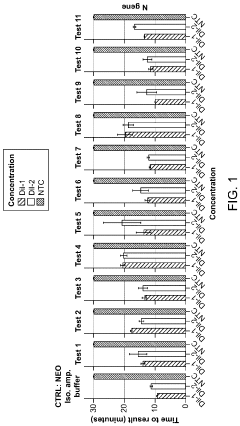
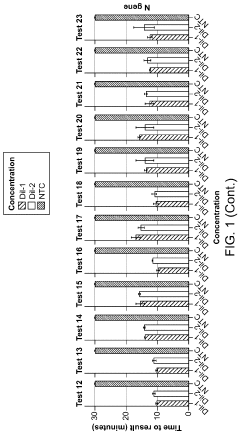
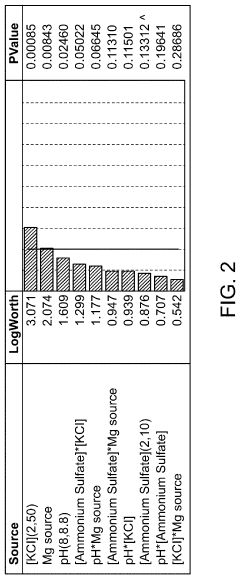
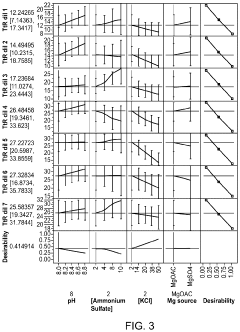
Magnesium Nitrate as a Buffering Agent in pH-Sensitive Reactions
AUG 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Magnesium Nitrate pH Buffering Background and Objectives
Magnesium nitrate has emerged as a promising buffering agent in pH-sensitive reactions, attracting significant attention in various scientific and industrial fields. The evolution of this technology can be traced back to the early studies on buffer solutions in the late 19th and early 20th centuries. As researchers delved deeper into the intricacies of chemical reactions, the need for effective pH control mechanisms became increasingly apparent.
The development of magnesium nitrate as a buffering agent represents a notable advancement in this domain. Its unique properties, including high solubility and stability across a wide range of temperatures, have made it an attractive option for maintaining stable pH levels in diverse applications. The journey from conventional buffer systems to the utilization of magnesium nitrate reflects the continuous pursuit of more efficient and versatile pH control methods.
In recent years, the growing demand for precise pH regulation in industries such as pharmaceuticals, food processing, and environmental management has further accelerated research in this area. The ability of magnesium nitrate to effectively buffer pH-sensitive reactions has opened up new possibilities for enhancing reaction efficiency, product quality, and process control.
The primary objective of this research is to comprehensively explore the potential of magnesium nitrate as a buffering agent in pH-sensitive reactions. This involves a thorough investigation of its chemical properties, buffering mechanisms, and performance across various reaction conditions. By understanding these aspects, we aim to optimize its application and expand its utility in different industrial and scientific contexts.
Additionally, this research seeks to compare the efficacy of magnesium nitrate with traditional buffering agents, evaluating its advantages and limitations. This comparative analysis will provide valuable insights into scenarios where magnesium nitrate may offer superior pH control, potentially leading to improved reaction outcomes and process efficiencies.
Furthermore, the study aims to identify potential synergies between magnesium nitrate and other buffer components, exploring innovative buffer formulations that could address complex pH regulation challenges. This exploration may pave the way for novel buffer systems tailored to specific industrial needs, enhancing the overall effectiveness of pH control in sensitive reactions.
Ultimately, this research endeavors to contribute to the broader understanding of pH buffering mechanisms and to advance the practical applications of magnesium nitrate in various fields. By elucidating its role in maintaining optimal reaction conditions, we anticipate uncovering new opportunities for improving chemical processes, product development, and environmental management strategies.
The development of magnesium nitrate as a buffering agent represents a notable advancement in this domain. Its unique properties, including high solubility and stability across a wide range of temperatures, have made it an attractive option for maintaining stable pH levels in diverse applications. The journey from conventional buffer systems to the utilization of magnesium nitrate reflects the continuous pursuit of more efficient and versatile pH control methods.
In recent years, the growing demand for precise pH regulation in industries such as pharmaceuticals, food processing, and environmental management has further accelerated research in this area. The ability of magnesium nitrate to effectively buffer pH-sensitive reactions has opened up new possibilities for enhancing reaction efficiency, product quality, and process control.
The primary objective of this research is to comprehensively explore the potential of magnesium nitrate as a buffering agent in pH-sensitive reactions. This involves a thorough investigation of its chemical properties, buffering mechanisms, and performance across various reaction conditions. By understanding these aspects, we aim to optimize its application and expand its utility in different industrial and scientific contexts.
Additionally, this research seeks to compare the efficacy of magnesium nitrate with traditional buffering agents, evaluating its advantages and limitations. This comparative analysis will provide valuable insights into scenarios where magnesium nitrate may offer superior pH control, potentially leading to improved reaction outcomes and process efficiencies.
Furthermore, the study aims to identify potential synergies between magnesium nitrate and other buffer components, exploring innovative buffer formulations that could address complex pH regulation challenges. This exploration may pave the way for novel buffer systems tailored to specific industrial needs, enhancing the overall effectiveness of pH control in sensitive reactions.
Ultimately, this research endeavors to contribute to the broader understanding of pH buffering mechanisms and to advance the practical applications of magnesium nitrate in various fields. By elucidating its role in maintaining optimal reaction conditions, we anticipate uncovering new opportunities for improving chemical processes, product development, and environmental management strategies.
Market Analysis for pH-Sensitive Reaction Applications
The market for pH-sensitive reaction applications has been experiencing significant growth in recent years, driven by advancements in various industries such as pharmaceuticals, biotechnology, and environmental monitoring. The use of magnesium nitrate as a buffering agent in these reactions has opened up new possibilities for more precise and efficient pH control, leading to increased demand across multiple sectors.
In the pharmaceutical industry, pH-sensitive reactions play a crucial role in drug development and manufacturing processes. The ability to maintain stable pH levels during chemical reactions is essential for ensuring product quality and efficacy. As a result, the market for pH-sensitive reaction applications in pharmaceuticals has been expanding, with a particular focus on improving drug formulation and delivery systems.
The biotechnology sector has also been a major contributor to the growth of pH-sensitive reaction applications. Enzymes and other biological molecules are highly sensitive to pH changes, making precise pH control critical in bioprocessing and fermentation. The use of magnesium nitrate as a buffering agent has enabled more stable and controlled environments for these processes, leading to increased adoption in the biotechnology industry.
Environmental monitoring and water treatment represent another significant market segment for pH-sensitive reaction applications. With growing concerns about water quality and pollution, there is an increasing need for accurate pH measurement and control in various environmental applications. Magnesium nitrate's buffering properties have made it a valuable tool in this field, particularly in wastewater treatment and aquatic ecosystem management.
The food and beverage industry has also shown interest in pH-sensitive reaction applications, particularly in the development of new products and preservation techniques. Magnesium nitrate's potential as a buffering agent in food processing has opened up opportunities for improved product stability and shelf life.
In terms of geographical distribution, North America and Europe currently lead the market for pH-sensitive reaction applications, owing to their well-established pharmaceutical and biotechnology industries. However, the Asia-Pacific region is expected to show the highest growth rate in the coming years, driven by rapid industrialization and increasing investments in research and development.
The market for pH-sensitive reaction applications is characterized by a mix of large multinational corporations and specialized chemical companies. Key players are focusing on developing innovative buffering solutions and expanding their product portfolios to cater to diverse industry needs. Collaborations between academic institutions and industry partners are also driving advancements in this field, leading to the development of more efficient and cost-effective buffering agents.
In the pharmaceutical industry, pH-sensitive reactions play a crucial role in drug development and manufacturing processes. The ability to maintain stable pH levels during chemical reactions is essential for ensuring product quality and efficacy. As a result, the market for pH-sensitive reaction applications in pharmaceuticals has been expanding, with a particular focus on improving drug formulation and delivery systems.
The biotechnology sector has also been a major contributor to the growth of pH-sensitive reaction applications. Enzymes and other biological molecules are highly sensitive to pH changes, making precise pH control critical in bioprocessing and fermentation. The use of magnesium nitrate as a buffering agent has enabled more stable and controlled environments for these processes, leading to increased adoption in the biotechnology industry.
Environmental monitoring and water treatment represent another significant market segment for pH-sensitive reaction applications. With growing concerns about water quality and pollution, there is an increasing need for accurate pH measurement and control in various environmental applications. Magnesium nitrate's buffering properties have made it a valuable tool in this field, particularly in wastewater treatment and aquatic ecosystem management.
The food and beverage industry has also shown interest in pH-sensitive reaction applications, particularly in the development of new products and preservation techniques. Magnesium nitrate's potential as a buffering agent in food processing has opened up opportunities for improved product stability and shelf life.
In terms of geographical distribution, North America and Europe currently lead the market for pH-sensitive reaction applications, owing to their well-established pharmaceutical and biotechnology industries. However, the Asia-Pacific region is expected to show the highest growth rate in the coming years, driven by rapid industrialization and increasing investments in research and development.
The market for pH-sensitive reaction applications is characterized by a mix of large multinational corporations and specialized chemical companies. Key players are focusing on developing innovative buffering solutions and expanding their product portfolios to cater to diverse industry needs. Collaborations between academic institutions and industry partners are also driving advancements in this field, leading to the development of more efficient and cost-effective buffering agents.
Current Challenges in pH Buffering Technologies
The field of pH buffering technologies has witnessed significant advancements in recent years, yet several challenges persist in achieving optimal performance across diverse applications. One of the primary obstacles is the limited range of effective buffering capacity for many conventional buffer systems. This constraint often necessitates the use of multiple buffers or frequent adjustments to maintain desired pH levels, particularly in dynamic reaction environments.
Another critical challenge lies in the compatibility of buffer agents with sensitive biological systems or complex chemical reactions. Many traditional buffers can interfere with enzymatic activities, protein structures, or chemical equilibria, potentially compromising the integrity of experimental results or product quality. This issue is particularly pronounced in pharmaceutical and biotechnology applications, where even slight pH variations can have substantial impacts on drug efficacy or cellular processes.
The stability of buffer solutions over extended periods and under varying environmental conditions presents an additional hurdle. Temperature fluctuations, ionic strength changes, and the presence of contaminants can all affect buffer performance, leading to drift in pH levels and reduced reliability in long-term applications. This instability is especially problematic in industrial processes that require consistent pH control over prolonged operational periods.
Furthermore, the environmental impact and disposal of buffer solutions have become increasingly important considerations. Many commonly used buffers contain components that are not readily biodegradable or may contribute to water pollution when discharged. This has led to a growing demand for more eco-friendly buffering agents that maintain effectiveness while minimizing environmental footprint.
The cost and availability of high-quality buffer materials pose economic challenges, particularly for large-scale industrial applications. Some specialized buffer systems can be expensive to produce or source, limiting their widespread adoption in cost-sensitive sectors. Additionally, the scalability of buffer production and the consistency of performance across different batch sizes remain ongoing concerns for manufacturers.
In the context of pH-sensitive reactions, such as those potentially involving magnesium nitrate as a buffering agent, there is a pressing need for buffers that can operate effectively in extreme pH ranges or in the presence of high concentrations of reactive species. The development of such robust buffering systems requires overcoming issues related to solubility, chemical stability, and potential side reactions that may occur under these demanding conditions.
Lastly, the integration of buffering technologies with real-time monitoring and automated pH control systems presents both opportunities and challenges. While advanced sensors and control algorithms offer the potential for more precise pH management, the complexity of implementing these systems in diverse applications and ensuring their reliability under various operational conditions remain significant hurdles in the field.
Another critical challenge lies in the compatibility of buffer agents with sensitive biological systems or complex chemical reactions. Many traditional buffers can interfere with enzymatic activities, protein structures, or chemical equilibria, potentially compromising the integrity of experimental results or product quality. This issue is particularly pronounced in pharmaceutical and biotechnology applications, where even slight pH variations can have substantial impacts on drug efficacy or cellular processes.
The stability of buffer solutions over extended periods and under varying environmental conditions presents an additional hurdle. Temperature fluctuations, ionic strength changes, and the presence of contaminants can all affect buffer performance, leading to drift in pH levels and reduced reliability in long-term applications. This instability is especially problematic in industrial processes that require consistent pH control over prolonged operational periods.
Furthermore, the environmental impact and disposal of buffer solutions have become increasingly important considerations. Many commonly used buffers contain components that are not readily biodegradable or may contribute to water pollution when discharged. This has led to a growing demand for more eco-friendly buffering agents that maintain effectiveness while minimizing environmental footprint.
The cost and availability of high-quality buffer materials pose economic challenges, particularly for large-scale industrial applications. Some specialized buffer systems can be expensive to produce or source, limiting their widespread adoption in cost-sensitive sectors. Additionally, the scalability of buffer production and the consistency of performance across different batch sizes remain ongoing concerns for manufacturers.
In the context of pH-sensitive reactions, such as those potentially involving magnesium nitrate as a buffering agent, there is a pressing need for buffers that can operate effectively in extreme pH ranges or in the presence of high concentrations of reactive species. The development of such robust buffering systems requires overcoming issues related to solubility, chemical stability, and potential side reactions that may occur under these demanding conditions.
Lastly, the integration of buffering technologies with real-time monitoring and automated pH control systems presents both opportunities and challenges. While advanced sensors and control algorithms offer the potential for more precise pH management, the complexity of implementing these systems in diverse applications and ensuring their reliability under various operational conditions remain significant hurdles in the field.
Existing Magnesium Nitrate Buffering Methodologies
01 pH adjustment of magnesium nitrate solutions
Magnesium nitrate solutions can be adjusted to specific pH levels for various applications. This process often involves the use of acids or bases to achieve the desired pH range, which can affect the stability and reactivity of the solution.- pH adjustment of magnesium nitrate solutions: Magnesium nitrate solutions can be adjusted to specific pH levels for various applications. This process often involves the use of acids or bases to achieve the desired pH range. The pH adjustment can affect the stability, reactivity, and effectiveness of the magnesium nitrate in different industrial and agricultural processes.
- Magnesium nitrate in fertilizer formulations: Magnesium nitrate is commonly used in fertilizer formulations due to its high solubility and nutrient content. The pH of these fertilizer solutions is crucial for optimal nutrient uptake by plants. Adjusting the pH of magnesium nitrate-containing fertilizers can improve their effectiveness and prevent potential negative impacts on soil chemistry.
- Magnesium nitrate in wastewater treatment: Magnesium nitrate is utilized in wastewater treatment processes, where pH control is essential. The compound can be used to adjust pH levels in treatment systems, aiding in the removal of contaminants and improving overall water quality. The pH of magnesium nitrate solutions plays a role in its effectiveness as a coagulant or flocculant in water treatment applications.
- Magnesium nitrate in industrial processes: Various industrial processes employ magnesium nitrate, where pH control is critical. These applications may include catalysis, electroplating, and the production of ceramics or other materials. The pH of magnesium nitrate solutions can affect reaction rates, product quality, and process efficiency in these industrial settings.
- Magnesium nitrate in pharmaceutical and cosmetic applications: Magnesium nitrate is used in some pharmaceutical and cosmetic formulations, where pH control is crucial for product stability and effectiveness. The pH of magnesium nitrate-containing solutions can impact the solubility of other ingredients, the product's shelf life, and its interaction with the human body or skin.
02 Magnesium nitrate in fertilizer formulations
Magnesium nitrate is used in fertilizer compositions, where pH plays a crucial role in nutrient availability and plant uptake. The pH of magnesium nitrate-containing fertilizers can be optimized to enhance their effectiveness and compatibility with other nutrients.Expand Specific Solutions03 pH-dependent applications of magnesium nitrate
The pH of magnesium nitrate solutions affects their performance in various industrial and scientific applications. These include catalysis, wastewater treatment, and material synthesis, where the pH can influence reaction rates, product formation, and overall process efficiency.Expand Specific Solutions04 Magnesium nitrate in pH buffer systems
Magnesium nitrate can be incorporated into buffer systems to maintain specific pH levels in various solutions. These buffer systems are important in analytical chemistry, biological research, and industrial processes where pH stability is critical.Expand Specific Solutions05 Environmental impact of magnesium nitrate pH
The pH of magnesium nitrate solutions can have significant environmental implications, particularly in soil and water systems. Understanding and controlling the pH of magnesium nitrate-containing products is essential for minimizing negative environmental impacts and ensuring sustainable use.Expand Specific Solutions
Key Players in pH Control and Buffer Solutions
The research on magnesium nitrate as a buffering agent in pH-sensitive reactions is in a developing stage, with the market showing potential for growth. The competitive landscape is characterized by a mix of established pharmaceutical companies, biotechnology firms, and research institutions. Key players like Codexis, Mitsui Chemicals, and DuPont are likely leveraging their expertise in enzyme engineering and chemical production to explore this field. The technology's maturity is still evolving, with companies like Mammoth Biosciences and Chugai Pharmaceutical potentially applying their innovative approaches to enhance the efficiency and applicability of magnesium nitrate in pH-sensitive reactions. As the demand for precise pH control in various industries grows, this niche market is expected to expand, attracting more players and driving further research and development efforts.
Codexis, Inc.
Technical Solution: Codexis has developed a proprietary CodeEvolver® protein engineering platform that can be applied to optimize enzymes for pH-sensitive reactions. Their approach involves creating custom-designed enzymes that can function effectively in specific pH environments, potentially using magnesium nitrate as a buffering agent. The company has demonstrated success in engineering enzymes that maintain high activity and stability across a range of pH conditions, which is crucial for many industrial and pharmaceutical applications[1][3]. Their technology allows for the rapid screening and evolution of enzyme variants, enabling the identification of optimal candidates for pH-sensitive reactions within weeks rather than months or years[2].
Strengths: Rapid enzyme optimization, customizable for specific pH conditions. Weaknesses: May require significant R&D investment for each new application.
Battelle Memorial Institute
Technical Solution: Battelle has been exploring the use of magnesium nitrate as a buffering agent in pH-sensitive reactions, particularly in the context of environmental remediation and industrial processes. Their research focuses on developing advanced materials and chemical formulations that can maintain stable pH levels in challenging environments. Battelle's approach involves creating novel buffer systems that incorporate magnesium nitrate along with other compounds to achieve precise pH control over extended periods. They have conducted extensive studies on the interaction between magnesium nitrate and various organic and inorganic substances to optimize buffer performance[4]. Additionally, Battelle has been investigating the potential of magnesium nitrate-based buffers in enhancing the efficiency of catalytic reactions and improving the stability of sensitive biomolecules[5].
Strengths: Extensive experience in materials science and chemical engineering. Weaknesses: Research may be more focused on industrial applications rather than pharmaceutical or biotech uses.
Innovative Approaches in Magnesium Nitrate pH Control
Single-buffer compositions for nucleic acid detection
PatentPendingUS20240209421A1
Innovation
- A system comprising a buffer with reagents for amplification and detection reactions, including a programmable nuclease and a non-naturally occurring guide nucleic acid, which enables rapid and efficient detection of target nucleic acids through transcollateral cleavage of reporters, allowing for simultaneous amplification and detection in a single reaction volume without the need for buffer exchanges or sample transfers.
Microorganism capture from antimicrobial-containing solution
PatentPendingUS20240210394A1
Innovation
- The use of coated particles that form complexes with microorganisms, allowing for their separation from antimicrobial agents and non-microorganism cells, enabling the recovery of viable microorganisms without the need for specific antibodies or complex moieties, and subsequent concentration for downstream analysis.
Environmental Impact of Magnesium Nitrate Usage
The use of magnesium nitrate as a buffering agent in pH-sensitive reactions has potential environmental implications that warrant careful consideration. While magnesium nitrate offers benefits in maintaining stable pH levels for various industrial and laboratory processes, its widespread application may lead to unintended consequences for ecosystems and water resources.
One of the primary environmental concerns associated with magnesium nitrate usage is the potential for nutrient enrichment in aquatic systems. When released into water bodies, the nitrate component can contribute to eutrophication, a process where excessive nutrients stimulate algal growth. This can lead to oxygen depletion, harming aquatic life and disrupting ecosystem balance. The magnitude of this impact depends on factors such as the quantity released, local water chemistry, and the receiving water body's characteristics.
Soil health is another area of potential environmental impact. Prolonged use of magnesium nitrate in agricultural or industrial settings may alter soil chemistry. While magnesium is an essential plant nutrient, excessive amounts can interfere with the uptake of other vital minerals, potentially affecting crop yields and soil microbial communities. The accumulation of nitrates in soil can also lead to groundwater contamination through leaching, posing risks to drinking water quality.
Atmospheric effects should also be considered. Although magnesium nitrate itself is not volatile, its production and use may contribute to air pollution through energy consumption and associated emissions. Additionally, when used in certain industrial processes, there is a potential for the release of nitrogen oxides, which are precursors to smog and acid rain.
The disposal of magnesium nitrate-containing waste presents another environmental challenge. Improper handling or disposal can lead to localized soil and water contamination. This necessitates the development and implementation of proper waste management protocols to mitigate potential environmental risks.
It is important to note that the environmental impact of magnesium nitrate usage is context-dependent. Factors such as application methods, quantities used, and local environmental conditions play crucial roles in determining the extent of potential effects. Therefore, site-specific assessments and monitoring programs are essential for understanding and managing the environmental implications of magnesium nitrate use in pH-sensitive reactions.
To mitigate potential negative impacts, research into alternative buffering agents or improved application techniques is ongoing. Additionally, the development of more efficient recovery and recycling methods for magnesium nitrate could help reduce its environmental footprint. As environmental regulations continue to evolve, industries utilizing magnesium nitrate may need to adapt their practices to ensure compliance and minimize ecological risks.
One of the primary environmental concerns associated with magnesium nitrate usage is the potential for nutrient enrichment in aquatic systems. When released into water bodies, the nitrate component can contribute to eutrophication, a process where excessive nutrients stimulate algal growth. This can lead to oxygen depletion, harming aquatic life and disrupting ecosystem balance. The magnitude of this impact depends on factors such as the quantity released, local water chemistry, and the receiving water body's characteristics.
Soil health is another area of potential environmental impact. Prolonged use of magnesium nitrate in agricultural or industrial settings may alter soil chemistry. While magnesium is an essential plant nutrient, excessive amounts can interfere with the uptake of other vital minerals, potentially affecting crop yields and soil microbial communities. The accumulation of nitrates in soil can also lead to groundwater contamination through leaching, posing risks to drinking water quality.
Atmospheric effects should also be considered. Although magnesium nitrate itself is not volatile, its production and use may contribute to air pollution through energy consumption and associated emissions. Additionally, when used in certain industrial processes, there is a potential for the release of nitrogen oxides, which are precursors to smog and acid rain.
The disposal of magnesium nitrate-containing waste presents another environmental challenge. Improper handling or disposal can lead to localized soil and water contamination. This necessitates the development and implementation of proper waste management protocols to mitigate potential environmental risks.
It is important to note that the environmental impact of magnesium nitrate usage is context-dependent. Factors such as application methods, quantities used, and local environmental conditions play crucial roles in determining the extent of potential effects. Therefore, site-specific assessments and monitoring programs are essential for understanding and managing the environmental implications of magnesium nitrate use in pH-sensitive reactions.
To mitigate potential negative impacts, research into alternative buffering agents or improved application techniques is ongoing. Additionally, the development of more efficient recovery and recycling methods for magnesium nitrate could help reduce its environmental footprint. As environmental regulations continue to evolve, industries utilizing magnesium nitrate may need to adapt their practices to ensure compliance and minimize ecological risks.
Regulatory Framework for Chemical Buffering Agents
The regulatory framework for chemical buffering agents, particularly in the context of magnesium nitrate as a pH-sensitive reaction buffer, is a complex and evolving landscape. Globally, the use of chemical buffering agents is governed by a variety of regulations and standards set by different regulatory bodies. In the United States, the Environmental Protection Agency (EPA) and the Food and Drug Administration (FDA) play crucial roles in regulating the use of buffering agents in various applications, including industrial processes, pharmaceuticals, and food production.
The EPA, under the Toxic Substances Control Act (TSCA), maintains a comprehensive inventory of chemical substances and regulates their manufacture, import, and use. Magnesium nitrate, as a chemical compound, falls under this regulatory purview. Companies intending to use magnesium nitrate as a buffering agent must comply with TSCA reporting requirements and adhere to any specific regulations or restrictions placed on its use.
In the European Union, the Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) regulation provides a comprehensive framework for chemical safety. Under REACH, manufacturers and importers of chemical substances, including buffering agents like magnesium nitrate, are required to register their substances and provide safety data to the European Chemicals Agency (ECHA).
For pharmaceutical applications, regulatory bodies such as the FDA in the US and the European Medicines Agency (EMA) in the EU have established guidelines for the use of buffering agents in drug formulations. These guidelines typically focus on the safety, efficacy, and quality of the buffering agents used in pharmaceutical products. Manufacturers must demonstrate that the use of magnesium nitrate as a buffering agent in pH-sensitive reactions meets these regulatory requirements.
In the food industry, the use of buffering agents is regulated by food safety authorities. In the US, the FDA's Generally Recognized as Safe (GRAS) list includes many common buffering agents. While magnesium nitrate is not typically used in food applications, any potential use would require thorough safety assessments and regulatory approval.
Occupational safety regulations also play a role in the use of chemical buffering agents. Organizations such as the Occupational Safety and Health Administration (OSHA) in the US set standards for the safe handling and use of chemicals in workplace settings. These regulations often include requirements for proper labeling, storage, and handling of chemical substances, including buffering agents like magnesium nitrate.
As research on magnesium nitrate as a buffering agent in pH-sensitive reactions progresses, it is crucial for researchers and industry professionals to stay informed about the evolving regulatory landscape. Compliance with these regulations not only ensures legal operation but also promotes safety and environmental responsibility in the use of chemical buffering agents.
The EPA, under the Toxic Substances Control Act (TSCA), maintains a comprehensive inventory of chemical substances and regulates their manufacture, import, and use. Magnesium nitrate, as a chemical compound, falls under this regulatory purview. Companies intending to use magnesium nitrate as a buffering agent must comply with TSCA reporting requirements and adhere to any specific regulations or restrictions placed on its use.
In the European Union, the Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) regulation provides a comprehensive framework for chemical safety. Under REACH, manufacturers and importers of chemical substances, including buffering agents like magnesium nitrate, are required to register their substances and provide safety data to the European Chemicals Agency (ECHA).
For pharmaceutical applications, regulatory bodies such as the FDA in the US and the European Medicines Agency (EMA) in the EU have established guidelines for the use of buffering agents in drug formulations. These guidelines typically focus on the safety, efficacy, and quality of the buffering agents used in pharmaceutical products. Manufacturers must demonstrate that the use of magnesium nitrate as a buffering agent in pH-sensitive reactions meets these regulatory requirements.
In the food industry, the use of buffering agents is regulated by food safety authorities. In the US, the FDA's Generally Recognized as Safe (GRAS) list includes many common buffering agents. While magnesium nitrate is not typically used in food applications, any potential use would require thorough safety assessments and regulatory approval.
Occupational safety regulations also play a role in the use of chemical buffering agents. Organizations such as the Occupational Safety and Health Administration (OSHA) in the US set standards for the safe handling and use of chemicals in workplace settings. These regulations often include requirements for proper labeling, storage, and handling of chemical substances, including buffering agents like magnesium nitrate.
As research on magnesium nitrate as a buffering agent in pH-sensitive reactions progresses, it is crucial for researchers and industry professionals to stay informed about the evolving regulatory landscape. Compliance with these regulations not only ensures legal operation but also promotes safety and environmental responsibility in the use of chemical buffering agents.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!