LFP Battery Cathode Engineering: Particle Morphology, Conductive Network And Rate
SEP 17, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
LFP Battery Evolution and Engineering Objectives
Lithium iron phosphate (LFP) batteries have undergone significant evolution since their initial development in the 1990s. The journey began with John Goodenough's team at the University of Texas discovering the LiFePO4 cathode material, which offered a safer and more environmentally friendly alternative to cobalt-based cathodes. Early LFP batteries faced substantial challenges, particularly regarding energy density and rate capability limitations stemming from the inherently poor electronic conductivity of the LFP material.
The technological progression of LFP batteries has been marked by several breakthrough innovations addressing these fundamental limitations. Carbon coating techniques emerged as the first major advancement, significantly improving electron transport across particle surfaces. This was followed by particle size reduction strategies, which shortened lithium-ion diffusion paths and enhanced rate performance. The development of hierarchical particle architectures represented another milestone, creating optimized pathways for both electron and ion transport.
Recent years have witnessed accelerated advancement in LFP technology, driven by increasing demand for safer, more sustainable energy storage solutions. Nano-engineering approaches have enabled precise control over particle morphology, while doping strategies with elements such as manganese, vanadium, and niobium have further enhanced intrinsic conductivity and structural stability. The integration of advanced conductive networks using graphene, carbon nanotubes, and specialized conductive polymers has pushed performance boundaries even further.
The primary engineering objectives for modern LFP cathode development center around overcoming the material's inherent limitations while capitalizing on its advantages. Key goals include increasing energy density to compete with higher-energy cathode chemistries, improving rate capability for fast-charging applications, and enhancing cycle life under demanding conditions. Engineers are particularly focused on optimizing three interconnected aspects: particle morphology control, conductive network design, and rate performance enhancement.
Looking forward, the trajectory of LFP battery development aims to position this chemistry as a dominant player in multiple market segments. Engineering efforts are increasingly targeting specific application requirements, with electric vehicles demanding high energy density and power, grid storage requiring exceptional cycle life and safety, and consumer electronics needing balanced performance characteristics. The ultimate objective is to develop LFP cathodes that maintain the chemistry's inherent safety and sustainability advantages while delivering performance comparable to or exceeding that of more resource-constrained alternatives.
The technological progression of LFP batteries has been marked by several breakthrough innovations addressing these fundamental limitations. Carbon coating techniques emerged as the first major advancement, significantly improving electron transport across particle surfaces. This was followed by particle size reduction strategies, which shortened lithium-ion diffusion paths and enhanced rate performance. The development of hierarchical particle architectures represented another milestone, creating optimized pathways for both electron and ion transport.
Recent years have witnessed accelerated advancement in LFP technology, driven by increasing demand for safer, more sustainable energy storage solutions. Nano-engineering approaches have enabled precise control over particle morphology, while doping strategies with elements such as manganese, vanadium, and niobium have further enhanced intrinsic conductivity and structural stability. The integration of advanced conductive networks using graphene, carbon nanotubes, and specialized conductive polymers has pushed performance boundaries even further.
The primary engineering objectives for modern LFP cathode development center around overcoming the material's inherent limitations while capitalizing on its advantages. Key goals include increasing energy density to compete with higher-energy cathode chemistries, improving rate capability for fast-charging applications, and enhancing cycle life under demanding conditions. Engineers are particularly focused on optimizing three interconnected aspects: particle morphology control, conductive network design, and rate performance enhancement.
Looking forward, the trajectory of LFP battery development aims to position this chemistry as a dominant player in multiple market segments. Engineering efforts are increasingly targeting specific application requirements, with electric vehicles demanding high energy density and power, grid storage requiring exceptional cycle life and safety, and consumer electronics needing balanced performance characteristics. The ultimate objective is to develop LFP cathodes that maintain the chemistry's inherent safety and sustainability advantages while delivering performance comparable to or exceeding that of more resource-constrained alternatives.
Market Demand Analysis for High-Rate LFP Batteries
The global market for high-rate LFP (Lithium Iron Phosphate) batteries has experienced significant growth in recent years, driven primarily by the expanding electric vehicle (EV) sector and stationary energy storage applications. The demand for batteries capable of rapid charging and discharging while maintaining safety and longevity has become increasingly critical as these markets mature.
In the EV segment, consumer expectations for faster charging times continue to rise, with many manufacturers now targeting 15-20 minute charging capabilities for 80% capacity. This requirement directly translates to a need for cathode materials that can facilitate high lithium-ion diffusion rates without structural degradation. Market research indicates that vehicles equipped with high-rate capability batteries command premium pricing, with consumers willing to pay 15-20% more for reduced charging times.
The grid-scale energy storage market presents another significant demand driver, as frequency regulation and peak shaving applications require batteries capable of rapid response to fluctuating grid conditions. This sector is projected to grow at a compound annual rate exceeding 20% through 2030, with high-rate batteries positioned to capture a substantial portion of this expansion.
Industrial applications represent a third major market segment, where high-power tools, automated guided vehicles, and material handling equipment benefit from batteries capable of delivering high current without excessive heating or capacity loss. This sector values cycle life and operational efficiency over energy density, making improved LFP formulations particularly attractive.
Consumer electronics manufacturers are also showing renewed interest in LFP chemistry as safety concerns with higher energy density chemistries persist. The ability to rapidly charge personal devices without compromising battery longevity represents a significant market opportunity, particularly in premium product segments.
Market analysis reveals geographic variations in demand patterns. Asian markets, particularly China, currently lead in high-rate LFP battery adoption, driven by government policies promoting electric vehicles and renewable energy integration. European markets show accelerating demand growth as regulatory frameworks increasingly favor sustainable energy solutions, while North American markets exhibit strong demand in the utility-scale storage segment.
Price sensitivity analysis indicates that while high-rate LFP batteries currently command a premium over standard LFP formulations, this gap is expected to narrow as manufacturing processes mature and economies of scale are realized. The market appears willing to absorb a 10-15% premium for significantly improved rate capability, particularly in applications where operational efficiency translates directly to economic benefits.
In the EV segment, consumer expectations for faster charging times continue to rise, with many manufacturers now targeting 15-20 minute charging capabilities for 80% capacity. This requirement directly translates to a need for cathode materials that can facilitate high lithium-ion diffusion rates without structural degradation. Market research indicates that vehicles equipped with high-rate capability batteries command premium pricing, with consumers willing to pay 15-20% more for reduced charging times.
The grid-scale energy storage market presents another significant demand driver, as frequency regulation and peak shaving applications require batteries capable of rapid response to fluctuating grid conditions. This sector is projected to grow at a compound annual rate exceeding 20% through 2030, with high-rate batteries positioned to capture a substantial portion of this expansion.
Industrial applications represent a third major market segment, where high-power tools, automated guided vehicles, and material handling equipment benefit from batteries capable of delivering high current without excessive heating or capacity loss. This sector values cycle life and operational efficiency over energy density, making improved LFP formulations particularly attractive.
Consumer electronics manufacturers are also showing renewed interest in LFP chemistry as safety concerns with higher energy density chemistries persist. The ability to rapidly charge personal devices without compromising battery longevity represents a significant market opportunity, particularly in premium product segments.
Market analysis reveals geographic variations in demand patterns. Asian markets, particularly China, currently lead in high-rate LFP battery adoption, driven by government policies promoting electric vehicles and renewable energy integration. European markets show accelerating demand growth as regulatory frameworks increasingly favor sustainable energy solutions, while North American markets exhibit strong demand in the utility-scale storage segment.
Price sensitivity analysis indicates that while high-rate LFP batteries currently command a premium over standard LFP formulations, this gap is expected to narrow as manufacturing processes mature and economies of scale are realized. The market appears willing to absorb a 10-15% premium for significantly improved rate capability, particularly in applications where operational efficiency translates directly to economic benefits.
Current Challenges in LFP Cathode Technology
Despite significant advancements in LFP (Lithium Iron Phosphate) cathode technology, several critical challenges continue to impede its widespread adoption and performance optimization. The inherently low electronic conductivity of LFP materials (approximately 10^-9 S/cm) represents a fundamental limitation, necessitating sophisticated conductive network engineering to facilitate efficient electron transport during charge-discharge cycles.
Particle morphology control remains problematic at industrial scale. While laboratory conditions can produce well-defined nano-sized particles with optimized shapes, translating these processes to mass production often results in inconsistent morphologies, agglomeration issues, and compromised electrochemical performance. The trade-off between particle size reduction (which shortens Li-ion diffusion paths) and increased surface area (which can promote unwanted side reactions) presents a persistent engineering dilemma.
The formation of robust conductive networks within LFP cathodes faces significant hurdles. Current carbon coating techniques often yield non-uniform coverage, creating "dead zones" where active material remains electronically isolated. Additionally, the carbon coating process can introduce impurities that negatively impact cycle life and rate capability. The optimal carbon content remains contentious, with excessive carbon reducing volumetric energy density while insufficient carbon compromises power performance.
Rate capability limitations stem from LFP's intrinsic properties, particularly its one-dimensional Li-ion diffusion channels. These channels are easily blocked by defects or impurities, severely restricting ionic transport. At high charge-discharge rates, this limitation becomes especially pronounced, resulting in capacity fading and voltage hysteresis that diminishes energy efficiency.
Temperature sensitivity presents another significant challenge. LFP cathodes exhibit markedly reduced performance at low temperatures (below 0°C), limiting their application in cold-climate regions. This temperature dependence stems from decreased Li-ion diffusion kinetics within the olivine structure and increased charge transfer resistance at electrode interfaces.
Manufacturing consistency and quality control issues persist across the industry. Variations in precursor purity, synthesis conditions, and post-processing treatments lead to batch-to-batch inconsistencies that affect electrochemical performance. The lack of standardized characterization protocols for evaluating particle morphology and conductive network quality further complicates quality assurance efforts.
Finally, the environmental impact of current LFP manufacturing processes remains concerning. High-temperature calcination steps consume significant energy, while certain synthesis routes utilize environmentally problematic reagents. Developing greener synthesis methods that maintain or enhance electrochemical performance represents an ongoing challenge for the industry.
Particle morphology control remains problematic at industrial scale. While laboratory conditions can produce well-defined nano-sized particles with optimized shapes, translating these processes to mass production often results in inconsistent morphologies, agglomeration issues, and compromised electrochemical performance. The trade-off between particle size reduction (which shortens Li-ion diffusion paths) and increased surface area (which can promote unwanted side reactions) presents a persistent engineering dilemma.
The formation of robust conductive networks within LFP cathodes faces significant hurdles. Current carbon coating techniques often yield non-uniform coverage, creating "dead zones" where active material remains electronically isolated. Additionally, the carbon coating process can introduce impurities that negatively impact cycle life and rate capability. The optimal carbon content remains contentious, with excessive carbon reducing volumetric energy density while insufficient carbon compromises power performance.
Rate capability limitations stem from LFP's intrinsic properties, particularly its one-dimensional Li-ion diffusion channels. These channels are easily blocked by defects or impurities, severely restricting ionic transport. At high charge-discharge rates, this limitation becomes especially pronounced, resulting in capacity fading and voltage hysteresis that diminishes energy efficiency.
Temperature sensitivity presents another significant challenge. LFP cathodes exhibit markedly reduced performance at low temperatures (below 0°C), limiting their application in cold-climate regions. This temperature dependence stems from decreased Li-ion diffusion kinetics within the olivine structure and increased charge transfer resistance at electrode interfaces.
Manufacturing consistency and quality control issues persist across the industry. Variations in precursor purity, synthesis conditions, and post-processing treatments lead to batch-to-batch inconsistencies that affect electrochemical performance. The lack of standardized characterization protocols for evaluating particle morphology and conductive network quality further complicates quality assurance efforts.
Finally, the environmental impact of current LFP manufacturing processes remains concerning. High-temperature calcination steps consume significant energy, while certain synthesis routes utilize environmentally problematic reagents. Developing greener synthesis methods that maintain or enhance electrochemical performance represents an ongoing challenge for the industry.
Current Approaches to LFP Conductive Network Design
01 Particle morphology optimization for LFP cathodes
The morphology of LiFePO4 (LFP) cathode particles significantly impacts battery performance. Optimizing particle size, shape, and surface characteristics can enhance lithium-ion diffusion pathways and improve electrochemical performance. Controlled synthesis methods can produce particles with specific morphologies such as nanoplates, microspheres, or hierarchical structures that offer improved rate capability and cycling stability. These optimized morphologies provide larger surface areas for lithium-ion exchange and shorter diffusion distances within the particles.- Particle morphology optimization for LFP cathodes: The morphology of LiFePO4 (LFP) particles significantly impacts battery performance. Controlling particle size, shape, and distribution can enhance lithium-ion diffusion pathways and increase active material utilization. Optimized particle morphologies, such as nanoplates, microspheres, or hierarchical structures, can reduce diffusion distances and improve rate capability. These morphological optimizations help overcome LFP's inherent limitations of low electronic conductivity and slow lithium-ion diffusion.
- Conductive network formation and carbon coating techniques: Establishing effective conductive networks within LFP cathodes is crucial for enhancing electronic conductivity. Various approaches include carbon coating of LFP particles, incorporation of conductive additives, and formation of interconnected conductive frameworks. Carbon coating techniques involve pyrolysis of organic precursors to create uniform carbon layers on particle surfaces. These conductive networks facilitate electron transport throughout the electrode, reducing internal resistance and improving rate performance, especially at high current densities.
- Doping strategies to enhance LFP conductivity: Doping LFP cathode materials with various elements can significantly improve their intrinsic electronic conductivity and lithium-ion diffusion properties. Common dopants include metal ions (such as Mg, Zn, Ni, Co) and non-metal elements that can substitute for Fe, P, or O sites in the crystal structure. These dopants can modify the band structure, create defects that enhance conductivity, and stabilize the crystal structure during cycling. Properly doped LFP materials demonstrate superior rate capability and cycling stability compared to undoped counterparts.
- Composite cathode structures for improved performance: Composite cathode structures combining LFP with other materials can overcome limitations of traditional LFP cathodes. These composites may incorporate conductive polymers, graphene, carbon nanotubes, or other high-conductivity materials to form hybrid structures. Some approaches include core-shell architectures, 3D interconnected networks, and hierarchical porous structures. These composite designs create synergistic effects that enhance electron transport, facilitate ion diffusion, and improve the utilization of active materials, resulting in better rate performance and energy density.
- Electrode engineering for optimized rate performance: Electrode engineering approaches focus on optimizing the entire cathode structure beyond individual particles. This includes controlling electrode porosity, thickness, calendering parameters, and binder systems. Advanced techniques involve creating gradient structures, tailored pore networks, and optimized active material-to-conductive additive ratios. Proper electrode engineering ensures efficient electrolyte penetration, minimizes ion transport limitations, and maintains electronic pathways throughout the electrode. These approaches are critical for achieving high rate performance while maintaining good energy density in LFP batteries.
02 Conductive network formation and carbon coating techniques
Establishing effective conductive networks in LFP cathodes is crucial for overcoming the inherent low electronic conductivity of LiFePO4 material. Various carbon coating techniques, including in-situ carbonization, hydrothermal carbon coating, and vapor deposition methods, can create uniform conductive layers around LFP particles. Additionally, incorporating conductive additives such as carbon nanotubes, graphene, or conductive polymers helps form three-dimensional conductive networks throughout the electrode. These approaches significantly enhance electron transport within the cathode structure, leading to improved rate performance and power density.Expand Specific Solutions03 Doping strategies to enhance LFP conductivity
Doping LFP cathode materials with various elements can significantly improve their intrinsic electronic and ionic conductivity. Metal ion doping (using elements like Mg, Zn, Ni, Co, or Mn) at Fe sites or anion doping at P or O sites can modify the crystal structure and electronic properties of LFP. These dopants create defects or alter the band structure, facilitating faster electron and lithium-ion transport. Optimized doping strategies can enhance rate capability without compromising the structural stability or capacity of LFP cathodes, making them more suitable for high-power applications.Expand Specific Solutions04 Composite cathode structures for improved rate performance
Developing composite cathode structures by combining LFP with other materials can significantly enhance rate performance. These composites may include LFP integrated with highly conductive materials like graphene, carbon nanotubes, or metal oxides. Hierarchical porous structures can be designed to facilitate both electron transport and lithium-ion diffusion. Core-shell architectures, where LFP particles are encapsulated in conductive shells, provide enhanced interface properties. These composite structures offer synergistic effects that address multiple performance limitations simultaneously, resulting in batteries with superior rate capability and cycling stability.Expand Specific Solutions05 Advanced electrode manufacturing techniques
Innovative electrode manufacturing techniques can optimize the distribution of active materials and conductive additives in LFP cathodes. Methods such as freeze-drying, spray pyrolysis, or template-assisted synthesis can create electrodes with controlled porosity and optimized particle arrangement. Advanced mixing and coating processes ensure homogeneous distribution of components throughout the electrode. These manufacturing approaches can reduce agglomeration of particles, create efficient ion transport channels, and establish robust conductive networks, ultimately enhancing the rate performance and power density of LFP batteries.Expand Specific Solutions
Key Industry Players in LFP Battery Development
The LFP battery cathode engineering market is currently in a growth phase, with increasing demand driven by electric vehicle adoption and energy storage applications. The global market size is projected to reach significant scale due to LFP's cost advantages and safety profile. Technologically, the field is advancing rapidly with companies at varying maturity levels. Industry leaders like CATL, LG Chem, and A123 Systems have established commercial-scale production with optimized particle morphology techniques, while innovative players such as StoreDot, Mitra Chem, and NOVONIX are developing next-generation LFP cathodes with enhanced rate capabilities. Research institutions including KAUST and Karlsruhe Institute of Technology are contributing fundamental advancements in conductive network engineering. The competitive landscape features both established battery manufacturers and specialized materials companies focusing on nano-engineering approaches to overcome LFP's inherent conductivity limitations.
Contemporary Amperex Technology Co., Ltd.
Technical Solution: CATL has developed advanced LFP cathode engineering solutions focusing on nano-crystallization and carbon coating techniques. Their proprietary spherical LFP particle morphology design optimizes ion diffusion pathways, significantly improving rate capability. CATL employs a hierarchical conductive network where nano-sized LFP particles are encapsulated in a carbon matrix with both primary and secondary conductive pathways. This design creates a three-dimensional electron transport network that addresses LFP's inherent low conductivity. Their Cell-to-Pack (CTP) technology specifically optimized for LFP chemistry has achieved energy densities of 160-170 Wh/kg at the pack level, representing a substantial improvement over traditional LFP implementations. CATL has also pioneered the use of doping elements (including Mn, Co, and Ni) in precise concentrations to modify the crystal structure of LFP particles, enhancing electronic conductivity while maintaining structural stability during cycling.
Strengths: Industry-leading energy density for LFP batteries, excellent thermal stability, and superior cycle life (4,000+ cycles). Their manufacturing scale enables cost-effective production. Weaknesses: Still lower energy density compared to NCM chemistries, and rate performance at extremely low temperatures remains challenging despite improvements.
Hefei Guoxuan High-Tech Power Energy Co., Ltd.
Technical Solution: Guoxuan has developed an advanced "nano-composite LFP" technology that addresses the inherent conductivity limitations of LFP materials. Their approach focuses on precise control of particle morphology through a modified solid-state reaction method that produces primary particles with optimized size (100-200nm) and crystallinity. These primary particles are engineered with specific surface features that enhance lithium-ion exchange kinetics. Guoxuan's conductive network design incorporates a multi-layer carbon coating strategy where different carbon precursors are applied sequentially to create a gradient conductive layer that optimizes both electronic conductivity and ionic transport. Their proprietary "conductive bridge" technology strategically positions nano-sized conductive additives between LFP particles to create dedicated electron transport pathways throughout the electrode structure. Additionally, Guoxuan has developed specialized binders and electrode formulations that maintain conductive network integrity during cycling. This comprehensive approach has enabled Guoxuan to achieve LFP batteries with improved rate performance (60% capacity retention at 5C) while maintaining excellent cycle stability (>2,500 cycles at 1C).
Strengths: Good balance of rate capability, cycle life, and cost-effectiveness. Their manufacturing process is highly scalable and environmentally friendly. Weaknesses: The complex particle engineering and conductive network design require sophisticated quality control measures, and energy density remains lower than competing cathode chemistries.
Critical Patents in LFP Particle Engineering
High performance cathode active material for lithium ion battery
PatentInactiveUS10629897B2
Innovation
- A lithium iron phosphate/sulfonated graphene oxide (LFP/SG) nanocomposite material is developed, synthesized via a microwave-assisted hydrothermal method, with a molar ratio of sulfonated graphene oxide to lithium iron phosphate of 0.1:1, enhancing electronic conductivity and ion diffusion.
Lithium iron phosphate (LFP) cathode active materials and method for deposition of the same
PatentInactiveUS20240072257A1
Innovation
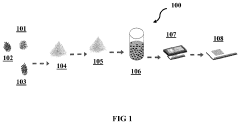
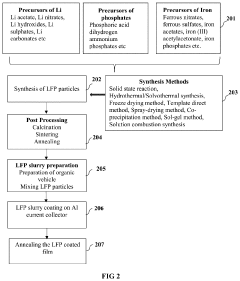
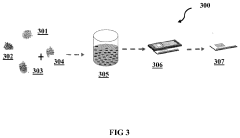
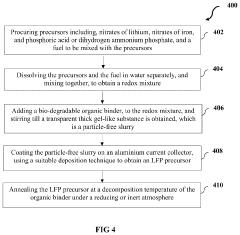
- A method using bio-degradable organic binders and solvents, specifically sodium carboxymethyl cellulose, sodium poly acrylic acid, or sodium alginate, to create an aqueous-based LFP precursor slurry, which is deposited on an aluminum foil current collector via blade coating, slot-die coating, or screen printing, followed by annealing under an inert atmosphere, bypassing energy-intensive synthesis and post-processing steps.
Sustainability Impact of Advanced LFP Technologies
The advancement of LFP battery cathode technologies represents a significant opportunity for enhancing sustainability across multiple dimensions of the global energy ecosystem. Advanced LFP cathode engineering, particularly innovations in particle morphology and conductive networks, contributes substantially to reducing environmental footprints throughout the battery lifecycle.
The sustainability advantages begin with raw material sourcing. LFP cathodes utilize iron and phosphate, which are abundant, widely distributed geographically, and require less energy-intensive mining compared to nickel and cobalt-based alternatives. This reduces extraction-related environmental degradation and carbon emissions by an estimated 30-40% compared to conventional cathode materials.
Manufacturing processes for optimized LFP particles with controlled morphology can be achieved at lower temperatures than competing chemistries, resulting in reduced energy consumption during production. Recent innovations in hydrothermal synthesis methods for nano-structured LFP particles have demonstrated energy requirement reductions of up to 25% compared to solid-state reactions traditionally employed.
The extended cycle life achieved through advanced conductive networks and optimized particle engineering translates directly to sustainability benefits. Modern LFP batteries with engineered cathodes can achieve 3,000-5,000 cycles, significantly outlasting consumer electronics batteries and reducing waste generation. This longevity reduces the materials and energy required for replacement batteries by approximately 60% over a 10-year application period.
End-of-life considerations further highlight LFP's sustainability advantages. The absence of cobalt and nickel simplifies recycling processes and reduces toxicity concerns. Emerging recycling technologies specifically designed for LFP chemistries can recover up to 90% of cathode materials with lower energy inputs than required for other lithium-ion chemistries.
From a broader environmental perspective, the improved rate capability of advanced LFP cathodes enables more efficient integration of renewable energy sources into grid systems. Fast-charging capabilities support the deployment of electric vehicles with smaller battery packs, reducing overall material requirements while maintaining performance.
The social sustainability dimension cannot be overlooked. LFP technology eliminates dependence on conflict minerals and reduces exposure to volatile supply chains associated with cobalt and nickel. This promotes more equitable distribution of economic benefits across the battery value chain and reduces geopolitical tensions related to critical mineral access.
The sustainability advantages begin with raw material sourcing. LFP cathodes utilize iron and phosphate, which are abundant, widely distributed geographically, and require less energy-intensive mining compared to nickel and cobalt-based alternatives. This reduces extraction-related environmental degradation and carbon emissions by an estimated 30-40% compared to conventional cathode materials.
Manufacturing processes for optimized LFP particles with controlled morphology can be achieved at lower temperatures than competing chemistries, resulting in reduced energy consumption during production. Recent innovations in hydrothermal synthesis methods for nano-structured LFP particles have demonstrated energy requirement reductions of up to 25% compared to solid-state reactions traditionally employed.
The extended cycle life achieved through advanced conductive networks and optimized particle engineering translates directly to sustainability benefits. Modern LFP batteries with engineered cathodes can achieve 3,000-5,000 cycles, significantly outlasting consumer electronics batteries and reducing waste generation. This longevity reduces the materials and energy required for replacement batteries by approximately 60% over a 10-year application period.
End-of-life considerations further highlight LFP's sustainability advantages. The absence of cobalt and nickel simplifies recycling processes and reduces toxicity concerns. Emerging recycling technologies specifically designed for LFP chemistries can recover up to 90% of cathode materials with lower energy inputs than required for other lithium-ion chemistries.
From a broader environmental perspective, the improved rate capability of advanced LFP cathodes enables more efficient integration of renewable energy sources into grid systems. Fast-charging capabilities support the deployment of electric vehicles with smaller battery packs, reducing overall material requirements while maintaining performance.
The social sustainability dimension cannot be overlooked. LFP technology eliminates dependence on conflict minerals and reduces exposure to volatile supply chains associated with cobalt and nickel. This promotes more equitable distribution of economic benefits across the battery value chain and reduces geopolitical tensions related to critical mineral access.
Manufacturing Scalability of Engineered LFP Cathodes
The scalability of engineered LFP cathode manufacturing processes represents a critical factor in the widespread adoption of this battery technology. Current laboratory-scale innovations in particle morphology and conductive network engineering face significant challenges when transitioning to industrial production volumes. The manufacturing scalability depends heavily on process consistency, equipment compatibility, and cost-effectiveness across increased production volumes.
Traditional LFP cathode manufacturing typically involves solid-state reactions or hydrothermal synthesis methods. When scaling these processes to accommodate engineered morphologies, maintaining precise control over particle size distribution becomes increasingly difficult. Production equipment designed for conventional spherical particles may require substantial modifications to handle novel morphologies such as nanoplates or hierarchical structures, adding capital expenditure barriers to implementation.
The integration of conductive networks at scale presents additional challenges. Laboratory techniques for creating intimate carbon coatings or conductive additive distributions often rely on batch processes with careful monitoring. Translating these to continuous production lines requires redesigning mixing sequences, drying parameters, and quality control protocols. Current industrial coating technologies struggle to achieve the nanoscale precision demonstrated in research settings while maintaining throughput rates necessary for commercial viability.
Economic considerations significantly impact scalability decisions. Enhanced rate performance through engineered cathodes must justify the increased production costs. Analysis indicates that manufacturing complexity increases exponentially with more sophisticated morphology control, potentially limiting adoption to premium battery applications initially. The cost-benefit equation improves as production volumes increase, suggesting a gradual implementation pathway beginning with high-value applications.
Raw material supply chains also influence scalability. Novel dopants or specialized carbon sources required for advanced conductive networks may have limited availability at industrial scales. Manufacturers must secure reliable supply agreements or develop alternative formulations compatible with more abundant materials to ensure production stability.
Quality control systems require adaptation for engineered cathodes. Conventional testing protocols may not adequately characterize the critical parameters of morphologically optimized materials. New inline measurement technologies capable of rapidly assessing particle geometry, carbon distribution, and electrochemical performance at production speeds are essential for maintaining consistency across scaled production.
Traditional LFP cathode manufacturing typically involves solid-state reactions or hydrothermal synthesis methods. When scaling these processes to accommodate engineered morphologies, maintaining precise control over particle size distribution becomes increasingly difficult. Production equipment designed for conventional spherical particles may require substantial modifications to handle novel morphologies such as nanoplates or hierarchical structures, adding capital expenditure barriers to implementation.
The integration of conductive networks at scale presents additional challenges. Laboratory techniques for creating intimate carbon coatings or conductive additive distributions often rely on batch processes with careful monitoring. Translating these to continuous production lines requires redesigning mixing sequences, drying parameters, and quality control protocols. Current industrial coating technologies struggle to achieve the nanoscale precision demonstrated in research settings while maintaining throughput rates necessary for commercial viability.
Economic considerations significantly impact scalability decisions. Enhanced rate performance through engineered cathodes must justify the increased production costs. Analysis indicates that manufacturing complexity increases exponentially with more sophisticated morphology control, potentially limiting adoption to premium battery applications initially. The cost-benefit equation improves as production volumes increase, suggesting a gradual implementation pathway beginning with high-value applications.
Raw material supply chains also influence scalability. Novel dopants or specialized carbon sources required for advanced conductive networks may have limited availability at industrial scales. Manufacturers must secure reliable supply agreements or develop alternative formulations compatible with more abundant materials to ensure production stability.
Quality control systems require adaptation for engineered cathodes. Conventional testing protocols may not adequately characterize the critical parameters of morphologically optimized materials. New inline measurement technologies capable of rapidly assessing particle geometry, carbon distribution, and electrochemical performance at production speeds are essential for maintaining consistency across scaled production.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!