
Comfort and wearability are paramount considerations in the design of these devices. Users expect seamless integration into their daily lives, with minimal disruption to their activities. This necessitates lightweight, flexible, and ergonomic designs that can be worn for extended periods without causing discomfort or irritation. The form factor of the device should be carefully considered to ensure it does not interfere with the user's range of motion or daily activities.
Ease of use is another critical factor influencing adoption. The device's interface should be intuitive and user-friendly, allowing for simple operation even by individuals with limited technological proficiency. Clear instructions and feedback mechanisms are essential to guide users through the therapeutic process and provide reassurance about the device's functionality.
Aesthetics also play a significant role in user adoption. As wearable devices become increasingly visible in everyday life, users seek products that align with their personal style and preferences. Offering customization options in terms of colors, materials, and designs can enhance the appeal of these therapeutic devices to a broader audience.
Battery life and charging convenience are crucial aspects of the user experience. Long-lasting battery performance reduces the frequency of charging, enhancing the device's usability. Wireless charging capabilities or easily accessible charging ports can further improve the user experience by minimizing downtime and simplifying the maintenance process.
Data visualization and feedback mechanisms are essential for user engagement and motivation. Providing clear, actionable insights into the therapeutic progress through companion mobile apps or integrated displays can help users track their progress and understand the benefits of the treatment. This data-driven approach can increase user compliance and long-term adoption of the technology.
Privacy and data security concerns must be addressed to build trust and encourage adoption. Users need assurance that their personal health data is protected and used responsibly. Implementing robust encryption methods and transparent data handling policies can alleviate these concerns and promote user confidence in the technology.
Lastly, the perceived value and effectiveness of the device significantly impact adoption rates. Clinical studies demonstrating the efficacy of piezoelectric therapeutic devices in treating specific conditions can bolster user confidence. Additionally, testimonials from healthcare professionals and early adopters can help overcome skepticism and encourage wider acceptance of this innovative technology in the healthcare and wellness sectors.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!