Longitudinal wave behavior in chemotherapeutic drug delivery
AUG 13, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Longitudinal Wave Drug Delivery Background
Longitudinal waves have emerged as a promising approach in the field of chemotherapeutic drug delivery, offering new possibilities for targeted and efficient treatment of various cancers. This innovative technique leverages the unique properties of longitudinal waves to enhance drug penetration and distribution within tumor tissues.
The concept of using longitudinal waves in drug delivery stems from the broader field of acoustic-based therapies, which have been explored for decades in medical applications. Longitudinal waves, characterized by their ability to propagate through media by alternating compression and rarefaction, have shown potential in manipulating biological tissues and enhancing molecular transport.
In the context of chemotherapeutic drug delivery, longitudinal waves are being investigated for their ability to overcome traditional barriers in cancer treatment. These barriers include poor drug penetration into solid tumors, limited drug distribution within tumor tissues, and the development of drug resistance by cancer cells.
The application of longitudinal waves in this field aims to address several key challenges in cancer therapy. Firstly, it seeks to improve the delivery of chemotherapeutic agents to tumor sites by enhancing their penetration through biological barriers. Secondly, it aims to increase the intracellular uptake of drugs by cancer cells, potentially overcoming mechanisms of drug resistance. Lastly, it explores the possibility of achieving more uniform drug distribution within tumor tissues, which is crucial for effective treatment.
Research in this area has been driven by the need for more effective and less toxic cancer treatments. Traditional chemotherapy often suffers from systemic toxicity and limited efficacy due to poor drug delivery to tumor sites. The use of longitudinal waves offers a potential solution by providing a non-invasive method to enhance drug delivery specifically to target areas.
The development of this technology intersects with various scientific disciplines, including physics, bioengineering, pharmacology, and oncology. It requires a deep understanding of wave mechanics, tissue interactions, drug pharmacokinetics, and tumor biology. This interdisciplinary nature has led to collaborations between researchers from diverse fields, fostering innovation and rapid advancement in the area.
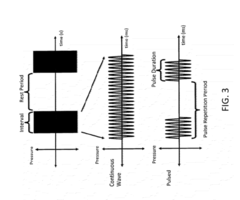
As research in this field progresses, there is growing interest in understanding the precise mechanisms by which longitudinal waves interact with biological tissues and drug molecules. This includes investigating how wave parameters such as frequency, amplitude, and duration affect drug delivery outcomes. Additionally, there is a focus on developing optimal wave generation and delivery systems that can be safely and effectively used in clinical settings.
The potential impact of this technology extends beyond cancer treatment. The principles and techniques developed for chemotherapeutic drug delivery using longitudinal waves may find applications in other areas of medicine, including the treatment of neurological disorders, cardiovascular diseases, and infectious diseases.
The concept of using longitudinal waves in drug delivery stems from the broader field of acoustic-based therapies, which have been explored for decades in medical applications. Longitudinal waves, characterized by their ability to propagate through media by alternating compression and rarefaction, have shown potential in manipulating biological tissues and enhancing molecular transport.
In the context of chemotherapeutic drug delivery, longitudinal waves are being investigated for their ability to overcome traditional barriers in cancer treatment. These barriers include poor drug penetration into solid tumors, limited drug distribution within tumor tissues, and the development of drug resistance by cancer cells.
The application of longitudinal waves in this field aims to address several key challenges in cancer therapy. Firstly, it seeks to improve the delivery of chemotherapeutic agents to tumor sites by enhancing their penetration through biological barriers. Secondly, it aims to increase the intracellular uptake of drugs by cancer cells, potentially overcoming mechanisms of drug resistance. Lastly, it explores the possibility of achieving more uniform drug distribution within tumor tissues, which is crucial for effective treatment.
Research in this area has been driven by the need for more effective and less toxic cancer treatments. Traditional chemotherapy often suffers from systemic toxicity and limited efficacy due to poor drug delivery to tumor sites. The use of longitudinal waves offers a potential solution by providing a non-invasive method to enhance drug delivery specifically to target areas.
The development of this technology intersects with various scientific disciplines, including physics, bioengineering, pharmacology, and oncology. It requires a deep understanding of wave mechanics, tissue interactions, drug pharmacokinetics, and tumor biology. This interdisciplinary nature has led to collaborations between researchers from diverse fields, fostering innovation and rapid advancement in the area.
As research in this field progresses, there is growing interest in understanding the precise mechanisms by which longitudinal waves interact with biological tissues and drug molecules. This includes investigating how wave parameters such as frequency, amplitude, and duration affect drug delivery outcomes. Additionally, there is a focus on developing optimal wave generation and delivery systems that can be safely and effectively used in clinical settings.
The potential impact of this technology extends beyond cancer treatment. The principles and techniques developed for chemotherapeutic drug delivery using longitudinal waves may find applications in other areas of medicine, including the treatment of neurological disorders, cardiovascular diseases, and infectious diseases.
Chemotherapy Market Analysis
The global chemotherapy market has experienced significant growth in recent years, driven by the increasing prevalence of cancer and advancements in drug delivery technologies. As of 2021, the market was valued at approximately $12.7 billion, with projections indicating a compound annual growth rate (CAGR) of 11.2% from 2022 to 2030. This growth is primarily attributed to the rising incidence of cancer worldwide, with an estimated 19.3 million new cancer cases reported in 2020 alone.
The market for chemotherapeutic drugs is segmented based on drug type, route of administration, and cancer type. Alkylating agents, antimetabolites, and plant alkaloids are among the most widely used drug types, collectively accounting for over 60% of the market share. Intravenous administration remains the dominant route, representing about 70% of the market, although oral chemotherapy is gaining traction due to its convenience and improved patient compliance.
Geographically, North America holds the largest market share, followed by Europe and Asia-Pacific. The United States, in particular, leads the market due to its advanced healthcare infrastructure and high healthcare expenditure. However, emerging economies in Asia-Pacific, such as China and India, are expected to witness the fastest growth rates in the coming years, driven by improving healthcare access and rising cancer awareness.
Key players in the chemotherapy market include F. Hoffmann-La Roche AG, Pfizer Inc., Merck & Co., Inc., and Bristol-Myers Squibb Company. These companies are investing heavily in research and development to introduce innovative drug delivery systems and targeted therapies. The trend towards personalized medicine and combination therapies is reshaping the competitive landscape, with a focus on improving efficacy while minimizing side effects.
The market is also witnessing a shift towards more targeted and less toxic treatments, such as immunotherapy and precision medicine. This trend is expected to impact the traditional chemotherapy market, potentially leading to a gradual decline in certain segments. However, the overall market is still projected to grow, driven by the increasing cancer burden and the development of novel drug delivery technologies, including those utilizing longitudinal wave behavior.
In conclusion, the chemotherapy market analysis reveals a dynamic and growing sector with significant opportunities for innovation in drug delivery methods. The integration of technologies like longitudinal wave behavior in chemotherapeutic drug delivery represents a promising avenue for improving treatment efficacy and patient outcomes, potentially reshaping the market landscape in the coming years.
The market for chemotherapeutic drugs is segmented based on drug type, route of administration, and cancer type. Alkylating agents, antimetabolites, and plant alkaloids are among the most widely used drug types, collectively accounting for over 60% of the market share. Intravenous administration remains the dominant route, representing about 70% of the market, although oral chemotherapy is gaining traction due to its convenience and improved patient compliance.
Geographically, North America holds the largest market share, followed by Europe and Asia-Pacific. The United States, in particular, leads the market due to its advanced healthcare infrastructure and high healthcare expenditure. However, emerging economies in Asia-Pacific, such as China and India, are expected to witness the fastest growth rates in the coming years, driven by improving healthcare access and rising cancer awareness.
Key players in the chemotherapy market include F. Hoffmann-La Roche AG, Pfizer Inc., Merck & Co., Inc., and Bristol-Myers Squibb Company. These companies are investing heavily in research and development to introduce innovative drug delivery systems and targeted therapies. The trend towards personalized medicine and combination therapies is reshaping the competitive landscape, with a focus on improving efficacy while minimizing side effects.
The market is also witnessing a shift towards more targeted and less toxic treatments, such as immunotherapy and precision medicine. This trend is expected to impact the traditional chemotherapy market, potentially leading to a gradual decline in certain segments. However, the overall market is still projected to grow, driven by the increasing cancer burden and the development of novel drug delivery technologies, including those utilizing longitudinal wave behavior.
In conclusion, the chemotherapy market analysis reveals a dynamic and growing sector with significant opportunities for innovation in drug delivery methods. The integration of technologies like longitudinal wave behavior in chemotherapeutic drug delivery represents a promising avenue for improving treatment efficacy and patient outcomes, potentially reshaping the market landscape in the coming years.
Current Challenges in Drug Delivery
Despite significant advancements in drug delivery systems, several challenges persist in the field of chemotherapeutic drug delivery, particularly when considering the application of longitudinal wave behavior. One of the primary obstacles is achieving precise targeting of cancer cells while minimizing damage to healthy tissues. The complex nature of tumor microenvironments and the heterogeneity of cancer cells make it difficult to design delivery systems that can effectively penetrate and distribute drugs throughout the tumor mass.
Another major challenge is overcoming biological barriers that hinder drug delivery. These include the blood-brain barrier, which restricts the passage of many chemotherapeutic agents to brain tumors, and the dense extracellular matrix of solid tumors, which impedes drug penetration. The use of longitudinal waves in drug delivery systems adds an additional layer of complexity to these challenges, as the interaction between waves and biological tissues must be carefully controlled to avoid unintended effects.
Drug resistance remains a significant hurdle in chemotherapy. Cancer cells often develop mechanisms to expel or neutralize drugs, reducing their efficacy over time. This necessitates the development of innovative delivery strategies that can overcome or bypass these resistance mechanisms. Incorporating longitudinal wave behavior into drug delivery systems presents both opportunities and challenges in addressing this issue, as it may offer new ways to enhance drug uptake and retention in cancer cells.
The stability and controlled release of drugs in delivery systems utilizing longitudinal waves is another area of concern. Ensuring that drugs remain stable during transport and are released at the desired rate and location within the body is crucial for effective treatment. The dynamic nature of wave-based delivery systems adds complexity to achieving this control, requiring advanced formulation techniques and precise engineering of delivery vehicles.
Furthermore, there is a need for improved real-time monitoring and feedback mechanisms in drug delivery systems. Current technologies often lack the ability to accurately track drug distribution and efficacy in real-time, making it challenging to optimize treatment protocols. Integrating sensing capabilities with longitudinal wave-based delivery systems could potentially address this issue but requires overcoming significant technical hurdles.
Lastly, the translation of promising laboratory results to clinical applications remains a significant challenge. Many innovative drug delivery approaches, including those utilizing longitudinal waves, show promise in preclinical studies but face difficulties in scaling up for human use. Regulatory hurdles, manufacturing complexities, and the need for extensive clinical trials can significantly delay or prevent the adoption of new delivery technologies in clinical practice.
Another major challenge is overcoming biological barriers that hinder drug delivery. These include the blood-brain barrier, which restricts the passage of many chemotherapeutic agents to brain tumors, and the dense extracellular matrix of solid tumors, which impedes drug penetration. The use of longitudinal waves in drug delivery systems adds an additional layer of complexity to these challenges, as the interaction between waves and biological tissues must be carefully controlled to avoid unintended effects.
Drug resistance remains a significant hurdle in chemotherapy. Cancer cells often develop mechanisms to expel or neutralize drugs, reducing their efficacy over time. This necessitates the development of innovative delivery strategies that can overcome or bypass these resistance mechanisms. Incorporating longitudinal wave behavior into drug delivery systems presents both opportunities and challenges in addressing this issue, as it may offer new ways to enhance drug uptake and retention in cancer cells.
The stability and controlled release of drugs in delivery systems utilizing longitudinal waves is another area of concern. Ensuring that drugs remain stable during transport and are released at the desired rate and location within the body is crucial for effective treatment. The dynamic nature of wave-based delivery systems adds complexity to achieving this control, requiring advanced formulation techniques and precise engineering of delivery vehicles.
Furthermore, there is a need for improved real-time monitoring and feedback mechanisms in drug delivery systems. Current technologies often lack the ability to accurately track drug distribution and efficacy in real-time, making it challenging to optimize treatment protocols. Integrating sensing capabilities with longitudinal wave-based delivery systems could potentially address this issue but requires overcoming significant technical hurdles.
Lastly, the translation of promising laboratory results to clinical applications remains a significant challenge. Many innovative drug delivery approaches, including those utilizing longitudinal waves, show promise in preclinical studies but face difficulties in scaling up for human use. Regulatory hurdles, manufacturing complexities, and the need for extensive clinical trials can significantly delay or prevent the adoption of new delivery technologies in clinical practice.
Existing Longitudinal Wave Solutions
01 Ultrasound-mediated drug delivery
Longitudinal waves, particularly ultrasound, can be used to enhance drug delivery. The acoustic waves can increase tissue permeability, allowing for better penetration of therapeutic agents. This method can be especially useful for targeted drug delivery in various medical applications.- Ultrasound-mediated drug delivery: Longitudinal waves, particularly ultrasound, are used to enhance drug delivery through various mechanisms. This approach can increase the permeability of biological barriers, improve drug penetration, and enable targeted delivery to specific tissues or organs. The technique can be applied to various drug formulations and delivery routes.
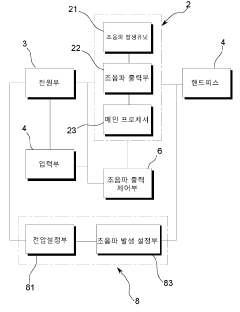
- Acoustic wave generators for drug delivery: Specialized devices and systems are developed to generate controlled longitudinal waves for drug delivery applications. These may include ultrasound transducers, piezoelectric elements, or other acoustic wave generators. The devices are designed to produce specific wave patterns, frequencies, and intensities optimized for drug delivery in different therapeutic contexts.
- Nanoparticle-based drug delivery with acoustic activation: Nanoparticles are engineered to respond to longitudinal waves, particularly ultrasound, for controlled drug release. These nanocarriers can be designed to release their payload upon exposure to specific acoustic stimuli, allowing for spatiotemporal control of drug delivery. This approach combines the benefits of nanoparticle drug carriers with the precision of acoustic activation.
- Transdermal drug delivery using longitudinal waves: Longitudinal waves are employed to enhance transdermal drug delivery by temporarily increasing skin permeability. This non-invasive approach can improve the absorption of topically applied medications, expand the range of drugs suitable for transdermal delivery, and provide an alternative to traditional injection methods for certain therapeutics.
- Combination of longitudinal waves with other drug delivery technologies: Longitudinal wave-based drug delivery is often combined with other technologies to enhance efficacy. This may include integration with electroporation, iontophoresis, or microneedle systems. Such combinations can synergistically improve drug penetration, targeting, and overall therapeutic outcomes across various medical applications.
02 Nanoparticle-based drug delivery systems
Nanoparticles can be designed to respond to longitudinal waves for controlled drug release. These systems can be triggered by external stimuli such as ultrasound or other forms of acoustic energy, allowing for precise spatial and temporal control of drug delivery.Expand Specific Solutions03 Transdermal drug delivery using acoustic waves
Longitudinal waves can be employed to enhance transdermal drug delivery. The acoustic energy can temporarily disrupt the skin barrier, allowing for improved penetration of drugs through the skin. This non-invasive approach can be beneficial for various therapeutic applications.Expand Specific Solutions04 Intracellular drug delivery using acoustic techniques
Longitudinal waves can be utilized to facilitate intracellular drug delivery. The acoustic energy can create temporary pores in cell membranes, allowing for the entry of therapeutic agents into cells. This approach can improve the efficacy of treatments targeting intracellular processes.Expand Specific Solutions05 Combination of longitudinal waves with other drug delivery technologies
Integrating longitudinal wave techniques with other drug delivery technologies can lead to synergistic effects. This may include combining acoustic methods with electroporation, microneedles, or other physical or chemical enhancers to improve drug delivery efficiency and targeting.Expand Specific Solutions
Key Players in Acoustic Drug Delivery
The longitudinal wave behavior in chemotherapeutic drug delivery is an emerging field with significant potential for improving cancer treatment. The market is in its early growth stage, with increasing research and development efforts. While the exact market size is not established, it is expected to expand rapidly due to the rising demand for targeted drug delivery systems. Technologically, the field is still evolving, with various companies and institutions at different stages of development. Key players like The Regents of the University of California, Intarcia Therapeutics, and Boston Scientific Scimed are making notable advancements, while academic institutions such as the University of North Carolina at Chapel Hill and Jilin University are contributing valuable research. The competitive landscape is diverse, with both established pharmaceutical companies and innovative startups vying for breakthroughs in this promising area.
The Regents of the University of California
Technical Solution: The University of California has developed a novel approach to longitudinal wave behavior in chemotherapeutic drug delivery using ultrasound-mediated drug release. Their technique employs focused ultrasound to trigger the release of drugs from nanocarriers at specific tumor sites. This method utilizes longitudinal acoustic waves to create localized pressure changes, causing the nanocarriers to release their payload in a controlled manner[1]. The university's researchers have also explored the use of phase-change nanodroplets that vaporize upon exposure to ultrasound, creating microbubbles that enhance drug penetration into tumor tissue[3]. This approach allows for precise spatial and temporal control of drug release, potentially improving the efficacy of chemotherapy while reducing systemic side effects[5].
Strengths: Highly targeted drug delivery, reduced systemic toxicity, and improved drug penetration into tumors. Weaknesses: Requires specialized equipment and may have limited effectiveness in deep-seated tumors.
Intarcia Therapeutics, Inc.
Technical Solution: Intarcia Therapeutics has developed the ITCA 650, a revolutionary osmotic mini-pump system for the continuous subcutaneous delivery of exenatide for type 2 diabetes treatment. While not directly related to longitudinal wave behavior in chemotherapeutic drug delivery, their technology demonstrates advanced controlled release mechanisms. The ITCA 650 utilizes osmotic pressure gradients to deliver a steady, long-term release of medication, which could potentially be adapted for chemotherapy applications[2]. The device is designed to be implanted subcutaneously and can deliver medication for up to 12 months, offering a novel approach to sustained drug delivery that could be explored for cancer treatment[4].
Strengths: Long-term continuous drug delivery, reduced patient adherence issues, and potential for adaptation to various therapeutic areas. Weaknesses: Invasive implantation procedure and limited flexibility in dosing adjustments.
Core Innovations in Wave Propagation
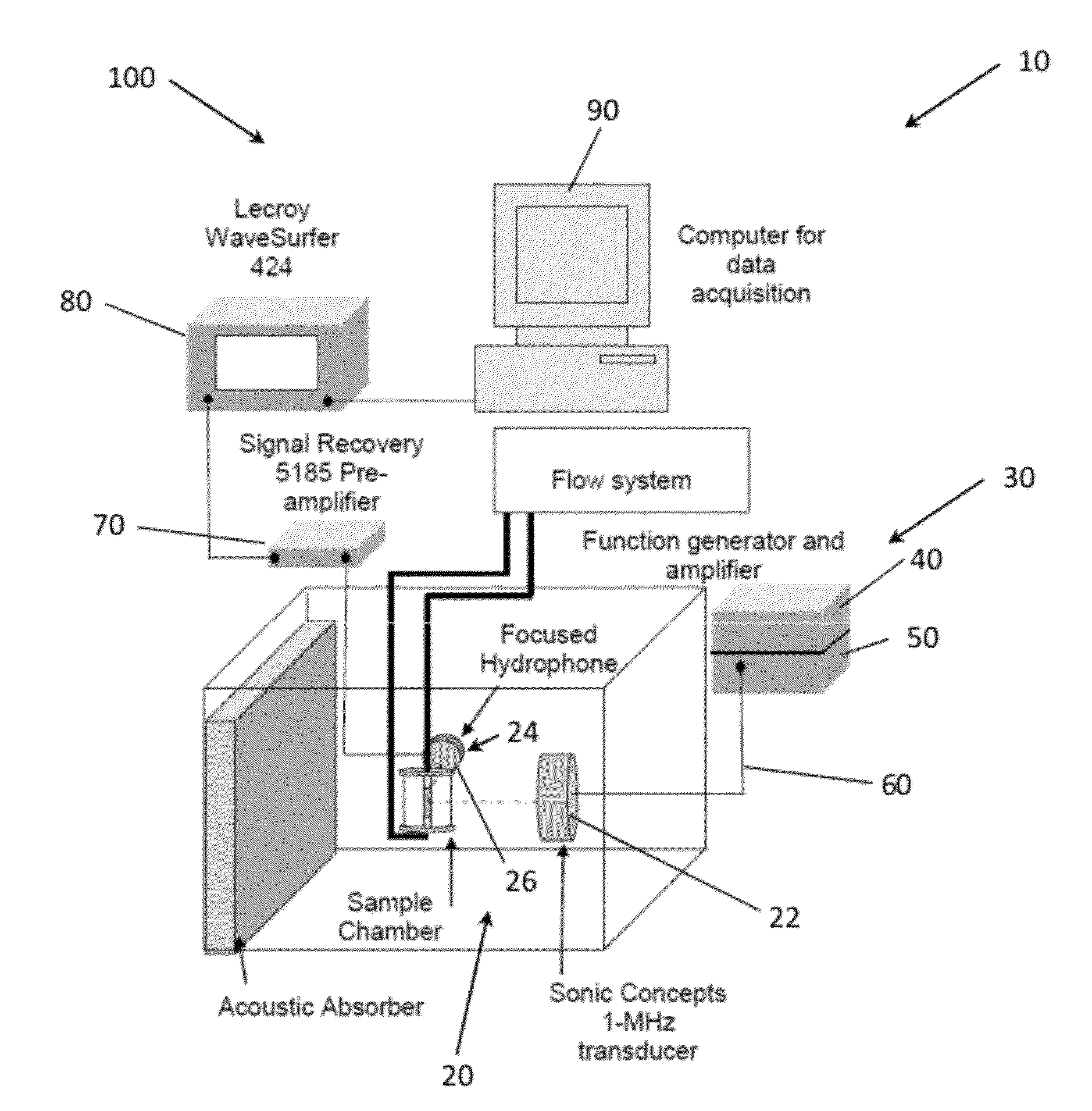
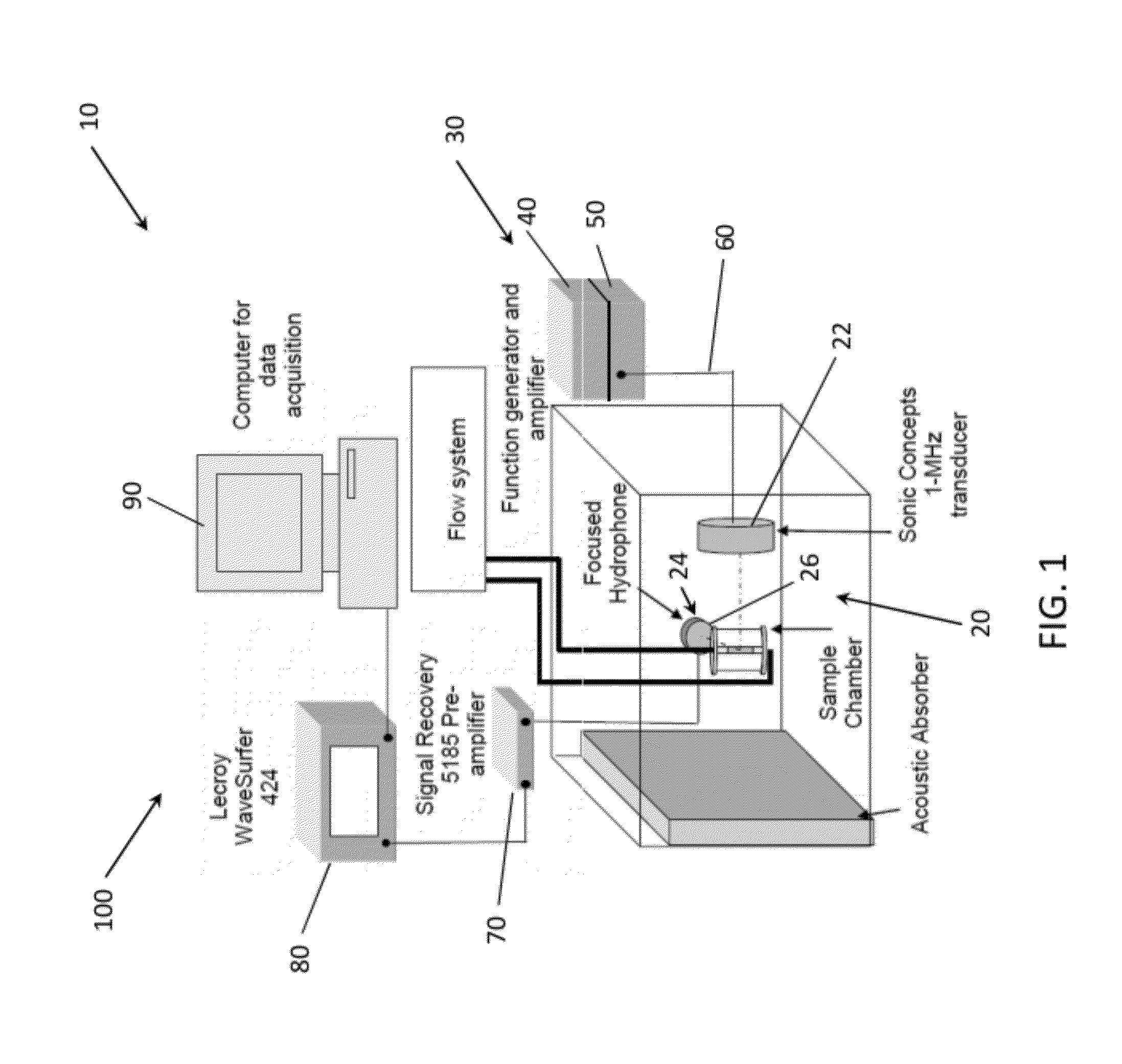
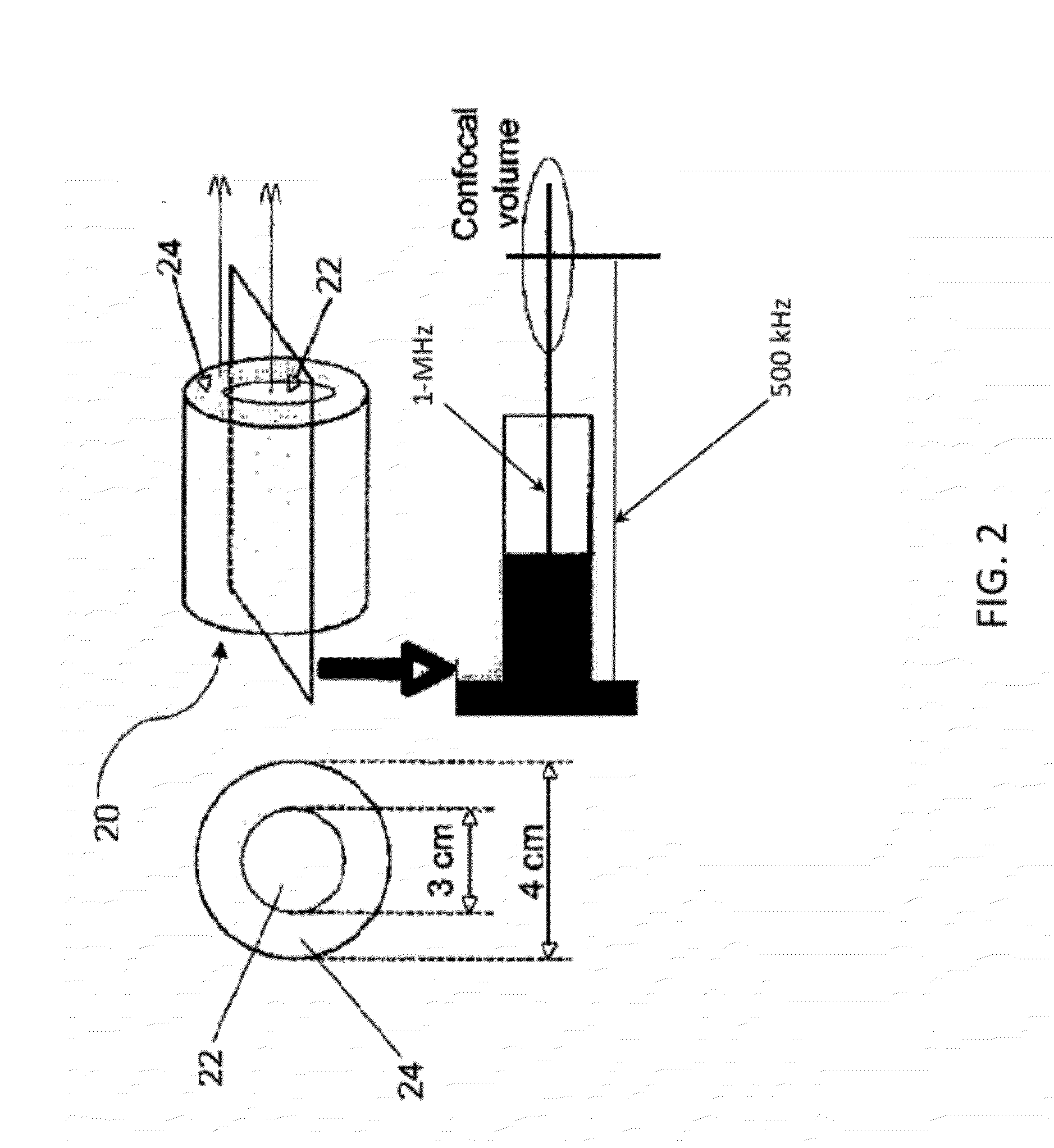
Methods of Enhancing Delivery of Drugs Using Ultrasonic Waves and Systems for Performing The Same
PatentActiveUS20120271167A1
Innovation
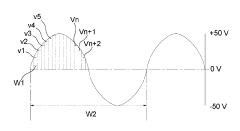
- The method involves administering vesicles containing a nucleating agent and therapeutic drug, combined with ultrasonic exposure at specific frequencies and amplitudes to induce and detect stable or inertial cavitation, enhancing drug absorption in targeted areas within the vascular system, using a system comprising a source transducer and detection transducer to intermittently provide ultrasonic exposure and monitor cavitation activity.
Drug delivery method using electrical stimulation and the system using the same
PatentActiveKR1020200129503A
Innovation
- A drug delivery system utilizing a sinusoidal waveform generated by dividing voltage into multiple sections and combining it with ultrasonic waves of varying frequencies to enhance skin permeation, featuring an ultrasonic oscillator, handpiece, and electrical stimulation generator with voltage and frequency control units.
Regulatory Framework for Novel Therapies
The regulatory framework for novel therapies in the context of longitudinal wave behavior in chemotherapeutic drug delivery is a complex and evolving landscape. As this innovative approach to drug delivery gains traction, regulatory bodies worldwide are adapting their guidelines to ensure patient safety while fostering scientific advancement.
In the United States, the Food and Drug Administration (FDA) has taken a proactive stance in developing regulatory pathways for such novel therapies. The FDA's Center for Drug Evaluation and Research (CDER) has established specific guidelines for the evaluation of drug delivery systems utilizing longitudinal wave technology. These guidelines emphasize the need for comprehensive preclinical studies to demonstrate the safety and efficacy of the delivery mechanism, as well as the drug itself.
The European Medicines Agency (EMA) has also recognized the potential of longitudinal wave-based drug delivery systems. The EMA's Committee for Medicinal Products for Human Use (CHMP) has issued guidance documents outlining the requirements for clinical trials and marketing authorization applications for these innovative therapies. These guidelines stress the importance of long-term safety data and the need for robust quality control measures in the manufacturing process.
In Japan, the Pharmaceuticals and Medical Devices Agency (PMDA) has implemented a fast-track approval process for breakthrough therapies, including those utilizing longitudinal wave technology for drug delivery. This approach aims to accelerate the development and approval of promising treatments while maintaining stringent safety standards.
Regulatory bodies are particularly focused on the potential off-target effects of longitudinal wave-based drug delivery systems. As a result, they require extensive toxicology studies and long-term follow-up data to assess any potential adverse effects on healthy tissues. Additionally, there is a growing emphasis on the need for standardized protocols for the calibration and quality assurance of devices used in longitudinal wave-based drug delivery.
The regulatory framework also addresses the unique challenges posed by the combination of a drug and a delivery device. Manufacturers must demonstrate the compatibility of the drug with the delivery system and provide evidence of consistent drug release profiles under various physiological conditions. This often necessitates the development of novel in vitro and in vivo models to accurately predict the behavior of the drug-device combination.
As the field of longitudinal wave-based drug delivery continues to advance, regulatory agencies are increasingly collaborating on a global scale to harmonize their approaches. The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) has initiated discussions to develop unified guidelines for the evaluation and approval of these novel therapies. This collaborative effort aims to streamline the regulatory process and facilitate the global development and availability of innovative treatments.
In the United States, the Food and Drug Administration (FDA) has taken a proactive stance in developing regulatory pathways for such novel therapies. The FDA's Center for Drug Evaluation and Research (CDER) has established specific guidelines for the evaluation of drug delivery systems utilizing longitudinal wave technology. These guidelines emphasize the need for comprehensive preclinical studies to demonstrate the safety and efficacy of the delivery mechanism, as well as the drug itself.
The European Medicines Agency (EMA) has also recognized the potential of longitudinal wave-based drug delivery systems. The EMA's Committee for Medicinal Products for Human Use (CHMP) has issued guidance documents outlining the requirements for clinical trials and marketing authorization applications for these innovative therapies. These guidelines stress the importance of long-term safety data and the need for robust quality control measures in the manufacturing process.
In Japan, the Pharmaceuticals and Medical Devices Agency (PMDA) has implemented a fast-track approval process for breakthrough therapies, including those utilizing longitudinal wave technology for drug delivery. This approach aims to accelerate the development and approval of promising treatments while maintaining stringent safety standards.
Regulatory bodies are particularly focused on the potential off-target effects of longitudinal wave-based drug delivery systems. As a result, they require extensive toxicology studies and long-term follow-up data to assess any potential adverse effects on healthy tissues. Additionally, there is a growing emphasis on the need for standardized protocols for the calibration and quality assurance of devices used in longitudinal wave-based drug delivery.
The regulatory framework also addresses the unique challenges posed by the combination of a drug and a delivery device. Manufacturers must demonstrate the compatibility of the drug with the delivery system and provide evidence of consistent drug release profiles under various physiological conditions. This often necessitates the development of novel in vitro and in vivo models to accurately predict the behavior of the drug-device combination.
As the field of longitudinal wave-based drug delivery continues to advance, regulatory agencies are increasingly collaborating on a global scale to harmonize their approaches. The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) has initiated discussions to develop unified guidelines for the evaluation and approval of these novel therapies. This collaborative effort aims to streamline the regulatory process and facilitate the global development and availability of innovative treatments.
Biocompatibility and Safety Considerations
Biocompatibility and safety considerations are paramount in the development and application of longitudinal wave-based chemotherapeutic drug delivery systems. The interaction between these acoustic waves and biological tissues must be carefully evaluated to ensure minimal adverse effects on healthy cells and organs.
One of the primary concerns is the potential for tissue damage caused by the mechanical forces exerted by longitudinal waves. Researchers must determine the optimal frequency and amplitude ranges that effectively deliver drugs without causing cellular disruption or necrosis. This involves extensive in vitro and in vivo studies to assess the impact of various wave parameters on different tissue types.
The generation of heat during wave propagation is another critical factor to consider. Excessive thermal energy can lead to unintended tissue ablation or protein denaturation. Implementing precise temperature monitoring and control mechanisms is essential to maintain safe operating conditions throughout the drug delivery process.
Potential immunological responses to the acoustic waves and their interaction with drug molecules must also be thoroughly investigated. The body's immune system may react to the physical stimuli or altered drug structures, potentially leading to inflammation or reduced therapeutic efficacy. Long-term studies are necessary to evaluate any chronic effects of repeated exposure to longitudinal waves.
The biocompatibility of materials used in wave-generating devices is equally important. Any components that come into direct contact with biological tissues must be non-toxic, non-immunogenic, and resistant to degradation in physiological environments. This includes transducer materials, coupling agents, and any implantable elements of the delivery system.
Safety considerations extend to the potential for unintended drug release or altered pharmacokinetics due to wave-induced changes in tissue permeability. Careful calibration of wave parameters and drug formulations is required to ensure controlled and targeted delivery without systemic side effects.
Regulatory compliance is a crucial aspect of developing longitudinal wave-based drug delivery systems. Adherence to FDA guidelines and international standards for medical devices and combination products is essential for clinical translation. This involves rigorous documentation of safety testing, risk assessments, and quality control measures throughout the development process.
One of the primary concerns is the potential for tissue damage caused by the mechanical forces exerted by longitudinal waves. Researchers must determine the optimal frequency and amplitude ranges that effectively deliver drugs without causing cellular disruption or necrosis. This involves extensive in vitro and in vivo studies to assess the impact of various wave parameters on different tissue types.
The generation of heat during wave propagation is another critical factor to consider. Excessive thermal energy can lead to unintended tissue ablation or protein denaturation. Implementing precise temperature monitoring and control mechanisms is essential to maintain safe operating conditions throughout the drug delivery process.
Potential immunological responses to the acoustic waves and their interaction with drug molecules must also be thoroughly investigated. The body's immune system may react to the physical stimuli or altered drug structures, potentially leading to inflammation or reduced therapeutic efficacy. Long-term studies are necessary to evaluate any chronic effects of repeated exposure to longitudinal waves.
The biocompatibility of materials used in wave-generating devices is equally important. Any components that come into direct contact with biological tissues must be non-toxic, non-immunogenic, and resistant to degradation in physiological environments. This includes transducer materials, coupling agents, and any implantable elements of the delivery system.
Safety considerations extend to the potential for unintended drug release or altered pharmacokinetics due to wave-induced changes in tissue permeability. Careful calibration of wave parameters and drug formulations is required to ensure controlled and targeted delivery without systemic side effects.
Regulatory compliance is a crucial aspect of developing longitudinal wave-based drug delivery systems. Adherence to FDA guidelines and international standards for medical devices and combination products is essential for clinical translation. This involves rigorous documentation of safety testing, risk assessments, and quality control measures throughout the development process.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!