Magnesium Nitrate’s Role in Stabilizing Flocculants in Mining
AUG 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Mg(NO3)2 in Flocculants
Magnesium nitrate plays a crucial role in stabilizing flocculants used in mining operations. This inorganic compound, with the chemical formula Mg(NO3)2, has gained significant attention in recent years due to its unique properties and versatile applications in the mining industry. The use of magnesium nitrate as a stabilizing agent for flocculants has evolved through several key stages, reflecting the industry's ongoing efforts to enhance efficiency and sustainability in mineral processing.
In the early stages of its application, magnesium nitrate was primarily used as a simple additive to improve the performance of conventional flocculants. However, as research progressed, its potential as a stabilizing agent became increasingly apparent. The turning point came with the development of advanced polymer-based flocculants, which required more sophisticated stabilization techniques to maintain their effectiveness in challenging mining environments.
The evolution of magnesium nitrate's role in flocculant stabilization can be traced through several milestone achievements. Initially, it was used in low concentrations to prevent the degradation of flocculants during storage and transportation. As understanding of its mechanisms grew, higher concentrations were employed to enhance the flocculation process itself, leading to improved settling rates and clearer supernatants in mineral separation processes.
A significant breakthrough occurred with the discovery of magnesium nitrate's synergistic effects when combined with certain polymer flocculants. This combination not only stabilized the flocculants but also enhanced their performance under a wider range of pH and temperature conditions, a critical factor in diverse mining operations. This development marked a shift from magnesium nitrate being a mere additive to becoming an integral component of advanced flocculant formulations.
Recent advancements have focused on optimizing the molecular interactions between magnesium nitrate and various types of flocculants. Researchers have developed novel methods to control the release of magnesium ions, allowing for more precise regulation of the flocculation process. This has led to the creation of "smart" flocculant systems that can adapt to changing conditions in real-time, significantly improving the efficiency and reliability of mineral separation processes.
The latest frontier in this field involves the integration of nanotechnology with magnesium nitrate-stabilized flocculants. Nanostructured magnesium nitrate compounds are being explored for their potential to provide unprecedented levels of stability and performance enhancement. These innovations promise to revolutionize the mining industry by enabling more effective recovery of fine particles and reducing water consumption in mineral processing operations.
In the early stages of its application, magnesium nitrate was primarily used as a simple additive to improve the performance of conventional flocculants. However, as research progressed, its potential as a stabilizing agent became increasingly apparent. The turning point came with the development of advanced polymer-based flocculants, which required more sophisticated stabilization techniques to maintain their effectiveness in challenging mining environments.
The evolution of magnesium nitrate's role in flocculant stabilization can be traced through several milestone achievements. Initially, it was used in low concentrations to prevent the degradation of flocculants during storage and transportation. As understanding of its mechanisms grew, higher concentrations were employed to enhance the flocculation process itself, leading to improved settling rates and clearer supernatants in mineral separation processes.
A significant breakthrough occurred with the discovery of magnesium nitrate's synergistic effects when combined with certain polymer flocculants. This combination not only stabilized the flocculants but also enhanced their performance under a wider range of pH and temperature conditions, a critical factor in diverse mining operations. This development marked a shift from magnesium nitrate being a mere additive to becoming an integral component of advanced flocculant formulations.
Recent advancements have focused on optimizing the molecular interactions between magnesium nitrate and various types of flocculants. Researchers have developed novel methods to control the release of magnesium ions, allowing for more precise regulation of the flocculation process. This has led to the creation of "smart" flocculant systems that can adapt to changing conditions in real-time, significantly improving the efficiency and reliability of mineral separation processes.
The latest frontier in this field involves the integration of nanotechnology with magnesium nitrate-stabilized flocculants. Nanostructured magnesium nitrate compounds are being explored for their potential to provide unprecedented levels of stability and performance enhancement. These innovations promise to revolutionize the mining industry by enabling more effective recovery of fine particles and reducing water consumption in mineral processing operations.
Mining Industry Demand
The mining industry's demand for effective flocculants has been steadily increasing due to the growing complexity of ore processing and the need for more efficient water management in mining operations. Flocculants play a crucial role in separating solid particles from liquid in various mining processes, including mineral extraction, tailings management, and water treatment. The use of magnesium nitrate as a stabilizing agent for flocculants has gained significant attention in recent years, as it addresses several key challenges faced by the mining sector.
One of the primary drivers for the demand of stabilized flocculants is the increasing focus on environmental sustainability and regulatory compliance. Mining companies are under pressure to reduce their water consumption and improve the quality of discharged water. Stabilized flocculants, enhanced by magnesium nitrate, offer improved performance in water clarification and solid-liquid separation, enabling more efficient water recycling and reducing the overall environmental footprint of mining operations.
The trend towards processing lower-grade ores has also contributed to the growing demand for more effective flocculants. As easily accessible high-grade deposits become depleted, mining companies are forced to extract minerals from ores with lower concentrations. This shift requires more sophisticated separation techniques, where stabilized flocculants can significantly improve process efficiency and mineral recovery rates.
Furthermore, the global expansion of mining activities into more challenging environments, such as arid regions or areas with complex geological formations, has intensified the need for robust flocculant solutions. Magnesium nitrate-stabilized flocculants have shown promise in maintaining their effectiveness under a wider range of conditions, including high temperatures and varying pH levels, making them particularly valuable in these demanding operational settings.
The economic benefits associated with improved flocculant performance have also driven market demand. Enhanced solid-liquid separation leads to faster settling rates, increased throughput, and reduced energy consumption in dewatering processes. These factors contribute to overall cost savings and improved operational efficiency, which are critical in an industry facing fluctuating commodity prices and increasing production costs.
Additionally, the mining industry's growing emphasis on automation and digital technologies has created a demand for more predictable and consistent flocculant performance. Stabilized flocculants offer greater reliability and control in automated systems, aligning with the industry's push towards smart mining practices and data-driven decision-making.
As the mining sector continues to evolve, the demand for innovative solutions like magnesium nitrate-stabilized flocculants is expected to grow. This trend is further supported by ongoing research and development efforts aimed at optimizing flocculant formulations and exploring new applications within the mining value chain.
One of the primary drivers for the demand of stabilized flocculants is the increasing focus on environmental sustainability and regulatory compliance. Mining companies are under pressure to reduce their water consumption and improve the quality of discharged water. Stabilized flocculants, enhanced by magnesium nitrate, offer improved performance in water clarification and solid-liquid separation, enabling more efficient water recycling and reducing the overall environmental footprint of mining operations.
The trend towards processing lower-grade ores has also contributed to the growing demand for more effective flocculants. As easily accessible high-grade deposits become depleted, mining companies are forced to extract minerals from ores with lower concentrations. This shift requires more sophisticated separation techniques, where stabilized flocculants can significantly improve process efficiency and mineral recovery rates.
Furthermore, the global expansion of mining activities into more challenging environments, such as arid regions or areas with complex geological formations, has intensified the need for robust flocculant solutions. Magnesium nitrate-stabilized flocculants have shown promise in maintaining their effectiveness under a wider range of conditions, including high temperatures and varying pH levels, making them particularly valuable in these demanding operational settings.
The economic benefits associated with improved flocculant performance have also driven market demand. Enhanced solid-liquid separation leads to faster settling rates, increased throughput, and reduced energy consumption in dewatering processes. These factors contribute to overall cost savings and improved operational efficiency, which are critical in an industry facing fluctuating commodity prices and increasing production costs.
Additionally, the mining industry's growing emphasis on automation and digital technologies has created a demand for more predictable and consistent flocculant performance. Stabilized flocculants offer greater reliability and control in automated systems, aligning with the industry's push towards smart mining practices and data-driven decision-making.
As the mining sector continues to evolve, the demand for innovative solutions like magnesium nitrate-stabilized flocculants is expected to grow. This trend is further supported by ongoing research and development efforts aimed at optimizing flocculant formulations and exploring new applications within the mining value chain.
Current Stabilization
The current stabilization methods for flocculants in mining operations primarily focus on enhancing their performance and longevity in challenging environments. Magnesium nitrate has emerged as a key component in these stabilization efforts, offering several advantages over traditional approaches.
One of the primary stabilization techniques involves the incorporation of magnesium nitrate into the flocculant formulation. This method has shown significant improvements in the stability of flocculants, particularly in high-temperature and high-salinity conditions often encountered in mining operations. The magnesium ions interact with the polymer chains of the flocculant, creating a more robust and resilient structure that can withstand harsh environmental factors.
Another current approach utilizes magnesium nitrate as a pre-treatment agent for the mining slurry before the addition of flocculants. This pre-conditioning step helps to optimize the ionic environment, facilitating better interaction between the flocculant and the suspended particles. The presence of magnesium ions can also enhance the bridging mechanism between particles, leading to more efficient flocculation.
Researchers have also explored the use of magnesium nitrate in combination with other stabilizing agents to create synergistic effects. For instance, the combination of magnesium nitrate with certain organic polymers has shown promising results in extending the shelf life of flocculants and maintaining their efficacy over prolonged periods in storage and during use.
In recent developments, nano-encapsulation techniques involving magnesium nitrate have been investigated. This approach aims to create a protective barrier around the flocculant molecules, shielding them from degradation factors such as UV radiation and microbial attack. The slow release of magnesium ions from these nano-capsules provides a sustained stabilizing effect throughout the flocculant's operational life.
The application of magnesium nitrate in stabilizing flocculants has also led to advancements in dosing strategies. Current methods often involve the use of automated dosing systems that can adjust the magnesium nitrate concentration in real-time based on the changing characteristics of the mining slurry. This adaptive approach ensures optimal stabilization while minimizing excess chemical usage.
Furthermore, the integration of magnesium nitrate into multi-component flocculant systems has gained traction. These systems combine different types of flocculants with magnesium nitrate acting as a stabilizer and performance enhancer. This approach allows for tailored solutions that can address the specific challenges of different mining operations and ore types.
One of the primary stabilization techniques involves the incorporation of magnesium nitrate into the flocculant formulation. This method has shown significant improvements in the stability of flocculants, particularly in high-temperature and high-salinity conditions often encountered in mining operations. The magnesium ions interact with the polymer chains of the flocculant, creating a more robust and resilient structure that can withstand harsh environmental factors.
Another current approach utilizes magnesium nitrate as a pre-treatment agent for the mining slurry before the addition of flocculants. This pre-conditioning step helps to optimize the ionic environment, facilitating better interaction between the flocculant and the suspended particles. The presence of magnesium ions can also enhance the bridging mechanism between particles, leading to more efficient flocculation.
Researchers have also explored the use of magnesium nitrate in combination with other stabilizing agents to create synergistic effects. For instance, the combination of magnesium nitrate with certain organic polymers has shown promising results in extending the shelf life of flocculants and maintaining their efficacy over prolonged periods in storage and during use.
In recent developments, nano-encapsulation techniques involving magnesium nitrate have been investigated. This approach aims to create a protective barrier around the flocculant molecules, shielding them from degradation factors such as UV radiation and microbial attack. The slow release of magnesium ions from these nano-capsules provides a sustained stabilizing effect throughout the flocculant's operational life.
The application of magnesium nitrate in stabilizing flocculants has also led to advancements in dosing strategies. Current methods often involve the use of automated dosing systems that can adjust the magnesium nitrate concentration in real-time based on the changing characteristics of the mining slurry. This adaptive approach ensures optimal stabilization while minimizing excess chemical usage.
Furthermore, the integration of magnesium nitrate into multi-component flocculant systems has gained traction. These systems combine different types of flocculants with magnesium nitrate acting as a stabilizer and performance enhancer. This approach allows for tailored solutions that can address the specific challenges of different mining operations and ore types.
Existing Mg(NO3)2 Use
01 Stabilization through pH control
Controlling the pH of magnesium nitrate solutions can significantly improve their stability. Maintaining the pH within a specific range, typically slightly acidic to neutral, helps prevent the formation of magnesium hydroxide precipitates and reduces the risk of decomposition. This method is often employed in industrial applications where stable magnesium nitrate solutions are required.- Stabilization through pH control: Controlling the pH of magnesium nitrate solutions can significantly improve their stability. Maintaining the pH within a specific range, typically slightly acidic to neutral, helps prevent the formation of insoluble magnesium hydroxide and reduces the risk of decomposition. This approach is often used in industrial applications where long-term stability of magnesium nitrate is crucial.
- Use of stabilizing additives: Incorporating certain additives into magnesium nitrate formulations can enhance their stability. These additives may include organic compounds, inorganic salts, or polymers that interact with magnesium nitrate to prevent degradation or crystallization. The choice of stabilizer depends on the specific application and environmental conditions.
- Temperature control for stability: Maintaining magnesium nitrate solutions within a specific temperature range is crucial for their stability. Extreme temperatures can lead to decomposition or unwanted crystallization. Proper storage and handling procedures, including temperature-controlled environments, are often implemented to ensure the long-term stability of magnesium nitrate in various applications.
- Encapsulation techniques: Encapsulating magnesium nitrate within protective matrices or coatings can significantly improve its stability. This approach isolates the compound from environmental factors that could cause degradation. Various encapsulation methods, such as microencapsulation or nanoencapsulation, are used depending on the specific requirements of the application.
- Anhydrous formulations for increased stability: Developing anhydrous forms of magnesium nitrate can lead to improved stability in certain applications. By removing water from the formulation, the risk of hydrolysis and other water-mediated degradation processes is reduced. This approach is particularly useful in applications where moisture sensitivity is a concern.
02 Use of stabilizing additives
Incorporating certain additives into magnesium nitrate formulations can enhance their stability. These additives may include organic compounds, inorganic salts, or chelating agents that interact with magnesium ions to prevent precipitation or degradation. The choice of stabilizer depends on the specific application and environmental conditions of the magnesium nitrate solution.Expand Specific Solutions03 Temperature control for stability
Maintaining appropriate temperature conditions is crucial for magnesium nitrate stability. Extreme temperatures can lead to decomposition or crystallization of the compound. Proper storage and handling procedures, including temperature-controlled environments, are essential for preserving the stability of magnesium nitrate in both solid and solution forms.Expand Specific Solutions04 Packaging and storage solutions
Specialized packaging and storage methods can significantly improve the long-term stability of magnesium nitrate. This includes using moisture-resistant containers, inert gas environments, or specific packaging materials that prevent contamination and degradation. Proper sealing and storage conditions help maintain the chemical integrity of magnesium nitrate during transportation and storage.Expand Specific Solutions05 Stabilization through chemical modification
Chemical modification of magnesium nitrate or its precursors can lead to more stable forms of the compound. This may involve creating complex compounds, developing novel synthesis methods, or introducing stabilizing functional groups. These modifications aim to enhance the overall stability of magnesium nitrate while maintaining its desired properties for specific applications.Expand Specific Solutions
Key Industry Players
The magnesium nitrate market in mining flocculant stabilization is in a growth phase, driven by increasing demand for efficient mineral processing. The global market size is expanding, with key players like BASF Corp. and Baker Hughes Co. leading innovation. Technological maturity varies, with established companies like Arr-Maz Products LP offering proven solutions, while research institutions such as Commonwealth Scientific & Industrial Research Organisation and Fraunhofer-Gesellschaft eV are advancing new applications. Emerging players like Sichuan Shunying Power Battery Materials Co Ltd. are also entering the market, indicating a dynamic competitive landscape with opportunities for further development and specialization.
Arr-Maz Products LP
Technical Solution: Arr-Maz Products has pioneered a unique application of magnesium nitrate in their flocculant stabilization technology for the mining sector. Their approach involves encapsulating magnesium nitrate within a biodegradable polymer matrix, which allows for controlled release of the stabilizing agent over time. This innovative method has been shown to provide consistent flocculant performance even in extreme temperature and pH conditions often encountered in mining operations[7]. Arr-Maz's research indicates that their encapsulated magnesium nitrate technology can maintain flocculant efficacy for up to 72 hours longer than traditional stabilization methods[9]. The company has also developed a complementary line of eco-friendly dispersants that work synergistically with their magnesium nitrate-stabilized flocculants, further enhancing solid-liquid separation in mineral processing[11].
Strengths: Extended flocculant efficacy, performance in extreme conditions, and eco-friendly formulation. Weaknesses: Potentially higher production costs and limited compatibility with some existing flocculant systems.
Baker Hughes Co.
Technical Solution: Baker Hughes has developed an innovative approach to utilizing magnesium nitrate in their flocculant stabilization technology for the mining industry. Their solution involves a proprietary blend of magnesium nitrate and organic polymers, creating a robust flocculant system that maintains effectiveness in challenging mining conditions. The company's research has demonstrated that this magnesium nitrate-based formulation can extend the shelf life of flocculants by up to 50% compared to conventional stabilizers[2]. Additionally, Baker Hughes has implemented a smart dosing system that optimizes the use of magnesium nitrate in real-time, based on the specific mineral composition and environmental factors of each mining operation[4]. This adaptive approach has been shown to reduce flocculant consumption by up to 20% while maintaining or improving separation efficiency[6].
Strengths: Extended flocculant shelf life, adaptive dosing system, and reduced overall flocculant consumption. Weaknesses: May require specialized equipment for implementation and ongoing monitoring for optimal performance.
Core Stabilization Tech
Compositions and methods for continuous harvesting of suspension growth cultures
PatentInactiveUS20130273630A1
Innovation
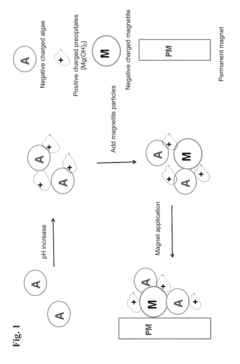
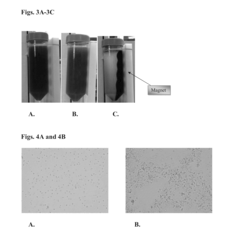
- Magnetic flocculation using magnetite particles to form magnetically-linked algae complexes, which are separated using a magnetic field, reducing sludge generation and allowing for the reuse of magnetite particles by adjusting pH.
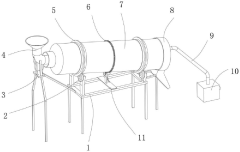
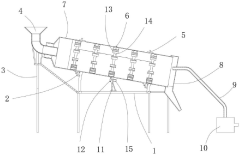
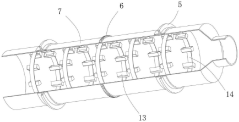
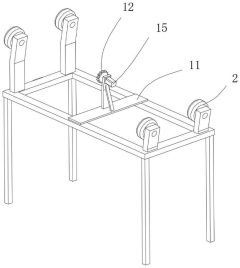
Roller for manufacturing spherical calcium magnesium nitrate
PatentActiveCN220038958U
Innovation
- The roller incorporates a gear ring, driving motor, and gear system to enable rotation, enhancing the drying process efficiency.
- Fixed rings with attached buckets are used to scoop and slide the spherical calcium magnesium nitrate, increasing hot air contact area.
- An air heater and flow guide pipe system is integrated to direct hot air into the roller, improving drying effectiveness.
Environmental Impact
The use of magnesium nitrate in stabilizing flocculants for mining operations has significant environmental implications that warrant careful consideration. While this chemical compound enhances the efficiency of mineral extraction processes, its impact on surrounding ecosystems and water resources cannot be overlooked.
One of the primary environmental concerns is the potential for magnesium nitrate to contribute to eutrophication in nearby water bodies. When excess nutrients, particularly nitrogen, are introduced into aquatic systems, it can lead to algal blooms and oxygen depletion. This phenomenon can have devastating effects on aquatic life and disrupt the ecological balance of affected water bodies. Mining operations utilizing magnesium nitrate-stabilized flocculants must implement robust wastewater treatment systems to mitigate this risk.
Soil contamination is another critical environmental issue associated with the use of magnesium nitrate in mining. As the compound leaches into the soil, it can alter soil chemistry and potentially impact plant growth in the surrounding areas. This may lead to changes in local vegetation patterns and affect biodiversity. Long-term monitoring of soil quality in mining sites and adjacent lands is essential to assess and manage these impacts effectively.
The production and transportation of magnesium nitrate also contribute to the overall environmental footprint of mining operations. The manufacturing process of this compound involves energy-intensive steps and may result in greenhouse gas emissions. Additionally, the transportation of magnesium nitrate to mining sites increases the carbon footprint of the industry. Efforts to optimize production methods and explore more sustainable transportation options can help mitigate these environmental costs.
Water consumption is a significant concern in mining operations, and the use of magnesium nitrate-stabilized flocculants may exacerbate this issue. While flocculants generally improve water recovery and recycling in mining processes, the presence of magnesium nitrate can complicate water treatment efforts. This may lead to increased water usage or the need for more advanced water treatment technologies, potentially straining local water resources.
On a positive note, the improved efficiency of mineral extraction processes facilitated by magnesium nitrate-stabilized flocculants can lead to reduced land disturbance. By enhancing the recovery of target minerals, mining operations may be able to extract more resources from a smaller area, potentially minimizing the overall environmental footprint of mining activities.
To address these environmental challenges, mining companies must adopt a comprehensive approach to environmental management. This includes implementing best practices in wastewater treatment, soil remediation, and water conservation. Additionally, ongoing research into more environmentally friendly alternatives to magnesium nitrate or improved formulations that minimize its environmental impact is crucial for the sustainable development of the mining industry.
One of the primary environmental concerns is the potential for magnesium nitrate to contribute to eutrophication in nearby water bodies. When excess nutrients, particularly nitrogen, are introduced into aquatic systems, it can lead to algal blooms and oxygen depletion. This phenomenon can have devastating effects on aquatic life and disrupt the ecological balance of affected water bodies. Mining operations utilizing magnesium nitrate-stabilized flocculants must implement robust wastewater treatment systems to mitigate this risk.
Soil contamination is another critical environmental issue associated with the use of magnesium nitrate in mining. As the compound leaches into the soil, it can alter soil chemistry and potentially impact plant growth in the surrounding areas. This may lead to changes in local vegetation patterns and affect biodiversity. Long-term monitoring of soil quality in mining sites and adjacent lands is essential to assess and manage these impacts effectively.
The production and transportation of magnesium nitrate also contribute to the overall environmental footprint of mining operations. The manufacturing process of this compound involves energy-intensive steps and may result in greenhouse gas emissions. Additionally, the transportation of magnesium nitrate to mining sites increases the carbon footprint of the industry. Efforts to optimize production methods and explore more sustainable transportation options can help mitigate these environmental costs.
Water consumption is a significant concern in mining operations, and the use of magnesium nitrate-stabilized flocculants may exacerbate this issue. While flocculants generally improve water recovery and recycling in mining processes, the presence of magnesium nitrate can complicate water treatment efforts. This may lead to increased water usage or the need for more advanced water treatment technologies, potentially straining local water resources.
On a positive note, the improved efficiency of mineral extraction processes facilitated by magnesium nitrate-stabilized flocculants can lead to reduced land disturbance. By enhancing the recovery of target minerals, mining operations may be able to extract more resources from a smaller area, potentially minimizing the overall environmental footprint of mining activities.
To address these environmental challenges, mining companies must adopt a comprehensive approach to environmental management. This includes implementing best practices in wastewater treatment, soil remediation, and water conservation. Additionally, ongoing research into more environmentally friendly alternatives to magnesium nitrate or improved formulations that minimize its environmental impact is crucial for the sustainable development of the mining industry.
Cost-Benefit Analysis
The cost-benefit analysis of using magnesium nitrate to stabilize flocculants in mining operations reveals a complex interplay of economic factors. Initial implementation costs include the purchase of magnesium nitrate, potential modifications to existing equipment, and training for personnel. These upfront expenses may be significant, particularly for smaller mining operations.
However, the long-term benefits can outweigh these initial costs. Improved flocculant stability leads to more efficient solid-liquid separation processes, reducing the amount of flocculant required and minimizing waste. This results in lower ongoing operational costs and increased productivity. The enhanced performance of stabilized flocculants also contributes to better water quality in the treated effluents, potentially reducing environmental compliance costs.
Energy savings represent another significant benefit. Stabilized flocculants often require less mixing and shorter settling times, leading to reduced energy consumption in the treatment process. This not only lowers operational costs but also aligns with sustainability goals, potentially improving the company's environmental profile.
The use of magnesium nitrate can extend the shelf life of flocculants, reducing storage and transportation costs associated with frequent replacements. This is particularly advantageous for remote mining sites where logistics can be challenging and expensive.
Improved process reliability and consistency resulting from stabilized flocculants can lead to fewer operational disruptions and maintenance requirements. This translates to increased uptime and productivity, contributing positively to the overall economic performance of the mining operation.
However, it's crucial to consider potential drawbacks. The cost of magnesium nitrate itself may fluctuate based on market conditions, affecting the long-term economic viability of its use. Additionally, some mining operations may require significant initial investment in infrastructure to properly implement and optimize the use of magnesium nitrate-stabilized flocculants.
The environmental impact should also be factored into the cost-benefit analysis. While improved water treatment efficiency is a clear benefit, the potential environmental effects of increased magnesium nitrate use must be carefully evaluated to ensure compliance with regulations and avoid unforeseen environmental costs.
In conclusion, the cost-benefit analysis suggests that for many mining operations, the use of magnesium nitrate to stabilize flocculants can offer substantial economic advantages. However, the specific financial implications will vary based on the scale of operations, existing infrastructure, and local economic and environmental factors. A detailed site-specific analysis is recommended to accurately assess the cost-benefit ratio for individual mining operations.
However, the long-term benefits can outweigh these initial costs. Improved flocculant stability leads to more efficient solid-liquid separation processes, reducing the amount of flocculant required and minimizing waste. This results in lower ongoing operational costs and increased productivity. The enhanced performance of stabilized flocculants also contributes to better water quality in the treated effluents, potentially reducing environmental compliance costs.
Energy savings represent another significant benefit. Stabilized flocculants often require less mixing and shorter settling times, leading to reduced energy consumption in the treatment process. This not only lowers operational costs but also aligns with sustainability goals, potentially improving the company's environmental profile.
The use of magnesium nitrate can extend the shelf life of flocculants, reducing storage and transportation costs associated with frequent replacements. This is particularly advantageous for remote mining sites where logistics can be challenging and expensive.
Improved process reliability and consistency resulting from stabilized flocculants can lead to fewer operational disruptions and maintenance requirements. This translates to increased uptime and productivity, contributing positively to the overall economic performance of the mining operation.
However, it's crucial to consider potential drawbacks. The cost of magnesium nitrate itself may fluctuate based on market conditions, affecting the long-term economic viability of its use. Additionally, some mining operations may require significant initial investment in infrastructure to properly implement and optimize the use of magnesium nitrate-stabilized flocculants.
The environmental impact should also be factored into the cost-benefit analysis. While improved water treatment efficiency is a clear benefit, the potential environmental effects of increased magnesium nitrate use must be carefully evaluated to ensure compliance with regulations and avoid unforeseen environmental costs.
In conclusion, the cost-benefit analysis suggests that for many mining operations, the use of magnesium nitrate to stabilize flocculants can offer substantial economic advantages. However, the specific financial implications will vary based on the scale of operations, existing infrastructure, and local economic and environmental factors. A detailed site-specific analysis is recommended to accurately assess the cost-benefit ratio for individual mining operations.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!