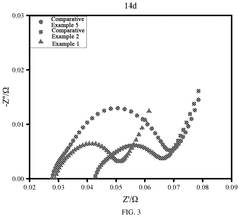
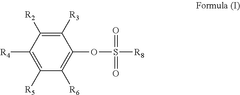
LFP Battery Low-Temperature Capability: Electrolyte Selection, SEI And Power
SEP 17, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
LFP Battery Low-Temperature Performance Background and Objectives
Lithium Iron Phosphate (LFP) batteries have emerged as a significant technology in the energy storage landscape due to their inherent safety, long cycle life, and cost-effectiveness. However, their performance at low temperatures remains a critical challenge that limits their widespread adoption in certain applications, particularly in electric vehicles operating in cold climates. The historical development of LFP batteries began in the late 1990s, with significant commercialization occurring in the early 2000s. Since then, continuous improvements have been made to enhance their energy density, cycle life, and temperature performance.
The evolution of LFP battery technology has been marked by several key advancements, including nano-sizing of active materials, carbon coating techniques, and doping strategies. Despite these improvements, the inherent limitations of LFP chemistry at low temperatures persist, primarily due to slower lithium-ion diffusion kinetics and increased electrolyte resistance when temperatures drop below 0°C. This results in reduced capacity, diminished power capability, and accelerated degradation during cold-weather operation.
Current market trends indicate a growing demand for batteries that can maintain performance across a wide temperature range, driven by the expanding electric vehicle market in regions with cold climates and the increasing deployment of stationary energy storage systems in diverse geographical locations. The ability to operate efficiently at low temperatures has become a key differentiator in battery technology selection for many applications.
The technical objectives for improving LFP battery low-temperature performance center around three critical areas: electrolyte optimization, solid electrolyte interphase (SEI) engineering, and power delivery enhancement. Electrolyte formulations must balance low-temperature fluidity with electrochemical stability. SEI formation and properties significantly impact lithium-ion transport at the electrode-electrolyte interface, particularly at reduced temperatures. Power capability at low temperatures requires addressing both materials and system-level design considerations.
Recent research indicates promising directions in developing novel electrolyte additives that can modify SEI composition and structure, creating more favorable conditions for ion transport at low temperatures. Additionally, advanced electrode designs incorporating hierarchical porosity and optimized conductive networks show potential for enhancing charge transfer kinetics in cold conditions.
The ultimate goal of this technical research is to develop LFP battery systems that maintain at least 80% of their room-temperature capacity and power capability at temperatures as low as -20°C, without compromising safety, cycle life, or cost advantages. Achieving this objective would significantly expand the application range of LFP batteries and strengthen their position in the competitive landscape of energy storage technologies.
The evolution of LFP battery technology has been marked by several key advancements, including nano-sizing of active materials, carbon coating techniques, and doping strategies. Despite these improvements, the inherent limitations of LFP chemistry at low temperatures persist, primarily due to slower lithium-ion diffusion kinetics and increased electrolyte resistance when temperatures drop below 0°C. This results in reduced capacity, diminished power capability, and accelerated degradation during cold-weather operation.
Current market trends indicate a growing demand for batteries that can maintain performance across a wide temperature range, driven by the expanding electric vehicle market in regions with cold climates and the increasing deployment of stationary energy storage systems in diverse geographical locations. The ability to operate efficiently at low temperatures has become a key differentiator in battery technology selection for many applications.
The technical objectives for improving LFP battery low-temperature performance center around three critical areas: electrolyte optimization, solid electrolyte interphase (SEI) engineering, and power delivery enhancement. Electrolyte formulations must balance low-temperature fluidity with electrochemical stability. SEI formation and properties significantly impact lithium-ion transport at the electrode-electrolyte interface, particularly at reduced temperatures. Power capability at low temperatures requires addressing both materials and system-level design considerations.
Recent research indicates promising directions in developing novel electrolyte additives that can modify SEI composition and structure, creating more favorable conditions for ion transport at low temperatures. Additionally, advanced electrode designs incorporating hierarchical porosity and optimized conductive networks show potential for enhancing charge transfer kinetics in cold conditions.
The ultimate goal of this technical research is to develop LFP battery systems that maintain at least 80% of their room-temperature capacity and power capability at temperatures as low as -20°C, without compromising safety, cycle life, or cost advantages. Achieving this objective would significantly expand the application range of LFP batteries and strengthen their position in the competitive landscape of energy storage technologies.
Market Demand Analysis for Low-Temperature LFP Batteries
The global market for low-temperature LFP (Lithium Iron Phosphate) batteries is experiencing significant growth driven by increasing demand across multiple sectors. Electric vehicles represent the primary market segment, with a strong push for batteries that can maintain performance in cold climates. This demand is particularly acute in regions with harsh winters such as Northern Europe, Canada, and Northern China, where conventional LFP batteries suffer from substantial capacity and power loss at temperatures below 0°C.
Commercial fleet operators are emerging as key stakeholders in this market, seeking energy storage solutions that can function reliably year-round without operational disruptions. The logistics and transportation sectors require batteries that maintain consistent range and charging capabilities regardless of seasonal temperature variations, making low-temperature performance a critical purchasing factor.
Energy storage systems for renewable integration constitute another rapidly expanding market segment. Grid-scale storage installations in cold-climate regions need batteries that can maintain efficiency during winter months to ensure consistent power supply and grid stability. The residential energy storage market in these regions similarly demands solutions that perform reliably during cold seasons.
Consumer electronics manufacturers are increasingly incorporating LFP chemistry into their products due to its safety profile, but require improved low-temperature formulations to maintain user satisfaction in all environments. This represents a smaller but growing market segment with specific requirements for miniaturized low-temperature electrolyte solutions.
Market analysis indicates that the performance gap between room temperature and sub-zero operation represents a significant competitive differentiator. Batteries that can deliver at least 80% of rated capacity at -20°C command premium pricing, with customers willing to pay 15-30% more for this capability compared to standard LFP formulations.
The regulatory landscape is also influencing market demand, with several countries implementing or considering minimum performance standards for batteries in cold conditions. These regulations are particularly relevant for electric vehicles and emergency backup systems, creating compliance-driven demand for improved low-temperature formulations.
Industry forecasts suggest that the market for specialized low-temperature LFP batteries will grow at a compound annual rate exceeding the broader battery market, driven by the electrification of transportation in cold-climate regions and the expansion of renewable energy infrastructure worldwide. This growth trajectory presents significant opportunities for companies that can effectively address the electrolyte selection and SEI formation challenges that currently limit low-temperature performance.
Commercial fleet operators are emerging as key stakeholders in this market, seeking energy storage solutions that can function reliably year-round without operational disruptions. The logistics and transportation sectors require batteries that maintain consistent range and charging capabilities regardless of seasonal temperature variations, making low-temperature performance a critical purchasing factor.
Energy storage systems for renewable integration constitute another rapidly expanding market segment. Grid-scale storage installations in cold-climate regions need batteries that can maintain efficiency during winter months to ensure consistent power supply and grid stability. The residential energy storage market in these regions similarly demands solutions that perform reliably during cold seasons.
Consumer electronics manufacturers are increasingly incorporating LFP chemistry into their products due to its safety profile, but require improved low-temperature formulations to maintain user satisfaction in all environments. This represents a smaller but growing market segment with specific requirements for miniaturized low-temperature electrolyte solutions.
Market analysis indicates that the performance gap between room temperature and sub-zero operation represents a significant competitive differentiator. Batteries that can deliver at least 80% of rated capacity at -20°C command premium pricing, with customers willing to pay 15-30% more for this capability compared to standard LFP formulations.
The regulatory landscape is also influencing market demand, with several countries implementing or considering minimum performance standards for batteries in cold conditions. These regulations are particularly relevant for electric vehicles and emergency backup systems, creating compliance-driven demand for improved low-temperature formulations.
Industry forecasts suggest that the market for specialized low-temperature LFP batteries will grow at a compound annual rate exceeding the broader battery market, driven by the electrification of transportation in cold-climate regions and the expansion of renewable energy infrastructure worldwide. This growth trajectory presents significant opportunities for companies that can effectively address the electrolyte selection and SEI formation challenges that currently limit low-temperature performance.
Current Challenges in LFP Battery Low-Temperature Operation
LFP (Lithium Iron Phosphate) batteries face significant performance degradation at low temperatures, primarily manifested as reduced capacity, diminished power capability, and accelerated aging. The fundamental challenge stems from the inherent physicochemical properties of LFP cathode materials and their interaction with electrolytes at reduced temperatures.
The ionic conductivity of conventional electrolytes decreases substantially below 0°C, resulting in higher internal resistance and limited lithium-ion transport. This effect is particularly pronounced in LFP batteries compared to other lithium-ion chemistries due to LFP's flat voltage profile and lower operating voltage, which provides less electrochemical driving force at low temperatures.
Solid Electrolyte Interphase (SEI) formation dynamics change dramatically in cold conditions. The SEI layer, critical for battery stability, becomes less permeable to lithium ions at low temperatures, further impeding charge transfer. Additionally, lithium plating becomes more probable during charging operations below 0°C, irreversibly consuming active lithium and potentially creating safety hazards.
The intercalation kinetics at the LFP cathode slow significantly at reduced temperatures, creating a bottleneck in the electrochemical reaction. This limitation is exacerbated by LFP's inherently lower electronic conductivity compared to other cathode materials like NMC or NCA.
Current electrolyte formulations struggle to balance low-temperature performance with other critical parameters such as cycle life, safety, and high-temperature stability. Conventional carbonate-based electrolytes with LiPF6 salt exhibit poor low-temperature performance, while alternatives that perform better in cold conditions often compromise other performance metrics or increase costs significantly.
The graphite anode used in most LFP batteries exhibits sluggish lithium intercalation kinetics at low temperatures, contributing to capacity fade and power limitations. This challenge is compounded by the tendency of lithium to plate on the anode surface rather than intercalate into the graphite structure when charging occurs at low temperatures.
Energy density trade-offs present another significant challenge. Modifications to improve low-temperature performance, such as electrolyte additives or alternative salts, often reduce the energy density of the battery system, creating a difficult engineering compromise.
Manufacturing consistency becomes more critical for low-temperature applications, as minor variations in electrode composition, electrolyte formulation, or cell assembly can disproportionately affect cold-weather performance. This increases production complexity and quality control requirements.
The ionic conductivity of conventional electrolytes decreases substantially below 0°C, resulting in higher internal resistance and limited lithium-ion transport. This effect is particularly pronounced in LFP batteries compared to other lithium-ion chemistries due to LFP's flat voltage profile and lower operating voltage, which provides less electrochemical driving force at low temperatures.
Solid Electrolyte Interphase (SEI) formation dynamics change dramatically in cold conditions. The SEI layer, critical for battery stability, becomes less permeable to lithium ions at low temperatures, further impeding charge transfer. Additionally, lithium plating becomes more probable during charging operations below 0°C, irreversibly consuming active lithium and potentially creating safety hazards.
The intercalation kinetics at the LFP cathode slow significantly at reduced temperatures, creating a bottleneck in the electrochemical reaction. This limitation is exacerbated by LFP's inherently lower electronic conductivity compared to other cathode materials like NMC or NCA.
Current electrolyte formulations struggle to balance low-temperature performance with other critical parameters such as cycle life, safety, and high-temperature stability. Conventional carbonate-based electrolytes with LiPF6 salt exhibit poor low-temperature performance, while alternatives that perform better in cold conditions often compromise other performance metrics or increase costs significantly.
The graphite anode used in most LFP batteries exhibits sluggish lithium intercalation kinetics at low temperatures, contributing to capacity fade and power limitations. This challenge is compounded by the tendency of lithium to plate on the anode surface rather than intercalate into the graphite structure when charging occurs at low temperatures.
Energy density trade-offs present another significant challenge. Modifications to improve low-temperature performance, such as electrolyte additives or alternative salts, often reduce the energy density of the battery system, creating a difficult engineering compromise.
Manufacturing consistency becomes more critical for low-temperature applications, as minor variations in electrode composition, electrolyte formulation, or cell assembly can disproportionately affect cold-weather performance. This increases production complexity and quality control requirements.
Current Electrolyte Formulations for Cold Climate Performance
01 Electrolyte modifications for low-temperature performance
Specialized electrolyte formulations can significantly improve the low-temperature capability of LFP batteries. These include using low-freezing-point solvents, electrolyte additives, and optimized salt concentrations that maintain ionic conductivity at low temperatures. Such modifications reduce electrolyte viscosity and improve lithium-ion transport kinetics in cold conditions, enabling better charge/discharge performance when conventional LFP batteries would typically suffer from reduced capacity and power output.- Electrolyte modifications for low-temperature performance: Specialized electrolyte formulations can significantly improve the low-temperature performance of LFP batteries. These include using low-freezing-point solvents, electrolyte additives, and optimized salt concentrations that maintain ionic conductivity at low temperatures. Such modifications reduce electrolyte viscosity and improve lithium-ion transport kinetics in cold conditions, enabling better charge/discharge capabilities when conventional LFP batteries would typically suffer from reduced capacity and power output.
- Cathode material engineering: Engineering the LFP cathode material structure can enhance low-temperature performance. Approaches include nano-sizing particles, surface coating, doping with conductive elements, and creating hierarchical structures. These modifications improve electron transport pathways, reduce charge transfer resistance, and facilitate lithium-ion diffusion at low temperatures, resulting in better capacity retention and rate capability when operating in cold environments.
- Battery thermal management systems: Advanced thermal management systems can maintain optimal operating temperatures for LFP batteries in cold environments. These include integrated heating elements, insulation materials, phase change materials, and intelligent temperature control systems. By keeping the battery within an ideal temperature range, these systems prevent the significant performance degradation typically experienced by LFP batteries at low temperatures, enabling consistent power output and extended cycle life.
- Composite electrode structures: Creating composite electrode structures by combining LFP with other materials can improve low-temperature performance. These composites often incorporate carbon-based materials (graphene, carbon nanotubes), conductive polymers, or other lithium-ion battery materials with better inherent low-temperature characteristics. The resulting hybrid electrodes provide enhanced electronic conductivity and ion diffusion pathways at low temperatures, mitigating the typical limitations of standard LFP cathodes.
- Cell design and manufacturing optimizations: Optimizing cell design and manufacturing processes can significantly improve LFP battery low-temperature capability. This includes adjusting electrode thickness and porosity, optimizing the ratio of active materials to conductive additives, enhancing electrode-electrolyte interfaces, and implementing specialized formation protocols. These refinements reduce internal resistance and improve charge transfer kinetics at low temperatures, resulting in better cold-weather performance without requiring changes to the basic LFP chemistry.
02 Cathode material engineering
Engineering the LFP cathode material structure can enhance low-temperature performance. Approaches include nano-sizing particles, carbon coating, doping with conductive elements, and creating hierarchical structures. These modifications improve electron transport pathways, reduce charge transfer resistance, and facilitate lithium-ion diffusion at low temperatures, resulting in better capacity retention and rate capability when operating in cold environments.Expand Specific Solutions03 Battery thermal management systems
Advanced thermal management systems can maintain optimal operating temperatures for LFP batteries in cold environments. These include integrated heating elements, insulation materials, phase change materials, and intelligent temperature control systems. By keeping the battery within an ideal temperature range, these systems prevent the significant performance degradation typically experienced by LFP batteries at low temperatures, enabling consistent power output and extended cycle life.Expand Specific Solutions04 Anode modifications and composite materials
Modifying the anode structure or using composite materials can improve low-temperature performance of LFP batteries. Techniques include using silicon-carbon composites, lithium titanate, or specially treated graphite with expanded interlayer spacing. These modifications facilitate lithium-ion intercalation at low temperatures, reduce lithium plating risk, and maintain higher capacity utilization when operating in cold conditions compared to conventional graphite anodes.Expand Specific Solutions05 Cell design and manufacturing optimizations
Optimized cell design and manufacturing processes can enhance low-temperature performance of LFP batteries. These include electrode thickness optimization, improved current collector designs, enhanced electrode-electrolyte interfaces, and specialized formation protocols. Such optimizations reduce internal resistance, improve ion transport pathways, and create more uniform current distribution, allowing LFP batteries to deliver better power and energy at low temperatures.Expand Specific Solutions
Key Industry Players in Advanced LFP Battery Technology
The LFP battery low-temperature capability market is currently in a growth phase, with increasing demand driven by electric vehicle adoption despite performance challenges in cold conditions. The global market for LFP batteries is projected to expand significantly as manufacturers address temperature limitations. Leading players like LG Energy Solution, BYD, and SK On are investing heavily in electrolyte optimization to improve low-temperature performance. Research institutions including Dalian Institute of Chemical Physics and California Institute of Technology are collaborating with companies such as Guangzhou Tinci Materials Technology and Central Glass to develop advanced electrolyte formulations that enhance SEI formation. The technology is approaching maturity with companies like Toyota and A123 Systems demonstrating promising results in stabilizing power output at sub-zero temperatures through innovative electrolyte solutions.
LG Chem Ltd.
Technical Solution: LG Chem has developed a comprehensive approach to improving LFP battery low-temperature performance through their "Cold-Optimized Electrolyte Technology." Their solution incorporates a carefully balanced electrolyte formulation with low-freezing-point linear carbonates and ethers that maintain fluidity below -20°C. The company utilizes dual-salt electrolyte systems combining conventional LiPF6 with lithium bis(fluorosulfonyl)imide (LiFSI) to enhance ionic conductivity at low temperatures. Their proprietary electrolyte additives, including fluorinated ethylene carbonate derivatives, form a more conductive and stable SEI layer that facilitates lithium-ion transport even in cold conditions. LG Chem employs nano-engineered carbon coatings on LFP particles that improve electronic conductivity throughout the electrode at all temperatures. Their cell design features optimized electrode architectures with controlled porosity and tortuosity specifically designed to maintain ion transport pathways in cold environments, while their thermal management system incorporates intelligent preheating strategies that efficiently prepare cells for operation in cold conditions.
Strengths: Comprehensive research capabilities across the entire battery value chain; excellent manufacturing quality control; strong integration with battery management systems. Weaknesses: Higher production costs compared to standard LFP formulations; slightly lower energy density due to cold-optimized design; requires sophisticated thermal management for optimal performance.
LG Energy Solution Ltd.
Technical Solution: LG Energy Solution has developed advanced LFP battery technology with enhanced low-temperature performance through their proprietary "Low-T Electrolyte System." This system employs a multi-solvent approach combining traditional carbonates with low-viscosity co-solvents like methyl acetate and fluoroethers that maintain fluidity at sub-zero temperatures. Their electrolyte formulation incorporates lithium bis(fluorosulfonyl)imide (LiFSI) salt which exhibits superior ionic conductivity at low temperatures compared to conventional LiPF6. LG's technology includes engineered electrolyte additives like vinylene carbonate derivatives that form a more ion-conductive SEI layer optimized for cold conditions. The company utilizes a gradient cathode structure with varying carbon coating thicknesses to balance low-temperature power delivery with overall energy density. Their cell design features optimized electrode porosity and tortuosity specifically engineered to maintain lithium-ion transport pathways in cold environments, while their advanced battery management system incorporates predictive temperature modeling to optimize performance across varying conditions.
Strengths: Excellent cold-cranking performance even at extreme temperatures; superior manufacturing consistency and quality control; comprehensive battery management capabilities. Weaknesses: Slightly higher cost compared to standard LFP formulations; trade-off between low-temperature performance and maximum energy density; requires sophisticated thermal management systems for optimal performance.
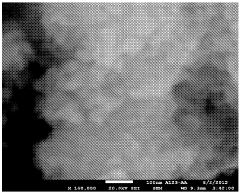
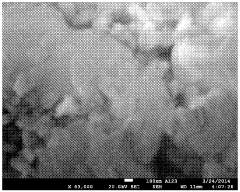
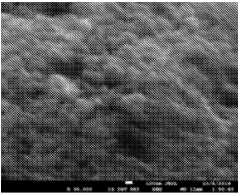
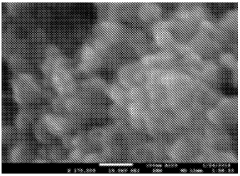
SEI Formation Mechanisms and Their Impact on Power Output
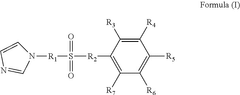
Electrolyte for lithium iron phosphate battery, and lithium iron phosphate battery
PatentPendingUS20250279473A1
Innovation
- An electrolytic solution for lithium iron phosphate batteries is formulated by incorporating a phenyl sulfonate compound and vinylene carbonate, forming a sulfur-rich solid electrolyte interface (SEI) film that enhances low-temperature cycle performance and reduces impedance.
High power electrode materials
PatentWO2015134948A1
Innovation
- A high-purity ammonium iron phosphate precursor, spheniscidite, is synthesized to produce LFP active materials with specific particle sizes and surface areas, resulting in improved electrochemical properties, including increased power and capacity retention at low temperatures.
Material Supply Chain Considerations for Advanced Electrolytes
The supply chain for advanced electrolytes represents a critical factor in the widespread adoption of LFP batteries with enhanced low-temperature capabilities. Current electrolyte formulations for low-temperature LFP applications typically incorporate additives such as fluoroethylene carbonate (FEC), vinylene carbonate (VC), and lithium difluoro(oxalato)borate (LiDFOB), which face varying degrees of supply constraints.
Key raw materials for these advanced electrolytes, particularly fluorinated compounds, rely heavily on mining operations concentrated in specific geographical regions. China currently dominates the production of lithium salts for electrolytes, controlling approximately 70% of global capacity, while Japan and South Korea lead in high-purity solvent manufacturing. This concentration creates potential vulnerabilities in the supply chain, especially as demand for low-temperature LFP batteries increases.
Production scaling presents another significant challenge. Many novel electrolyte components that show promising low-temperature performance in laboratory settings face substantial hurdles in scaling to commercial production volumes. The specialized equipment and stringent quality control requirements for high-purity electrolyte production further complicate capacity expansion efforts.
Price volatility remains a persistent concern across the electrolyte supply chain. The cost of lithium salts, particularly LiPF6 and newer alternatives like LiFSI, has experienced significant fluctuations over the past five years. These price instabilities directly impact the economic viability of advanced low-temperature electrolyte formulations, potentially slowing their market penetration.
Environmental and regulatory considerations also influence supply chain dynamics. The production of fluorinated compounds for electrolytes generates substantial environmental impacts, leading to increasing regulatory scrutiny. Several regions have implemented or proposed restrictions on certain manufacturing processes, potentially limiting future supply availability.
Strategic partnerships between battery manufacturers and chemical suppliers have emerged as a mitigation strategy for supply chain risks. Companies like CATL and BYD have established joint ventures with electrolyte producers to secure stable supply channels for their low-temperature LFP battery production. These vertical integration efforts may reshape traditional supply chain structures in the coming years.
Recycling infrastructure for advanced electrolytes remains underdeveloped compared to other battery components. While lithium and transition metals receive significant attention in battery recycling efforts, electrolyte recovery technologies are still nascent. Developing efficient recycling pathways could alleviate long-term supply constraints for critical electrolyte components.
Key raw materials for these advanced electrolytes, particularly fluorinated compounds, rely heavily on mining operations concentrated in specific geographical regions. China currently dominates the production of lithium salts for electrolytes, controlling approximately 70% of global capacity, while Japan and South Korea lead in high-purity solvent manufacturing. This concentration creates potential vulnerabilities in the supply chain, especially as demand for low-temperature LFP batteries increases.
Production scaling presents another significant challenge. Many novel electrolyte components that show promising low-temperature performance in laboratory settings face substantial hurdles in scaling to commercial production volumes. The specialized equipment and stringent quality control requirements for high-purity electrolyte production further complicate capacity expansion efforts.
Price volatility remains a persistent concern across the electrolyte supply chain. The cost of lithium salts, particularly LiPF6 and newer alternatives like LiFSI, has experienced significant fluctuations over the past five years. These price instabilities directly impact the economic viability of advanced low-temperature electrolyte formulations, potentially slowing their market penetration.
Environmental and regulatory considerations also influence supply chain dynamics. The production of fluorinated compounds for electrolytes generates substantial environmental impacts, leading to increasing regulatory scrutiny. Several regions have implemented or proposed restrictions on certain manufacturing processes, potentially limiting future supply availability.
Strategic partnerships between battery manufacturers and chemical suppliers have emerged as a mitigation strategy for supply chain risks. Companies like CATL and BYD have established joint ventures with electrolyte producers to secure stable supply channels for their low-temperature LFP battery production. These vertical integration efforts may reshape traditional supply chain structures in the coming years.
Recycling infrastructure for advanced electrolytes remains underdeveloped compared to other battery components. While lithium and transition metals receive significant attention in battery recycling efforts, electrolyte recovery technologies are still nascent. Developing efficient recycling pathways could alleviate long-term supply constraints for critical electrolyte components.
Environmental Impact and Sustainability of Low-Temperature Solutions
The environmental implications of enhancing LFP battery low-temperature performance must be carefully considered as the industry pursues technological advancements. Current electrolyte modifications often involve fluorinated compounds and additives that, while effective for performance, may pose significant environmental challenges throughout their lifecycle.
Electrolyte formulations designed for low-temperature applications frequently contain fluorinated solvents and lithium salts that demonstrate persistence in the environment. These compounds resist natural degradation processes and can accumulate in ecosystems when improperly disposed of. The manufacturing processes for specialized low-temperature electrolytes also typically require more energy-intensive synthesis routes and purification steps, contributing to higher carbon footprints compared to standard electrolyte production.
The sustainability profile of SEI-modifying additives presents another area of concern. Many effective additives contain rare elements or environmentally problematic chemical structures. Life cycle assessments indicate that the environmental benefits of extended battery life and improved cold-weather performance must be weighed against the ecological impact of these specialized materials.
Recent research has begun exploring bio-derived solvents and naturally occurring compounds as alternatives to traditional electrolyte components. These green chemistry approaches show promise for reducing environmental impact while maintaining acceptable low-temperature performance. Several research groups have demonstrated carbonate alternatives derived from biomass that offer comparable conductivity at low temperatures with significantly reduced environmental persistence.
Recycling considerations also factor prominently in sustainability evaluations. Batteries optimized for low-temperature operation often contain more complex chemical mixtures, potentially complicating end-of-life recycling processes. The industry is developing specialized recycling protocols for these advanced formulations, though recovery rates for some specialized additives remain suboptimal.
From a holistic perspective, the environmental impact assessment must consider the entire application context. Improved low-temperature performance extends battery utility in cold climates, potentially reducing the need for replacement batteries and associated manufacturing impacts. Additionally, better cold-weather performance in electric vehicles reduces reliance on fossil fuel alternatives that might otherwise be preferred in cold regions, offering significant net environmental benefits despite the more complex chemistry involved.
Electrolyte formulations designed for low-temperature applications frequently contain fluorinated solvents and lithium salts that demonstrate persistence in the environment. These compounds resist natural degradation processes and can accumulate in ecosystems when improperly disposed of. The manufacturing processes for specialized low-temperature electrolytes also typically require more energy-intensive synthesis routes and purification steps, contributing to higher carbon footprints compared to standard electrolyte production.
The sustainability profile of SEI-modifying additives presents another area of concern. Many effective additives contain rare elements or environmentally problematic chemical structures. Life cycle assessments indicate that the environmental benefits of extended battery life and improved cold-weather performance must be weighed against the ecological impact of these specialized materials.
Recent research has begun exploring bio-derived solvents and naturally occurring compounds as alternatives to traditional electrolyte components. These green chemistry approaches show promise for reducing environmental impact while maintaining acceptable low-temperature performance. Several research groups have demonstrated carbonate alternatives derived from biomass that offer comparable conductivity at low temperatures with significantly reduced environmental persistence.
Recycling considerations also factor prominently in sustainability evaluations. Batteries optimized for low-temperature operation often contain more complex chemical mixtures, potentially complicating end-of-life recycling processes. The industry is developing specialized recycling protocols for these advanced formulations, though recovery rates for some specialized additives remain suboptimal.
From a holistic perspective, the environmental impact assessment must consider the entire application context. Improved low-temperature performance extends battery utility in cold climates, potentially reducing the need for replacement batteries and associated manufacturing impacts. Additionally, better cold-weather performance in electric vehicles reduces reliance on fossil fuel alternatives that might otherwise be preferred in cold regions, offering significant net environmental benefits despite the more complex chemistry involved.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!