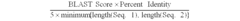Magnesium Nitrate’s Interaction with Biological Membrane Transport
AUG 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Mg(NO3)2 and Biomembrane Transport: Background and Objectives
Magnesium nitrate's interaction with biological membrane transport has emerged as a critical area of study in the fields of biochemistry and cellular biology. This research domain has evolved significantly over the past decades, driven by the growing recognition of magnesium's essential role in numerous physiological processes and the complex mechanisms governing its transport across cell membranes.
The historical context of this research can be traced back to the mid-20th century when scientists began to unravel the importance of magnesium in cellular functions. Initial studies focused on understanding the basic principles of ion transport across biological membranes, with magnesium gradually gaining attention as a vital player in these processes. As analytical techniques advanced, researchers were able to delve deeper into the specific mechanisms of magnesium transport and its interactions with various membrane components.
The evolution of this field has been marked by several key milestones. Early discoveries highlighted the presence of specific magnesium transporters in cell membranes, leading to the identification and characterization of proteins such as TRPM6 and TRPM7. Subsequent research revealed the intricate interplay between magnesium and other ions, particularly calcium, in cellular signaling and homeostasis.
In recent years, the focus has shifted towards understanding the role of magnesium nitrate specifically in membrane transport processes. This compound, composed of magnesium cations and nitrate anions, presents unique challenges and opportunities in biological systems. The nitrate component adds an additional layer of complexity to the transport mechanisms, potentially influencing cellular processes beyond those typically associated with magnesium alone.
The primary objectives of current research in this area are multifaceted. Firstly, there is a pressing need to elucidate the precise mechanisms by which magnesium nitrate interacts with and traverses biological membranes. This includes identifying specific transport proteins or channels involved, as well as understanding how the presence of nitrate affects these processes.
Secondly, researchers aim to investigate the physiological implications of magnesium nitrate transport. This encompasses studying its effects on cellular metabolism, signaling pathways, and overall cell function. Of particular interest is how the dual nature of magnesium nitrate – as both a source of magnesium and nitrate – influences cellular processes differently compared to other magnesium compounds.
Another key objective is to explore the potential applications of this knowledge in medical and agricultural fields. Understanding magnesium nitrate's interaction with biological membranes could lead to novel therapeutic approaches for magnesium-related disorders or innovative strategies for enhancing nutrient uptake in plants.
As research in this field progresses, it is anticipated that new technologies and methodologies will emerge, enabling more precise measurements and manipulations of magnesium nitrate transport at the cellular and molecular levels. This ongoing evolution of the field promises to yield valuable insights that could have far-reaching implications for our understanding of cellular biology and the development of new biotechnological applications.
The historical context of this research can be traced back to the mid-20th century when scientists began to unravel the importance of magnesium in cellular functions. Initial studies focused on understanding the basic principles of ion transport across biological membranes, with magnesium gradually gaining attention as a vital player in these processes. As analytical techniques advanced, researchers were able to delve deeper into the specific mechanisms of magnesium transport and its interactions with various membrane components.
The evolution of this field has been marked by several key milestones. Early discoveries highlighted the presence of specific magnesium transporters in cell membranes, leading to the identification and characterization of proteins such as TRPM6 and TRPM7. Subsequent research revealed the intricate interplay between magnesium and other ions, particularly calcium, in cellular signaling and homeostasis.
In recent years, the focus has shifted towards understanding the role of magnesium nitrate specifically in membrane transport processes. This compound, composed of magnesium cations and nitrate anions, presents unique challenges and opportunities in biological systems. The nitrate component adds an additional layer of complexity to the transport mechanisms, potentially influencing cellular processes beyond those typically associated with magnesium alone.
The primary objectives of current research in this area are multifaceted. Firstly, there is a pressing need to elucidate the precise mechanisms by which magnesium nitrate interacts with and traverses biological membranes. This includes identifying specific transport proteins or channels involved, as well as understanding how the presence of nitrate affects these processes.
Secondly, researchers aim to investigate the physiological implications of magnesium nitrate transport. This encompasses studying its effects on cellular metabolism, signaling pathways, and overall cell function. Of particular interest is how the dual nature of magnesium nitrate – as both a source of magnesium and nitrate – influences cellular processes differently compared to other magnesium compounds.
Another key objective is to explore the potential applications of this knowledge in medical and agricultural fields. Understanding magnesium nitrate's interaction with biological membranes could lead to novel therapeutic approaches for magnesium-related disorders or innovative strategies for enhancing nutrient uptake in plants.
As research in this field progresses, it is anticipated that new technologies and methodologies will emerge, enabling more precise measurements and manipulations of magnesium nitrate transport at the cellular and molecular levels. This ongoing evolution of the field promises to yield valuable insights that could have far-reaching implications for our understanding of cellular biology and the development of new biotechnological applications.
Market Analysis for Mg(NO3)2 in Biological Applications
The market for magnesium nitrate in biological applications has shown significant growth potential in recent years, driven by increasing research and development activities in the life sciences sector. The global market for magnesium compounds, including magnesium nitrate, is expected to expand steadily due to their diverse applications in pharmaceuticals, agriculture, and biotechnology.
In the pharmaceutical industry, magnesium nitrate has gained attention for its potential role in drug delivery systems and as a component in various formulations. The growing focus on personalized medicine and targeted drug delivery has created new opportunities for magnesium-based compounds, including magnesium nitrate, in the development of novel therapeutic approaches.
The agricultural sector represents another key market for magnesium nitrate, particularly in the production of fertilizers and plant growth enhancers. As global food demand continues to rise, there is an increasing need for efficient nutrient delivery systems in crop production. Magnesium nitrate's ability to provide both magnesium and nitrogen to plants makes it a valuable component in precision agriculture and sustainable farming practices.
Biotechnology and research laboratories constitute a growing market segment for magnesium nitrate. Its use in cell culture media, buffer solutions, and as a reagent in various biological assays has contributed to the steady demand from academic and industrial research institutions. The expansion of the biotechnology sector, particularly in emerging economies, is expected to further drive the market growth for magnesium nitrate in biological applications.
The healthcare sector, including nutraceuticals and dietary supplements, represents another potential growth area for magnesium nitrate. With increasing consumer awareness of the importance of magnesium in human health, there is a rising demand for magnesium-based supplements. While magnesium nitrate is not commonly used in dietary supplements due to its high nitrate content, research into its potential applications in this field may open new market opportunities.
Geographically, North America and Europe currently dominate the market for magnesium nitrate in biological applications, owing to their well-established life sciences industries and research infrastructure. However, the Asia-Pacific region is expected to witness the fastest growth in the coming years, driven by increasing investments in biotechnology, agriculture, and healthcare sectors in countries like China and India.
Despite the positive market outlook, challenges such as regulatory constraints and competition from alternative magnesium compounds may impact the growth trajectory of magnesium nitrate in biological applications. Ongoing research into its interactions with biological membrane transport systems will play a crucial role in expanding its market potential and addressing any safety concerns.
In the pharmaceutical industry, magnesium nitrate has gained attention for its potential role in drug delivery systems and as a component in various formulations. The growing focus on personalized medicine and targeted drug delivery has created new opportunities for magnesium-based compounds, including magnesium nitrate, in the development of novel therapeutic approaches.
The agricultural sector represents another key market for magnesium nitrate, particularly in the production of fertilizers and plant growth enhancers. As global food demand continues to rise, there is an increasing need for efficient nutrient delivery systems in crop production. Magnesium nitrate's ability to provide both magnesium and nitrogen to plants makes it a valuable component in precision agriculture and sustainable farming practices.
Biotechnology and research laboratories constitute a growing market segment for magnesium nitrate. Its use in cell culture media, buffer solutions, and as a reagent in various biological assays has contributed to the steady demand from academic and industrial research institutions. The expansion of the biotechnology sector, particularly in emerging economies, is expected to further drive the market growth for magnesium nitrate in biological applications.
The healthcare sector, including nutraceuticals and dietary supplements, represents another potential growth area for magnesium nitrate. With increasing consumer awareness of the importance of magnesium in human health, there is a rising demand for magnesium-based supplements. While magnesium nitrate is not commonly used in dietary supplements due to its high nitrate content, research into its potential applications in this field may open new market opportunities.
Geographically, North America and Europe currently dominate the market for magnesium nitrate in biological applications, owing to their well-established life sciences industries and research infrastructure. However, the Asia-Pacific region is expected to witness the fastest growth in the coming years, driven by increasing investments in biotechnology, agriculture, and healthcare sectors in countries like China and India.
Despite the positive market outlook, challenges such as regulatory constraints and competition from alternative magnesium compounds may impact the growth trajectory of magnesium nitrate in biological applications. Ongoing research into its interactions with biological membrane transport systems will play a crucial role in expanding its market potential and addressing any safety concerns.
Current Understanding and Challenges in Biomembrane Transport
The current understanding of magnesium nitrate's interaction with biological membrane transport is rooted in the broader context of ion transport mechanisms across cell membranes. Magnesium, as a divalent cation, plays crucial roles in various cellular processes, including enzyme activation, protein synthesis, and energy metabolism. The nitrate component, while less studied in this context, may influence membrane permeability and cellular ionic balance.
Recent research has elucidated several key aspects of magnesium transport across biological membranes. Dedicated magnesium transporters, such as TRPM6 and TRPM7 channels, have been identified as primary mediators of magnesium influx. These channels exhibit complex regulation mechanisms, responding to intracellular magnesium levels, pH, and other signaling molecules. Additionally, the SLC41 family of transporters has been implicated in magnesium homeostasis, although their exact mechanisms remain under investigation.
The interaction of magnesium nitrate with these transport systems presents unique challenges. The presence of nitrate ions may alter the electrochemical gradient across the membrane, potentially affecting the driving force for magnesium transport. Furthermore, the dissociation of magnesium nitrate in aqueous solutions creates a complex ionic environment that can influence the activity and selectivity of membrane transporters.
One of the primary challenges in understanding magnesium nitrate's membrane interactions lies in distinguishing between the effects of magnesium ions and those of the nitrate anion. While magnesium transport mechanisms are relatively well-characterized, the specific impact of nitrate on these processes is less clear. Some studies suggest that nitrate may compete with other anions for transport pathways, potentially modulating the overall ionic balance of the cell.
Another significant challenge is the variability of membrane composition across different cell types and organisms. The lipid and protein composition of membranes can dramatically affect their permeability to ions and small molecules. This heterogeneity complicates the development of a unified model for magnesium nitrate's interaction with biological membranes.
The dynamic nature of biological membranes adds another layer of complexity to this research area. Membrane fluidity, lipid raft formation, and protein-lipid interactions can all influence the behavior of ion transporters and channels. Understanding how these factors modulate magnesium nitrate's interaction with membrane transport systems requires sophisticated experimental approaches and modeling techniques.
Recent research has elucidated several key aspects of magnesium transport across biological membranes. Dedicated magnesium transporters, such as TRPM6 and TRPM7 channels, have been identified as primary mediators of magnesium influx. These channels exhibit complex regulation mechanisms, responding to intracellular magnesium levels, pH, and other signaling molecules. Additionally, the SLC41 family of transporters has been implicated in magnesium homeostasis, although their exact mechanisms remain under investigation.
The interaction of magnesium nitrate with these transport systems presents unique challenges. The presence of nitrate ions may alter the electrochemical gradient across the membrane, potentially affecting the driving force for magnesium transport. Furthermore, the dissociation of magnesium nitrate in aqueous solutions creates a complex ionic environment that can influence the activity and selectivity of membrane transporters.
One of the primary challenges in understanding magnesium nitrate's membrane interactions lies in distinguishing between the effects of magnesium ions and those of the nitrate anion. While magnesium transport mechanisms are relatively well-characterized, the specific impact of nitrate on these processes is less clear. Some studies suggest that nitrate may compete with other anions for transport pathways, potentially modulating the overall ionic balance of the cell.
Another significant challenge is the variability of membrane composition across different cell types and organisms. The lipid and protein composition of membranes can dramatically affect their permeability to ions and small molecules. This heterogeneity complicates the development of a unified model for magnesium nitrate's interaction with biological membranes.
The dynamic nature of biological membranes adds another layer of complexity to this research area. Membrane fluidity, lipid raft formation, and protein-lipid interactions can all influence the behavior of ion transporters and channels. Understanding how these factors modulate magnesium nitrate's interaction with membrane transport systems requires sophisticated experimental approaches and modeling techniques.
Existing Methodologies for Studying Mg(NO3)2-Membrane Interactions
01 Membrane-based magnesium nitrate separation
Membrane technology is utilized for the separation and purification of magnesium nitrate solutions. This process involves the use of specialized membranes that allow selective transport of magnesium and nitrate ions while rejecting impurities. The membrane-based separation can be applied in various industrial processes for magnesium nitrate production or recovery.- Membrane-based magnesium nitrate separation: Membrane technology is utilized for the separation and purification of magnesium nitrate solutions. This process involves the use of specialized membranes that allow selective transport of magnesium and nitrate ions, enabling efficient separation from other components in the solution. The membrane-based approach offers advantages in terms of energy efficiency and process control.
- Electrochemical transport of magnesium nitrate: Electrochemical methods are employed to facilitate the transport of magnesium nitrate across membranes. This technique involves the application of an electric field to drive the movement of magnesium and nitrate ions through ion-selective membranes. The process can be used for concentration, purification, or recovery of magnesium nitrate from various solutions.
- Magnesium nitrate transport in water treatment: Membrane transport of magnesium nitrate plays a crucial role in water treatment processes. This includes the removal of magnesium and nitrate ions from contaminated water sources, as well as the controlled addition of these ions for water quality adjustment. The transport mechanisms are optimized for efficient water purification and mineral balancing.
- Nanostructured membranes for magnesium nitrate transport: Advanced nanostructured membranes are developed to enhance the transport of magnesium nitrate. These membranes feature precisely engineered pore sizes and surface properties that improve selectivity and flux for magnesium and nitrate ions. The use of nanomaterials in membrane fabrication leads to improved performance in separation and purification processes.
- Magnesium nitrate transport in agricultural applications: Membrane transport systems are utilized for controlled delivery of magnesium nitrate in agricultural settings. This includes the use of semi-permeable membranes for slow-release fertilizer applications and precision nutrient delivery systems. The controlled transport of magnesium nitrate helps optimize plant nutrition and reduce environmental impact in agricultural practices.
02 Magnesium nitrate transport in biological membranes
Research focuses on the mechanisms of magnesium nitrate transport across biological membranes, such as cell membranes or plant tissues. This includes studies on ion channels, transporters, and other membrane proteins involved in facilitating the movement of magnesium and nitrate ions. Understanding these processes is crucial for applications in agriculture and biotechnology.Expand Specific Solutions03 Nanocomposite membranes for magnesium nitrate filtration
Development of nanocomposite membranes with enhanced properties for magnesium nitrate filtration and separation. These membranes incorporate nanomaterials to improve selectivity, permeability, and durability. The nanocomposite structure allows for better control of pore size and surface properties, leading to more efficient magnesium nitrate transport and separation.Expand Specific Solutions04 Electrochemical transport of magnesium nitrate
Electrochemical methods are employed to facilitate the transport of magnesium nitrate across membranes. This approach utilizes electric fields or potential differences to drive ion movement, enabling more controlled and efficient separation processes. Electrochemical transport can be applied in various applications, including water treatment and chemical processing.Expand Specific Solutions05 Magnesium nitrate transport in gas separation membranes
Investigation of magnesium nitrate transport phenomena in gas separation membranes. This research explores the behavior of magnesium nitrate in membrane-based gas separation processes, including its impact on membrane performance, selectivity, and stability. Understanding these interactions is essential for optimizing membrane materials and designs for specific gas separation applications.Expand Specific Solutions
Key Players in Magnesium Compound and Membrane Research
The field of magnesium nitrate's interaction with biological membrane transport is in its early developmental stages, with a growing market driven by increasing research in pharmaceutical and agricultural applications. The technology's maturity is still evolving, as evidenced by the diverse range of companies involved, from established pharmaceutical giants like Vertex Pharmaceuticals and Incyte Corp to agricultural leaders such as Pioneer Hi-Bred International. Academic institutions like the University of Copenhagen and Nanjing Agricultural University are also contributing significantly to the research. The competitive landscape is characterized by a mix of large corporations and specialized biotech firms, indicating a dynamic and potentially lucrative market with opportunities for both breakthrough innovations and incremental advancements in understanding biological membrane transport mechanisms.
Vertex Pharmaceuticals, Inc.
Technical Solution: Vertex Pharmaceuticals has applied its expertise in cystic fibrosis (CF) research to investigate magnesium nitrate's interaction with biological membrane transport. Their approach leverages their understanding of the CFTR protein, a chloride channel crucial for maintaining proper ion balance across cell membranes. By studying how magnesium nitrate influences CFTR function and other ion channels, Vertex aims to develop novel therapeutic strategies. Their research involves high-throughput screening of compound libraries to identify molecules that can modulate magnesium transport in conjunction with nitrate ions[3]. Additionally, Vertex employs advanced structural biology techniques, including cryo-EM, to visualize the molecular interactions between magnesium nitrate and membrane proteins[4].
Strengths: Extensive experience in membrane protein research, particularly ion channels. Advanced structural biology capabilities. Weaknesses: Primary focus on CF may limit broader applications of magnesium nitrate research.
University of Copenhagen
Technical Solution: The University of Copenhagen has made significant contributions to understanding magnesium nitrate's interaction with biological membrane transport through its Department of Plant and Environmental Sciences. Their research focuses on the role of magnesium and nitrate in plant nutrition and stress responses. Using advanced imaging techniques such as fluorescence microscopy and X-ray fluorescence, researchers have mapped the distribution and transport of magnesium and nitrate ions across plant cell membranes[7]. The university has also developed transgenic plant lines with altered magnesium transport capabilities to study the effects of magnesium nitrate on plant growth and development. Their findings have implications for improving crop nutrient use efficiency and understanding the environmental impact of nitrate fertilizers on magnesium uptake in plants[8].
Strengths: Specialized expertise in plant biology and environmental sciences. Strong focus on practical applications in agriculture. Weaknesses: Research primarily centered on plant systems, may require additional studies to extend findings to animal or human biology.
Breakthrough Studies on Mg(NO3)2 and Membrane Transport
Transporters and ion channels
PatentInactiveUS20040014945A1
Innovation
- The development of purified polypeptides and ion channels, referred to as TRICH, along with their encoding polynucleotides, which can be used for diagnostic, therapeutic, and preventive purposes, including the production of antibodies and recombinant polynucleotides for expression in cells or transgenic organisms.
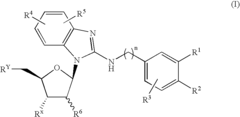
Benzimidazole derivatives and medical uses thereof
PatentInactiveUS20080038242A1
Innovation
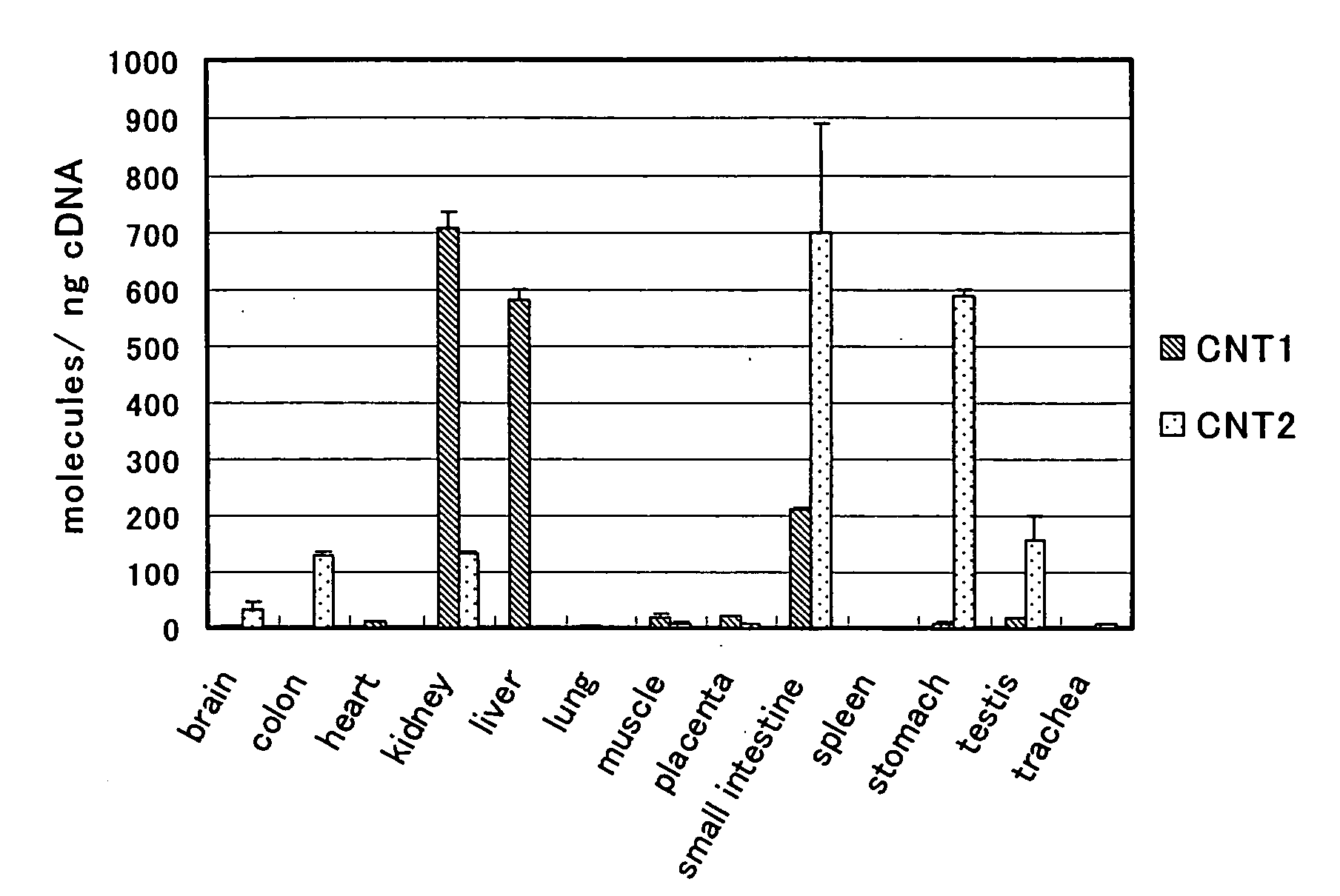
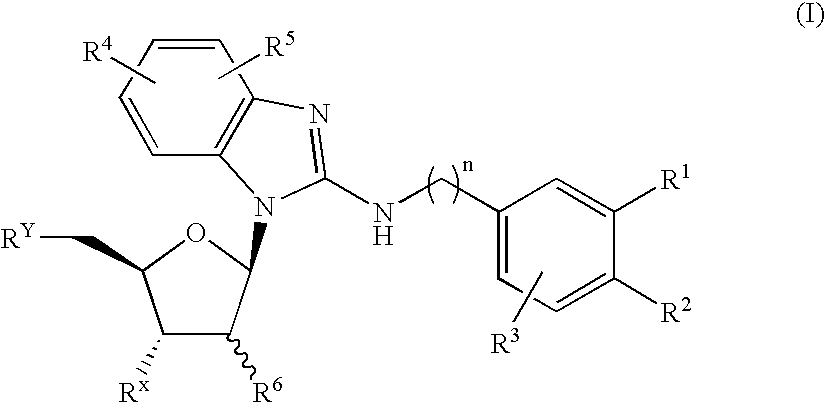
- A benzimidazole derivative glycosylated with D-ribose, specifically designed to inhibit sodium-dependent nucleoside transporter 2 (CNT2), which is predominantly expressed in the human small intestines, thereby reducing purine nucleoside absorption and plasma uric acid levels.
Safety and Toxicology Considerations of Mg(NO3)2 in Biological Systems
The safety and toxicology considerations of magnesium nitrate (Mg(NO3)2) in biological systems are crucial aspects to evaluate when studying its interaction with biological membrane transport. Magnesium nitrate, while essential for various physiological processes, can pose potential risks if not properly managed within biological systems.
One primary concern is the potential for nitrate toxicity. When magnesium nitrate dissociates in aqueous solutions, it releases nitrate ions, which can be harmful in high concentrations. Excessive nitrate intake has been associated with methemoglobinemia, a condition where the oxygen-carrying capacity of blood is reduced. This is particularly dangerous for infants and young children, whose digestive systems are more susceptible to nitrate conversion to nitrite.
The accumulation of nitrates in biological systems can also lead to eutrophication in aquatic environments, disrupting ecosystem balance. This indirect effect highlights the importance of considering not only immediate toxicological impacts but also broader environmental consequences of magnesium nitrate use.
Magnesium itself, while an essential nutrient, can cause adverse effects when present in excess. Hypermagnesemia, a condition characterized by abnormally high levels of magnesium in the blood, can lead to neuromuscular dysfunction, cardiac arrhythmias, and in severe cases, respiratory depression. Therefore, the dosage and administration of magnesium nitrate must be carefully controlled to prevent these potential complications.
The interaction of magnesium nitrate with biological membranes is another critical area of toxicological consideration. High concentrations of magnesium ions can alter membrane permeability and affect the function of various ion channels and transporters. This can disrupt cellular homeostasis and potentially lead to cell death if prolonged or severe.
Furthermore, the potential for magnesium nitrate to generate reactive nitrogen species (RNS) under certain conditions must be considered. RNS can cause oxidative stress and damage to cellular components, including proteins, lipids, and DNA. This oxidative damage can contribute to various pathological conditions and may have long-term health implications.
In assessing the safety of magnesium nitrate in biological systems, it is essential to consider its potential interactions with other substances. For instance, magnesium can interfere with the absorption and efficacy of certain medications, such as antibiotics and bisphosphonates. These interactions underscore the need for comprehensive toxicological evaluations in the context of diverse biological environments.
Lastly, the route of exposure to magnesium nitrate significantly influences its toxicological profile. Inhalation, ingestion, and dermal contact each present unique risks and require specific safety considerations. Occupational exposure limits and proper handling protocols must be established to minimize potential health hazards in industrial and research settings.
One primary concern is the potential for nitrate toxicity. When magnesium nitrate dissociates in aqueous solutions, it releases nitrate ions, which can be harmful in high concentrations. Excessive nitrate intake has been associated with methemoglobinemia, a condition where the oxygen-carrying capacity of blood is reduced. This is particularly dangerous for infants and young children, whose digestive systems are more susceptible to nitrate conversion to nitrite.
The accumulation of nitrates in biological systems can also lead to eutrophication in aquatic environments, disrupting ecosystem balance. This indirect effect highlights the importance of considering not only immediate toxicological impacts but also broader environmental consequences of magnesium nitrate use.
Magnesium itself, while an essential nutrient, can cause adverse effects when present in excess. Hypermagnesemia, a condition characterized by abnormally high levels of magnesium in the blood, can lead to neuromuscular dysfunction, cardiac arrhythmias, and in severe cases, respiratory depression. Therefore, the dosage and administration of magnesium nitrate must be carefully controlled to prevent these potential complications.
The interaction of magnesium nitrate with biological membranes is another critical area of toxicological consideration. High concentrations of magnesium ions can alter membrane permeability and affect the function of various ion channels and transporters. This can disrupt cellular homeostasis and potentially lead to cell death if prolonged or severe.
Furthermore, the potential for magnesium nitrate to generate reactive nitrogen species (RNS) under certain conditions must be considered. RNS can cause oxidative stress and damage to cellular components, including proteins, lipids, and DNA. This oxidative damage can contribute to various pathological conditions and may have long-term health implications.
In assessing the safety of magnesium nitrate in biological systems, it is essential to consider its potential interactions with other substances. For instance, magnesium can interfere with the absorption and efficacy of certain medications, such as antibiotics and bisphosphonates. These interactions underscore the need for comprehensive toxicological evaluations in the context of diverse biological environments.
Lastly, the route of exposure to magnesium nitrate significantly influences its toxicological profile. Inhalation, ingestion, and dermal contact each present unique risks and require specific safety considerations. Occupational exposure limits and proper handling protocols must be established to minimize potential health hazards in industrial and research settings.
Potential Applications in Pharmaceutical and Agricultural Industries
The potential applications of magnesium nitrate's interaction with biological membrane transport in pharmaceutical and agricultural industries are vast and promising. In the pharmaceutical sector, this interaction could be leveraged to enhance drug delivery systems. By understanding how magnesium nitrate affects membrane permeability, researchers can develop more efficient methods for transporting therapeutic compounds across cellular barriers. This could lead to improved bioavailability of drugs, potentially reducing dosage requirements and minimizing side effects.
Furthermore, the interaction between magnesium nitrate and biological membranes could be exploited to create novel drug formulations. For instance, magnesium nitrate-based nanocarriers could be designed to facilitate targeted drug delivery, allowing for more precise and effective treatments. This approach could be particularly beneficial in cancer therapies, where targeted delivery is crucial for maximizing therapeutic efficacy while minimizing damage to healthy tissues.
In the agricultural industry, the insights gained from studying magnesium nitrate's interaction with biological membranes could revolutionize crop protection and nutrient delivery systems. Magnesium nitrate-based fertilizers could be formulated to enhance nutrient uptake by plants, improving crop yields and resource efficiency. By optimizing the transport of essential nutrients across plant cell membranes, farmers could potentially reduce the amount of fertilizer needed while maintaining or even increasing crop productivity.
Moreover, this knowledge could be applied to develop more effective pesticides and herbicides. By manipulating the interaction between magnesium nitrate and cellular membranes, researchers could create formulations that selectively target pest organisms while minimizing impact on beneficial insects and plants. This could lead to more sustainable and environmentally friendly agricultural practices.
The potential applications extend to seed coating technologies as well. Magnesium nitrate-based coatings could be designed to enhance seed germination and early plant growth by facilitating the transport of essential nutrients across seed membranes. This could result in stronger, more resilient crops that are better equipped to withstand environmental stresses.
In both pharmaceutical and agricultural sectors, the study of magnesium nitrate's interaction with biological membrane transport opens up possibilities for developing smart delivery systems. These systems could be designed to respond to specific environmental triggers, releasing their payload only under predetermined conditions. Such precision in delivery mechanisms could significantly improve the efficiency and effectiveness of both medical treatments and agricultural inputs.
Furthermore, the interaction between magnesium nitrate and biological membranes could be exploited to create novel drug formulations. For instance, magnesium nitrate-based nanocarriers could be designed to facilitate targeted drug delivery, allowing for more precise and effective treatments. This approach could be particularly beneficial in cancer therapies, where targeted delivery is crucial for maximizing therapeutic efficacy while minimizing damage to healthy tissues.
In the agricultural industry, the insights gained from studying magnesium nitrate's interaction with biological membranes could revolutionize crop protection and nutrient delivery systems. Magnesium nitrate-based fertilizers could be formulated to enhance nutrient uptake by plants, improving crop yields and resource efficiency. By optimizing the transport of essential nutrients across plant cell membranes, farmers could potentially reduce the amount of fertilizer needed while maintaining or even increasing crop productivity.
Moreover, this knowledge could be applied to develop more effective pesticides and herbicides. By manipulating the interaction between magnesium nitrate and cellular membranes, researchers could create formulations that selectively target pest organisms while minimizing impact on beneficial insects and plants. This could lead to more sustainable and environmentally friendly agricultural practices.
The potential applications extend to seed coating technologies as well. Magnesium nitrate-based coatings could be designed to enhance seed germination and early plant growth by facilitating the transport of essential nutrients across seed membranes. This could result in stronger, more resilient crops that are better equipped to withstand environmental stresses.
In both pharmaceutical and agricultural sectors, the study of magnesium nitrate's interaction with biological membrane transport opens up possibilities for developing smart delivery systems. These systems could be designed to respond to specific environmental triggers, releasing their payload only under predetermined conditions. Such precision in delivery mechanisms could significantly improve the efficiency and effectiveness of both medical treatments and agricultural inputs.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!