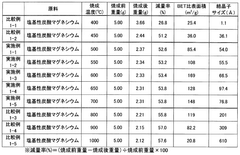
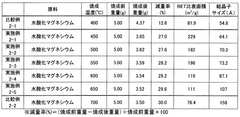
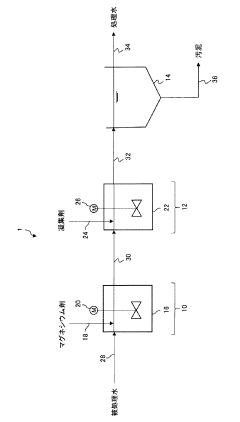
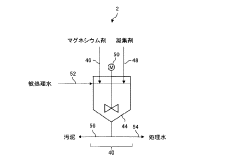
Investigating Magnesium Nitrate as a Coagulant in Water Treatment
AUG 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Mg(NO3)2 Coagulation Background and Objectives
Water treatment has been a critical concern for human societies throughout history, with the need for clean and safe water becoming increasingly urgent as populations grow and industrialization advances. In recent years, the search for more efficient and environmentally friendly coagulants has led researchers to explore various alternatives to traditional options. Magnesium nitrate (Mg(NO3)2) has emerged as a promising candidate in this field, drawing attention for its potential to address some of the limitations associated with conventional coagulants.
The use of coagulants in water treatment dates back to ancient times, with documented evidence of alum being used in Egypt as early as 1500 BC. However, the scientific understanding and systematic application of coagulation processes in water treatment began to develop significantly in the 19th and 20th centuries. Traditional coagulants such as aluminum sulfate (alum) and ferric chloride have been widely used due to their effectiveness and relatively low cost. Nevertheless, concerns about their environmental impact and potential health effects have spurred the search for alternatives.
Magnesium nitrate, a compound composed of magnesium and nitrate ions, has gained interest as a potential coagulant due to its unique properties. Magnesium, being an essential nutrient for human health, presents a more benign option compared to aluminum-based coagulants. Additionally, the nitrate component, while requiring careful management, can potentially serve as a nutrient source for biological processes in certain applications.
The primary objective of investigating magnesium nitrate as a coagulant in water treatment is to evaluate its efficacy in removing various contaminants, including suspended solids, organic matter, and potentially certain dissolved pollutants. Researchers aim to determine the optimal conditions for Mg(NO3)2 coagulation, such as dosage, pH range, and mixing parameters, to maximize its performance in different water matrices.
Another crucial goal is to assess the environmental implications of using magnesium nitrate as a coagulant. This includes studying the fate of magnesium and nitrate ions in treated water and sludge, as well as their potential impacts on aquatic ecosystems. Comparisons with conventional coagulants in terms of sludge production, biodegradability, and overall environmental footprint are essential aspects of this investigation.
Furthermore, the research aims to explore the economic viability of magnesium nitrate as a coagulant. This involves analyzing production costs, required dosages, and potential synergies with existing water treatment processes. The ultimate objective is to determine whether Mg(NO3)2 can offer a sustainable and cost-effective alternative to traditional coagulants while maintaining or improving water treatment efficiency.
The use of coagulants in water treatment dates back to ancient times, with documented evidence of alum being used in Egypt as early as 1500 BC. However, the scientific understanding and systematic application of coagulation processes in water treatment began to develop significantly in the 19th and 20th centuries. Traditional coagulants such as aluminum sulfate (alum) and ferric chloride have been widely used due to their effectiveness and relatively low cost. Nevertheless, concerns about their environmental impact and potential health effects have spurred the search for alternatives.
Magnesium nitrate, a compound composed of magnesium and nitrate ions, has gained interest as a potential coagulant due to its unique properties. Magnesium, being an essential nutrient for human health, presents a more benign option compared to aluminum-based coagulants. Additionally, the nitrate component, while requiring careful management, can potentially serve as a nutrient source for biological processes in certain applications.
The primary objective of investigating magnesium nitrate as a coagulant in water treatment is to evaluate its efficacy in removing various contaminants, including suspended solids, organic matter, and potentially certain dissolved pollutants. Researchers aim to determine the optimal conditions for Mg(NO3)2 coagulation, such as dosage, pH range, and mixing parameters, to maximize its performance in different water matrices.
Another crucial goal is to assess the environmental implications of using magnesium nitrate as a coagulant. This includes studying the fate of magnesium and nitrate ions in treated water and sludge, as well as their potential impacts on aquatic ecosystems. Comparisons with conventional coagulants in terms of sludge production, biodegradability, and overall environmental footprint are essential aspects of this investigation.
Furthermore, the research aims to explore the economic viability of magnesium nitrate as a coagulant. This involves analyzing production costs, required dosages, and potential synergies with existing water treatment processes. The ultimate objective is to determine whether Mg(NO3)2 can offer a sustainable and cost-effective alternative to traditional coagulants while maintaining or improving water treatment efficiency.
Water Treatment Market Analysis
The global water treatment market has been experiencing significant growth in recent years, driven by increasing water scarcity, stringent environmental regulations, and growing awareness of water quality issues. The market is expected to continue its upward trajectory, with projections indicating a compound annual growth rate (CAGR) of around 7% over the next five years.
Industrialization and urbanization have led to increased water pollution, creating a pressing need for effective water treatment solutions. This has resulted in a surge in demand for advanced water treatment technologies, including coagulants like magnesium nitrate. The coagulant segment of the water treatment market is particularly robust, as these chemicals play a crucial role in removing contaminants and improving water quality.
Geographically, North America and Europe currently dominate the water treatment market, owing to their well-established infrastructure and stringent environmental regulations. However, the Asia-Pacific region is emerging as the fastest-growing market, driven by rapid industrialization, population growth, and increasing government initiatives to improve water quality.
The municipal sector remains the largest end-user of water treatment solutions, accounting for a significant portion of the market share. However, industrial applications are gaining traction, particularly in sectors such as power generation, oil and gas, and food and beverage processing.
Key market players in the water treatment industry include Veolia, Suez, Ecolab, and Kemira. These companies are investing heavily in research and development to introduce innovative solutions and gain a competitive edge. The market is characterized by intense competition, with companies focusing on strategic partnerships, mergers, and acquisitions to expand their product portfolios and geographical presence.
The adoption of sustainable and eco-friendly water treatment solutions is a growing trend in the market. This has led to increased interest in alternative coagulants like magnesium nitrate, which offer potential environmental benefits compared to traditional options. As sustainability becomes a key focus for both consumers and regulators, the demand for such innovative solutions is expected to rise.
Technological advancements, such as the integration of artificial intelligence and IoT in water treatment processes, are reshaping the market landscape. These innovations are improving the efficiency and effectiveness of water treatment systems, driving further market growth and creating new opportunities for industry players.
Industrialization and urbanization have led to increased water pollution, creating a pressing need for effective water treatment solutions. This has resulted in a surge in demand for advanced water treatment technologies, including coagulants like magnesium nitrate. The coagulant segment of the water treatment market is particularly robust, as these chemicals play a crucial role in removing contaminants and improving water quality.
Geographically, North America and Europe currently dominate the water treatment market, owing to their well-established infrastructure and stringent environmental regulations. However, the Asia-Pacific region is emerging as the fastest-growing market, driven by rapid industrialization, population growth, and increasing government initiatives to improve water quality.
The municipal sector remains the largest end-user of water treatment solutions, accounting for a significant portion of the market share. However, industrial applications are gaining traction, particularly in sectors such as power generation, oil and gas, and food and beverage processing.
Key market players in the water treatment industry include Veolia, Suez, Ecolab, and Kemira. These companies are investing heavily in research and development to introduce innovative solutions and gain a competitive edge. The market is characterized by intense competition, with companies focusing on strategic partnerships, mergers, and acquisitions to expand their product portfolios and geographical presence.
The adoption of sustainable and eco-friendly water treatment solutions is a growing trend in the market. This has led to increased interest in alternative coagulants like magnesium nitrate, which offer potential environmental benefits compared to traditional options. As sustainability becomes a key focus for both consumers and regulators, the demand for such innovative solutions is expected to rise.
Technological advancements, such as the integration of artificial intelligence and IoT in water treatment processes, are reshaping the market landscape. These innovations are improving the efficiency and effectiveness of water treatment systems, driving further market growth and creating new opportunities for industry players.
Current Coagulants and Challenges
Water treatment processes have long relied on coagulants to remove impurities and contaminants from water sources. Currently, the most widely used coagulants in the industry are aluminum-based compounds, such as aluminum sulfate (alum) and polyaluminum chloride (PAC). These coagulants have proven effective in removing suspended particles, colloids, and dissolved organic matter from water.
Iron-based coagulants, including ferric chloride and ferric sulfate, are also commonly employed in water treatment facilities. These coagulants are particularly effective in removing phosphorus and heavy metals from water sources. Additionally, organic polymers, both synthetic and natural, have gained popularity as coagulants or coagulant aids due to their ability to enhance flocculation and improve settling characteristics.
Despite the widespread use of these coagulants, several challenges persist in the water treatment industry. One of the primary concerns is the potential health and environmental impacts associated with aluminum-based coagulants. Studies have suggested a possible link between aluminum exposure and neurological disorders, prompting research into alternative coagulants.
Another significant challenge is the variability in raw water quality, which can affect coagulant performance. Fluctuations in pH, temperature, and organic matter content can impact the efficiency of coagulation processes, requiring constant monitoring and adjustment of dosage rates. This variability also necessitates the development of more robust and adaptable coagulation technologies.
The increasing presence of emerging contaminants, such as pharmaceuticals, personal care products, and microplastics, poses a new challenge for traditional coagulants. Many of these substances are not effectively removed by conventional coagulation processes, highlighting the need for innovative treatment solutions.
Cost-effectiveness and sustainability are also major concerns in the water treatment industry. The production and transportation of chemical coagulants can be energy-intensive and expensive, particularly for remote or developing regions. This has led to a growing interest in locally sourced and environmentally friendly coagulant alternatives.
Sludge management is another challenge associated with current coagulants. The use of metal-based coagulants often results in the production of large volumes of chemical sludge, which can be difficult and costly to dispose of. Developing coagulants that produce less sludge or generate more easily treatable residuals is an ongoing area of research.
In light of these challenges, the investigation of magnesium nitrate as a potential coagulant in water treatment represents an opportunity to address some of the limitations of current technologies. As a non-aluminum based compound, magnesium nitrate may offer a safer alternative while potentially providing comparable or superior treatment efficacy. Its exploration aligns with the industry's ongoing efforts to develop more sustainable, efficient, and environmentally friendly water treatment solutions.
Iron-based coagulants, including ferric chloride and ferric sulfate, are also commonly employed in water treatment facilities. These coagulants are particularly effective in removing phosphorus and heavy metals from water sources. Additionally, organic polymers, both synthetic and natural, have gained popularity as coagulants or coagulant aids due to their ability to enhance flocculation and improve settling characteristics.
Despite the widespread use of these coagulants, several challenges persist in the water treatment industry. One of the primary concerns is the potential health and environmental impacts associated with aluminum-based coagulants. Studies have suggested a possible link between aluminum exposure and neurological disorders, prompting research into alternative coagulants.
Another significant challenge is the variability in raw water quality, which can affect coagulant performance. Fluctuations in pH, temperature, and organic matter content can impact the efficiency of coagulation processes, requiring constant monitoring and adjustment of dosage rates. This variability also necessitates the development of more robust and adaptable coagulation technologies.
The increasing presence of emerging contaminants, such as pharmaceuticals, personal care products, and microplastics, poses a new challenge for traditional coagulants. Many of these substances are not effectively removed by conventional coagulation processes, highlighting the need for innovative treatment solutions.
Cost-effectiveness and sustainability are also major concerns in the water treatment industry. The production and transportation of chemical coagulants can be energy-intensive and expensive, particularly for remote or developing regions. This has led to a growing interest in locally sourced and environmentally friendly coagulant alternatives.
Sludge management is another challenge associated with current coagulants. The use of metal-based coagulants often results in the production of large volumes of chemical sludge, which can be difficult and costly to dispose of. Developing coagulants that produce less sludge or generate more easily treatable residuals is an ongoing area of research.
In light of these challenges, the investigation of magnesium nitrate as a potential coagulant in water treatment represents an opportunity to address some of the limitations of current technologies. As a non-aluminum based compound, magnesium nitrate may offer a safer alternative while potentially providing comparable or superior treatment efficacy. Its exploration aligns with the industry's ongoing efforts to develop more sustainable, efficient, and environmentally friendly water treatment solutions.
Mg(NO3)2 Coagulation Mechanisms
01 Magnesium nitrate as a coagulant in wastewater treatment
Magnesium nitrate is used as an effective coagulant in wastewater treatment processes. It helps in the removal of suspended solids, organic matter, and other pollutants from various types of wastewater. The coagulation process using magnesium nitrate can improve water clarity and reduce contaminants, making it suitable for industrial and municipal wastewater treatment applications.- Magnesium nitrate as a coagulant in water treatment: Magnesium nitrate is used as an effective coagulant in water treatment processes. It helps in removing impurities, suspended particles, and contaminants from water by promoting the formation of larger, more easily filterable particles. This method is particularly useful in industrial and municipal water treatment applications.
- Magnesium nitrate in soil stabilization: Magnesium nitrate is employed in soil stabilization techniques, particularly for improving the properties of expansive soils. It helps in reducing soil plasticity, increasing soil strength, and enhancing overall soil stability. This application is beneficial in construction and geotechnical engineering projects.
- Use of magnesium nitrate in flame retardants: Magnesium nitrate is utilized as a component in flame retardant formulations. It contributes to the fire-resistant properties of materials by releasing non-flammable gases when exposed to high temperatures. This application is particularly important in the manufacturing of fire-resistant textiles, plastics, and building materials.
- Magnesium nitrate in fertilizer production: Magnesium nitrate is used as a raw material in the production of specialized fertilizers. It provides both magnesium and nitrogen, essential nutrients for plant growth. These fertilizers are particularly beneficial for crops with high magnesium requirements and are often used in hydroponic systems and foliar applications.
- Magnesium nitrate in wastewater treatment: Magnesium nitrate is employed in advanced wastewater treatment processes, particularly for the removal of phosphates and heavy metals. It acts as a precipitating agent, forming insoluble compounds with these contaminants, which can then be easily separated from the water. This application is crucial in improving the quality of treated wastewater before discharge.
02 Combination of magnesium nitrate with other coagulants
Magnesium nitrate is often used in combination with other coagulants to enhance the overall coagulation process. This synergistic approach can lead to improved flocculation, better settling of particles, and more efficient removal of impurities from water. The combination of magnesium nitrate with other coagulants can be tailored to specific water treatment requirements.Expand Specific Solutions03 Magnesium nitrate in industrial wastewater treatment
Magnesium nitrate coagulation is particularly effective in treating industrial wastewater from various sectors such as textile, chemical, and manufacturing industries. It can help in removing heavy metals, dyes, and other industrial pollutants. The process can be optimized for different types of industrial effluents to meet environmental discharge standards.Expand Specific Solutions04 Optimization of magnesium nitrate coagulation process
Research focuses on optimizing the magnesium nitrate coagulation process by adjusting parameters such as dosage, pH, temperature, and mixing conditions. Advanced techniques like rapid mixing, flocculation, and sedimentation are employed to enhance the efficiency of the coagulation process. These optimizations aim to improve the overall performance and cost-effectiveness of magnesium nitrate as a coagulant.Expand Specific Solutions05 Environmental and cost-effective aspects of magnesium nitrate coagulation
Magnesium nitrate coagulation is considered an environmentally friendly and cost-effective method for water treatment. It produces less sludge compared to some traditional coagulants and can be easily integrated into existing water treatment systems. The process also helps in recovering valuable resources from wastewater, contributing to sustainable water management practices.Expand Specific Solutions
Key Water Treatment Industry Players
The investigation of magnesium nitrate as a coagulant in water treatment is currently in an emerging phase, with growing interest from both academic institutions and industry players. The market for this technology is expanding, driven by increasing demand for efficient and cost-effective water treatment solutions. Companies like Organo Corp., Kemira Oyj, and Kurita Water Industries are at the forefront of developing and implementing advanced coagulation technologies. While magnesium nitrate's application as a coagulant is still evolving, research institutions such as Hebei University of Science & Technology and Nanjing University are contributing to its technological maturity through ongoing studies and collaborations with industry partners.
CSIR South Africa
Technical Solution: The Council for Scientific and Industrial Research (CSIR) in South Africa has conducted extensive research on the use of magnesium nitrate as a coagulant in water treatment, particularly focusing on its application in treating acid mine drainage (AMD). Their approach involves using magnesium nitrate in conjunction with limestone to neutralize acidity and remove heavy metals from AMD[10]. CSIR's studies have shown that this method can achieve up to 99% removal of sulfate and heavy metals such as iron, aluminum, and manganese from AMD[11]. The research team has also developed a novel fluidized bed reactor system that enhances the efficiency of the magnesium nitrate coagulation process, allowing for higher treatment capacities and reduced retention times[12]. Additionally, CSIR has explored the potential of recovering valuable minerals from the sludge produced by the magnesium nitrate treatment process, contributing to the circular economy approach in water treatment[13].
Strengths: Highly effective in treating acid mine drainage, potential for resource recovery, and innovative reactor design for improved efficiency. Weaknesses: May be limited to specific types of wastewater and potential for high operational costs due to the need for additional reagents.
Kemira Oyj
Technical Solution: Kemira Oyj has developed an innovative approach to using magnesium nitrate as a coagulant in water treatment. Their process involves a two-step coagulation-flocculation method, where magnesium nitrate is first added as a primary coagulant, followed by a polymer-based flocculant. This combination has shown to be particularly effective in removing suspended solids, phosphorus, and organic matter from wastewater[1]. The company has also integrated this technology into their KemConnect™ digital platform, which allows for real-time monitoring and optimization of the coagulation process, ensuring optimal dosage and performance[2]. Kemira's research has demonstrated that magnesium nitrate can achieve up to 95% phosphorus removal in municipal wastewater treatment plants, outperforming traditional aluminum-based coagulants in certain conditions[3].
Strengths: High efficiency in phosphorus removal, integration with digital monitoring systems, and potential for cost savings due to lower sludge production. Weaknesses: May require pH adjustment in some water sources and potential for increased magnesium levels in treated water.
Innovative Mg(NO3)2 Coagulation Research
Water treatment method, magnesium agent for water treatment, and method for producing magnesium agent for water treatment
PatentWO2018168558A1
Innovation
- A water treatment method involving a magnesium agent produced by calcining basic magnesium carbonate at 500-700°C or magnesium hydroxide at 450-650°C, resulting in a product with a high BET specific surface area and small crystallite size, which enhances the insolubilization and separation of substances like boron, fluorine, and silica in water, improving treatment efficiency and reducing the volume of separated solids.
Water treatment method and water treatment apparatus
PatentActiveJP2020081951A
Innovation
- A water treatment method involving the use of a magnesium agent to insolubilize substances, followed by the addition of a flocculant with a colloid equivalent value of +0.5 meq/g and a weight average molecular weight of 1 million to 10 million, to enhance flocculation and solid-liquid separation, reducing turbidity and sludge retention.
Environmental Impact Assessment
The environmental impact assessment of using magnesium nitrate as a coagulant in water treatment is a critical aspect of evaluating its feasibility and sustainability. Magnesium nitrate, while effective in water treatment processes, may have both positive and negative environmental implications that need to be carefully considered.
One of the primary environmental benefits of using magnesium nitrate as a coagulant is its potential to reduce the overall chemical load in water treatment systems. Compared to traditional coagulants like aluminum sulfate, magnesium nitrate may require lower dosages to achieve similar treatment results, potentially reducing the amount of chemicals released into the environment.
However, the increased concentration of nitrates in treated water and subsequent discharge into natural water bodies is a significant concern. Elevated nitrate levels can lead to eutrophication, causing algal blooms and oxygen depletion in aquatic ecosystems. This impact needs to be carefully monitored and managed to prevent ecological imbalances in receiving water bodies.
The production process of magnesium nitrate should also be considered in the environmental assessment. Depending on the manufacturing methods, there may be associated energy consumption and greenhouse gas emissions. A comprehensive life cycle analysis would be necessary to compare the overall environmental footprint of magnesium nitrate with alternative coagulants.
Another important factor is the potential for magnesium accumulation in treated sludge. While magnesium is generally considered less toxic than aluminum, its long-term effects on soil and plant life when sludge is used for land application need to be studied. This aspect is particularly relevant for agricultural areas where treated sludge may be used as fertilizer.
The impact on aquatic life directly exposed to treated water containing residual magnesium nitrate should also be assessed. While magnesium is an essential nutrient, changes in water chemistry could affect sensitive aquatic species. Long-term ecological studies would be valuable in understanding these potential impacts.
From a broader perspective, the use of magnesium nitrate as a coagulant may have implications for water resource management. If it proves to be more effective in removing certain pollutants, it could lead to improved water quality in treated effluents, potentially reducing the strain on natural water sources and supporting water reuse initiatives.
In conclusion, while magnesium nitrate shows promise as an alternative coagulant, its environmental impact must be thoroughly evaluated. This assessment should encompass its effects on aquatic ecosystems, soil health, and overall environmental sustainability, balancing its benefits against potential risks to ensure responsible implementation in water treatment processes.
One of the primary environmental benefits of using magnesium nitrate as a coagulant is its potential to reduce the overall chemical load in water treatment systems. Compared to traditional coagulants like aluminum sulfate, magnesium nitrate may require lower dosages to achieve similar treatment results, potentially reducing the amount of chemicals released into the environment.
However, the increased concentration of nitrates in treated water and subsequent discharge into natural water bodies is a significant concern. Elevated nitrate levels can lead to eutrophication, causing algal blooms and oxygen depletion in aquatic ecosystems. This impact needs to be carefully monitored and managed to prevent ecological imbalances in receiving water bodies.
The production process of magnesium nitrate should also be considered in the environmental assessment. Depending on the manufacturing methods, there may be associated energy consumption and greenhouse gas emissions. A comprehensive life cycle analysis would be necessary to compare the overall environmental footprint of magnesium nitrate with alternative coagulants.
Another important factor is the potential for magnesium accumulation in treated sludge. While magnesium is generally considered less toxic than aluminum, its long-term effects on soil and plant life when sludge is used for land application need to be studied. This aspect is particularly relevant for agricultural areas where treated sludge may be used as fertilizer.
The impact on aquatic life directly exposed to treated water containing residual magnesium nitrate should also be assessed. While magnesium is an essential nutrient, changes in water chemistry could affect sensitive aquatic species. Long-term ecological studies would be valuable in understanding these potential impacts.
From a broader perspective, the use of magnesium nitrate as a coagulant may have implications for water resource management. If it proves to be more effective in removing certain pollutants, it could lead to improved water quality in treated effluents, potentially reducing the strain on natural water sources and supporting water reuse initiatives.
In conclusion, while magnesium nitrate shows promise as an alternative coagulant, its environmental impact must be thoroughly evaluated. This assessment should encompass its effects on aquatic ecosystems, soil health, and overall environmental sustainability, balancing its benefits against potential risks to ensure responsible implementation in water treatment processes.
Regulatory Framework for Water Treatment Chemicals
The regulatory framework for water treatment chemicals is a critical aspect of ensuring safe and effective water treatment processes. In the context of investigating magnesium nitrate as a coagulant, it is essential to understand the existing regulations and standards that govern the use of chemicals in water treatment.
At the international level, organizations such as the World Health Organization (WHO) provide guidelines for drinking water quality, which include recommendations for the use of treatment chemicals. These guidelines serve as a basis for many national regulatory frameworks and are regularly updated to reflect the latest scientific knowledge and best practices in water treatment.
In the United States, the Environmental Protection Agency (EPA) plays a central role in regulating water treatment chemicals under the Safe Drinking Water Act (SDWA). The EPA sets maximum contaminant levels (MCLs) for various substances in drinking water and approves treatment technologies and chemicals through the National Sanitation Foundation (NSF) certification process. Magnesium nitrate, if considered for use as a coagulant, would need to undergo rigorous testing and evaluation to meet these standards.
The European Union has established the Drinking Water Directive, which sets quality standards for drinking water across member states. This directive includes provisions for the use of treatment chemicals and requires that they do not compromise water safety or quality. The European Chemicals Agency (ECHA) also plays a role in regulating chemicals used in water treatment through the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation.
In many countries, specific regulations govern the use of coagulants in water treatment. These regulations often require that coagulants meet certain purity standards, have proven efficacy, and do not introduce harmful substances into the treated water. For magnesium nitrate to be considered as a coagulant, it would need to comply with these regulations and demonstrate its safety and effectiveness through extensive testing and documentation.
Regulatory bodies also typically require water treatment facilities to maintain detailed records of chemical usage, including dosage rates, treatment effectiveness, and any observed impacts on water quality. This documentation is crucial for ensuring compliance with regulations and for ongoing monitoring of treatment processes.
As research into magnesium nitrate as a coagulant progresses, it will be necessary to engage with regulatory agencies to determine the specific requirements for its approval and use. This may involve conducting toxicological studies, assessing potential environmental impacts, and demonstrating its effectiveness compared to existing coagulants. The regulatory process can be lengthy and complex, requiring substantial investment in research and development to meet all necessary standards and requirements.
At the international level, organizations such as the World Health Organization (WHO) provide guidelines for drinking water quality, which include recommendations for the use of treatment chemicals. These guidelines serve as a basis for many national regulatory frameworks and are regularly updated to reflect the latest scientific knowledge and best practices in water treatment.
In the United States, the Environmental Protection Agency (EPA) plays a central role in regulating water treatment chemicals under the Safe Drinking Water Act (SDWA). The EPA sets maximum contaminant levels (MCLs) for various substances in drinking water and approves treatment technologies and chemicals through the National Sanitation Foundation (NSF) certification process. Magnesium nitrate, if considered for use as a coagulant, would need to undergo rigorous testing and evaluation to meet these standards.
The European Union has established the Drinking Water Directive, which sets quality standards for drinking water across member states. This directive includes provisions for the use of treatment chemicals and requires that they do not compromise water safety or quality. The European Chemicals Agency (ECHA) also plays a role in regulating chemicals used in water treatment through the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation.
In many countries, specific regulations govern the use of coagulants in water treatment. These regulations often require that coagulants meet certain purity standards, have proven efficacy, and do not introduce harmful substances into the treated water. For magnesium nitrate to be considered as a coagulant, it would need to comply with these regulations and demonstrate its safety and effectiveness through extensive testing and documentation.
Regulatory bodies also typically require water treatment facilities to maintain detailed records of chemical usage, including dosage rates, treatment effectiveness, and any observed impacts on water quality. This documentation is crucial for ensuring compliance with regulations and for ongoing monitoring of treatment processes.
As research into magnesium nitrate as a coagulant progresses, it will be necessary to engage with regulatory agencies to determine the specific requirements for its approval and use. This may involve conducting toxicological studies, assessing potential environmental impacts, and demonstrating its effectiveness compared to existing coagulants. The regulatory process can be lengthy and complex, requiring substantial investment in research and development to meet all necessary standards and requirements.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!