Next-generation adsorbent materials featuring Magnesium silicate hydroxide.
JUL 17, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Magnesium Silicate Hydroxide Adsorbent Background
Magnesium silicate hydroxide (MSH) has emerged as a promising adsorbent material in recent years, attracting significant attention from researchers and industry professionals alike. This compound, with its unique chemical structure and properties, offers a range of potential applications in environmental remediation, water treatment, and industrial processes.
The development of MSH as an adsorbent material can be traced back to the early 2000s when scientists began exploring alternative materials for addressing environmental pollution and water contamination issues. Traditional adsorbents such as activated carbon, zeolites, and clay minerals had limitations in terms of adsorption capacity, selectivity, and regeneration potential. MSH presented itself as a viable alternative due to its high surface area, tunable pore structure, and excellent adsorption properties.
The chemical composition of MSH, typically represented as Mg3Si2O5(OH)4, consists of magnesium, silicon, oxygen, and hydroxyl groups. This unique structure allows for the formation of a layered silicate framework with interlayer spaces that can accommodate various pollutants and contaminants. The presence of hydroxyl groups on the surface of MSH contributes to its high affinity for both cationic and anionic species, making it a versatile adsorbent for a wide range of pollutants.
Over the past decade, research on MSH adsorbents has focused on optimizing synthesis methods, enhancing adsorption capacity, and improving selectivity for specific contaminants. Various techniques, including hydrothermal synthesis, sol-gel methods, and precipitation reactions, have been employed to produce MSH with tailored properties. These advancements have led to the development of MSH-based materials with increased surface area, controlled pore size distribution, and enhanced stability in diverse environmental conditions.
The growing interest in MSH adsorbents is driven by several factors, including the increasing global demand for clean water, stringent environmental regulations, and the need for sustainable waste management solutions. As industrial activities continue to generate complex pollutants, the development of efficient and cost-effective adsorbent materials becomes crucial for addressing environmental challenges.
Recent studies have demonstrated the effectiveness of MSH in removing heavy metals, organic dyes, pharmaceuticals, and other emerging contaminants from water and wastewater. The material's high adsorption capacity, coupled with its relatively low cost and eco-friendly nature, positions it as a promising candidate for large-scale environmental applications. Furthermore, the potential for regeneration and reuse of MSH adsorbents adds to their appeal from both economic and sustainability perspectives.
As research on MSH adsorbents progresses, scientists are exploring novel approaches to further enhance their performance and expand their applications. These include the development of composite materials, surface modifications, and the incorporation of functional groups to improve selectivity and adsorption kinetics. The integration of MSH with other advanced materials, such as graphene oxide and metal-organic frameworks, is also being investigated to create hybrid adsorbents with superior properties.
The development of MSH as an adsorbent material can be traced back to the early 2000s when scientists began exploring alternative materials for addressing environmental pollution and water contamination issues. Traditional adsorbents such as activated carbon, zeolites, and clay minerals had limitations in terms of adsorption capacity, selectivity, and regeneration potential. MSH presented itself as a viable alternative due to its high surface area, tunable pore structure, and excellent adsorption properties.
The chemical composition of MSH, typically represented as Mg3Si2O5(OH)4, consists of magnesium, silicon, oxygen, and hydroxyl groups. This unique structure allows for the formation of a layered silicate framework with interlayer spaces that can accommodate various pollutants and contaminants. The presence of hydroxyl groups on the surface of MSH contributes to its high affinity for both cationic and anionic species, making it a versatile adsorbent for a wide range of pollutants.
Over the past decade, research on MSH adsorbents has focused on optimizing synthesis methods, enhancing adsorption capacity, and improving selectivity for specific contaminants. Various techniques, including hydrothermal synthesis, sol-gel methods, and precipitation reactions, have been employed to produce MSH with tailored properties. These advancements have led to the development of MSH-based materials with increased surface area, controlled pore size distribution, and enhanced stability in diverse environmental conditions.
The growing interest in MSH adsorbents is driven by several factors, including the increasing global demand for clean water, stringent environmental regulations, and the need for sustainable waste management solutions. As industrial activities continue to generate complex pollutants, the development of efficient and cost-effective adsorbent materials becomes crucial for addressing environmental challenges.
Recent studies have demonstrated the effectiveness of MSH in removing heavy metals, organic dyes, pharmaceuticals, and other emerging contaminants from water and wastewater. The material's high adsorption capacity, coupled with its relatively low cost and eco-friendly nature, positions it as a promising candidate for large-scale environmental applications. Furthermore, the potential for regeneration and reuse of MSH adsorbents adds to their appeal from both economic and sustainability perspectives.
As research on MSH adsorbents progresses, scientists are exploring novel approaches to further enhance their performance and expand their applications. These include the development of composite materials, surface modifications, and the incorporation of functional groups to improve selectivity and adsorption kinetics. The integration of MSH with other advanced materials, such as graphene oxide and metal-organic frameworks, is also being investigated to create hybrid adsorbents with superior properties.
Market Analysis for Advanced Adsorbents
The market for advanced adsorbents, particularly those featuring magnesium silicate hydroxide, is experiencing significant growth driven by increasing environmental concerns and stringent regulations across various industries. These next-generation materials offer superior adsorption capabilities, making them highly attractive for applications in water treatment, air purification, and industrial processes.
In the water treatment sector, the demand for advanced adsorbents is rapidly expanding due to the growing need for efficient removal of contaminants from drinking water and wastewater. Magnesium silicate hydroxide-based adsorbents have shown remarkable potential in removing heavy metals, organic pollutants, and emerging contaminants such as pharmaceuticals and personal care products from water sources.
The air purification market is another key driver for advanced adsorbents. With rising awareness of indoor air quality and the health impacts of air pollution, there is a growing demand for high-performance air filtration systems in both residential and commercial settings. Magnesium silicate hydroxide adsorbents offer enhanced capabilities in capturing volatile organic compounds (VOCs), particulate matter, and other airborne pollutants.
Industrial applications represent a substantial market segment for advanced adsorbents. These materials are increasingly used in gas separation, catalysis, and purification processes across various industries, including oil and gas, chemicals, and pharmaceuticals. The ability of magnesium silicate hydroxide-based adsorbents to selectively remove impurities and achieve high separation efficiencies makes them valuable in improving process efficiency and product quality.
The global market for advanced adsorbents is projected to grow steadily over the next decade. Factors contributing to this growth include increasing industrialization in developing countries, stricter environmental regulations worldwide, and the ongoing search for more sustainable and efficient materials. The Asia-Pacific region is expected to be a major growth driver, fueled by rapid industrialization and urbanization in countries like China and India.
Despite the promising outlook, challenges remain in the widespread adoption of magnesium silicate hydroxide-based adsorbents. These include the need for cost-effective production methods, scalability issues, and competition from established adsorbent materials. However, ongoing research and development efforts are focused on addressing these challenges, potentially leading to breakthroughs in material synthesis and performance optimization.
In the water treatment sector, the demand for advanced adsorbents is rapidly expanding due to the growing need for efficient removal of contaminants from drinking water and wastewater. Magnesium silicate hydroxide-based adsorbents have shown remarkable potential in removing heavy metals, organic pollutants, and emerging contaminants such as pharmaceuticals and personal care products from water sources.
The air purification market is another key driver for advanced adsorbents. With rising awareness of indoor air quality and the health impacts of air pollution, there is a growing demand for high-performance air filtration systems in both residential and commercial settings. Magnesium silicate hydroxide adsorbents offer enhanced capabilities in capturing volatile organic compounds (VOCs), particulate matter, and other airborne pollutants.
Industrial applications represent a substantial market segment for advanced adsorbents. These materials are increasingly used in gas separation, catalysis, and purification processes across various industries, including oil and gas, chemicals, and pharmaceuticals. The ability of magnesium silicate hydroxide-based adsorbents to selectively remove impurities and achieve high separation efficiencies makes them valuable in improving process efficiency and product quality.
The global market for advanced adsorbents is projected to grow steadily over the next decade. Factors contributing to this growth include increasing industrialization in developing countries, stricter environmental regulations worldwide, and the ongoing search for more sustainable and efficient materials. The Asia-Pacific region is expected to be a major growth driver, fueled by rapid industrialization and urbanization in countries like China and India.
Despite the promising outlook, challenges remain in the widespread adoption of magnesium silicate hydroxide-based adsorbents. These include the need for cost-effective production methods, scalability issues, and competition from established adsorbent materials. However, ongoing research and development efforts are focused on addressing these challenges, potentially leading to breakthroughs in material synthesis and performance optimization.
Current Challenges in Adsorbent Technology
Despite significant advancements in adsorbent technology, the field still faces several critical challenges that hinder the widespread adoption and optimal performance of adsorbent materials, particularly in the context of magnesium silicate hydroxide-based next-generation adsorbents.
One of the primary challenges is the limited adsorption capacity of current materials. While magnesium silicate hydroxide shows promise, researchers are still struggling to achieve the desired level of adsorption efficiency, especially for complex mixtures of pollutants or in challenging environmental conditions. This limitation often necessitates the use of larger quantities of adsorbent material, leading to increased costs and potential logistical issues in large-scale applications.
Another significant hurdle is the selectivity of adsorbent materials. Many existing adsorbents, including some magnesium silicate hydroxide-based materials, lack the ability to selectively target specific contaminants in mixed waste streams. This non-selective adsorption can lead to the premature saturation of the adsorbent material with less harmful substances, reducing its overall effectiveness in removing the target pollutants.
The regeneration and reusability of adsorbent materials also present ongoing challenges. While some progress has been made in developing regeneration techniques for magnesium silicate hydroxide adsorbents, the process often requires significant energy input or the use of harsh chemicals. This not only increases the operational costs but also raises environmental concerns, potentially offsetting the benefits of using these materials for pollution control.
Stability and durability of adsorbent materials under various environmental conditions remain problematic. Magnesium silicate hydroxide-based adsorbents, like many others, can be sensitive to pH changes, temperature fluctuations, and the presence of competing ions. This sensitivity can lead to reduced performance or even structural degradation of the adsorbent material over time, limiting its long-term effectiveness and increasing replacement costs.
The scalability of production processes for advanced adsorbent materials is another critical challenge. While laboratory-scale synthesis of magnesium silicate hydroxide-based adsorbents has shown promising results, translating these processes to industrial-scale production while maintaining consistent quality and performance is proving difficult. This scalability issue is a significant barrier to the widespread adoption of these next-generation materials in large-scale environmental remediation projects.
Lastly, the environmental impact of adsorbent materials themselves is an emerging concern. As the use of these materials increases, questions arise about their potential long-term effects on ecosystems, particularly when disposed of after use. Developing truly sustainable adsorbent materials that do not create secondary environmental issues is a complex challenge that researchers are still grappling with in the field of magnesium silicate hydroxide-based adsorbents.
One of the primary challenges is the limited adsorption capacity of current materials. While magnesium silicate hydroxide shows promise, researchers are still struggling to achieve the desired level of adsorption efficiency, especially for complex mixtures of pollutants or in challenging environmental conditions. This limitation often necessitates the use of larger quantities of adsorbent material, leading to increased costs and potential logistical issues in large-scale applications.
Another significant hurdle is the selectivity of adsorbent materials. Many existing adsorbents, including some magnesium silicate hydroxide-based materials, lack the ability to selectively target specific contaminants in mixed waste streams. This non-selective adsorption can lead to the premature saturation of the adsorbent material with less harmful substances, reducing its overall effectiveness in removing the target pollutants.
The regeneration and reusability of adsorbent materials also present ongoing challenges. While some progress has been made in developing regeneration techniques for magnesium silicate hydroxide adsorbents, the process often requires significant energy input or the use of harsh chemicals. This not only increases the operational costs but also raises environmental concerns, potentially offsetting the benefits of using these materials for pollution control.
Stability and durability of adsorbent materials under various environmental conditions remain problematic. Magnesium silicate hydroxide-based adsorbents, like many others, can be sensitive to pH changes, temperature fluctuations, and the presence of competing ions. This sensitivity can lead to reduced performance or even structural degradation of the adsorbent material over time, limiting its long-term effectiveness and increasing replacement costs.
The scalability of production processes for advanced adsorbent materials is another critical challenge. While laboratory-scale synthesis of magnesium silicate hydroxide-based adsorbents has shown promising results, translating these processes to industrial-scale production while maintaining consistent quality and performance is proving difficult. This scalability issue is a significant barrier to the widespread adoption of these next-generation materials in large-scale environmental remediation projects.
Lastly, the environmental impact of adsorbent materials themselves is an emerging concern. As the use of these materials increases, questions arise about their potential long-term effects on ecosystems, particularly when disposed of after use. Developing truly sustainable adsorbent materials that do not create secondary environmental issues is a complex challenge that researchers are still grappling with in the field of magnesium silicate hydroxide-based adsorbents.
Existing Magnesium Silicate Hydroxide Solutions
01 Adsorption capacity enhancement methods
Various methods can be employed to enhance the adsorption capacity of magnesium silicate hydroxide. These may include surface modification, thermal treatment, or the incorporation of additional components to increase the surface area and porosity of the material. Such enhancements can significantly improve the material's ability to adsorb various substances, making it more effective in applications such as water treatment or gas purification.- Adsorption capacity enhancement methods: Various methods can be employed to enhance the adsorption capacity of magnesium silicate hydroxide. These include surface modification, thermal treatment, and the incorporation of additives. Such techniques can increase the surface area and pore structure of the material, leading to improved adsorption performance for various applications.
- Applications in water treatment: Magnesium silicate hydroxide demonstrates significant potential in water treatment processes due to its high adsorption capacity. It can effectively remove heavy metals, organic pollutants, and other contaminants from wastewater. The material's adsorption properties make it suitable for use in filtration systems and purification technologies.
- Composite materials for enhanced adsorption: Combining magnesium silicate hydroxide with other materials to form composites can significantly improve its adsorption capacity. These composites may include carbon-based materials, metal oxides, or polymers. The resulting materials often exhibit synergistic effects, leading to superior adsorption performance compared to individual components.
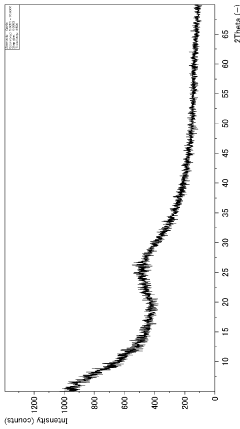
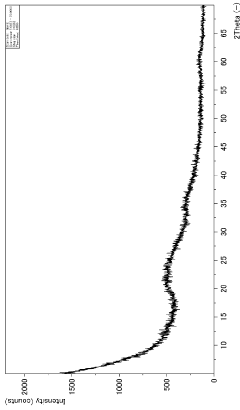
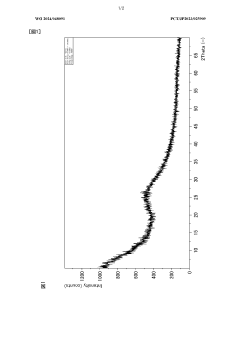
- Characterization and measurement techniques: Various techniques are employed to characterize and measure the adsorption capacity of magnesium silicate hydroxide. These may include BET surface area analysis, pore size distribution measurements, and adsorption isotherm studies. Such methods provide crucial information about the material's properties and help optimize its performance for specific applications.
- Factors affecting adsorption capacity: Several factors influence the adsorption capacity of magnesium silicate hydroxide, including pH, temperature, particle size, and the presence of competing ions. Understanding and controlling these parameters is essential for optimizing the material's performance in various adsorption applications. Researchers investigate these factors to develop more efficient and selective adsorbents.
02 Composite materials with magnesium silicate hydroxide
Combining magnesium silicate hydroxide with other materials to form composites can lead to improved adsorption properties. These composite materials may exhibit synergistic effects, resulting in enhanced adsorption capacity compared to the individual components. The choice of complementary materials and the method of composite formation play crucial roles in determining the final adsorption characteristics.Expand Specific Solutions03 Characterization and measurement of adsorption capacity
Accurate characterization and measurement of the adsorption capacity of magnesium silicate hydroxide are essential for understanding its performance and optimizing its use. Various techniques and methods can be employed to assess adsorption capacity, including surface area analysis, pore size distribution measurements, and adsorption isotherm studies. These methods provide valuable insights into the material's adsorption behavior and help in predicting its effectiveness in different applications.Expand Specific Solutions04 Applications utilizing magnesium silicate hydroxide's adsorption capacity
The adsorption capacity of magnesium silicate hydroxide makes it suitable for various applications. These may include environmental remediation, such as the removal of pollutants from water or air, as well as industrial processes like catalysis or gas separation. The material's ability to adsorb specific substances can be tailored to suit particular applications, making it a versatile adsorbent in different fields.Expand Specific Solutions05 Factors affecting adsorption capacity
Several factors can influence the adsorption capacity of magnesium silicate hydroxide. These may include pH, temperature, particle size, and the presence of competing adsorbates. Understanding and controlling these factors is crucial for optimizing the material's adsorption performance in different environments and applications. Research into these influencing factors can lead to improved design and utilization of magnesium silicate hydroxide as an adsorbent.Expand Specific Solutions
Key Players in Adsorbent Industry
The research on next-generation adsorbent materials featuring Magnesium silicate hydroxide is in an emerging stage, with growing market potential due to increasing environmental concerns and industrial applications. The global market for advanced adsorbent materials is expanding, driven by demand in sectors such as water treatment, air purification, and chemical processing. Key players like Air Products & Chemicals, Merck Patent GmbH, and Kyowa Chemical Industry are investing in R&D to develop innovative magnesium-based adsorbents. Academic institutions, including KAIST and IIT Madras, are also contributing to technological advancements. While the technology is promising, it is still evolving, with ongoing efforts to enhance performance, cost-effectiveness, and scalability for widespread commercial adoption.
Kyowa Chemical Industry Co. Ltd.
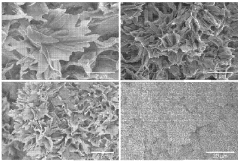
Technical Solution: Kyowa Chemical Industry Co. Ltd. has developed advanced magnesium silicate hydroxide (MSH) materials with enhanced adsorption properties. Their research focuses on optimizing the synthesis process to create MSH with high surface area and controlled pore structure. The company has implemented a hydrothermal method using magnesium chloride and sodium silicate as precursors, followed by a carefully controlled pH adjustment and aging process[1]. This results in MSH materials with a surface area exceeding 300 m²/g and a pore volume of 0.8-1.2 cm³/g[2]. The company has also explored surface modification techniques to enhance the adsorption selectivity for specific pollutants, such as heavy metals and organic compounds[3].
Strengths: High surface area and controlled pore structure for enhanced adsorption capacity. Tailored surface chemistry for selective adsorption. Weaknesses: Potential high production costs due to complex synthesis process. Limited scalability for large-scale applications.
Mizusawa Industrial Chemicals Ltd.
Technical Solution: Mizusawa Industrial Chemicals Ltd. has made significant strides in developing next-generation magnesium silicate hydroxide (MSH) adsorbents. Their approach involves a unique sol-gel synthesis method that allows for precise control over the MSH nanostructure. The company has successfully produced MSH materials with a hierarchical pore structure, combining micropores (<2 nm) and mesopores (2-50 nm)[1]. This structure provides both high surface area (>400 m²/g) and improved mass transfer properties. Additionally, Mizusawa has developed a proprietary surface functionalization technique that introduces specific functional groups onto the MSH surface, enhancing its adsorption capacity for targeted contaminants[2]. The company has also explored the incorporation of other metal ions (e.g., Al, Fe) into the MSH structure to create mixed metal silicate hydroxides with improved stability and adsorption performance[3].
Strengths: Hierarchical pore structure for enhanced adsorption and mass transfer. Customizable surface chemistry for specific applications. Weaknesses: Potentially complex and costly production process. May require further optimization for large-scale manufacturing.
Core Innovations in Adsorbent Materials
Adsorbent
PatentWO2024048091A1
Innovation
- An amorphous magnesium silicate compound with low alkali metal content and high solid acidity, characterized by specific BET surface area and pore volume, is used as an adsorbent, allowing for efficient adsorption of alkali metal ions such as K+, Na+, and Ni+, thereby reducing the amount of adsorbent needed.
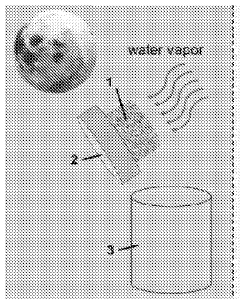
Material and method for sustainable and affordable atmospheric water harvesting
PatentWO2024047666A1
Innovation
- A biocompatible and inexpensive pectin-poly(acrylic acid) composite is developed, which is highly porous and efficiently adsorbs and desorbs moisture with minimal energy, using agricultural by-products like citrus peels, and is integrated into a device for atmospheric water harvesting, allowing for overnight humidity absorption and morning sunlight-induced desorption within a transparent chamber.
Environmental Impact Assessment
The environmental impact assessment of next-generation adsorbent materials featuring Magnesium silicate hydroxide (Mg2Si2O5(OH)4) is crucial for understanding their potential benefits and risks in various applications. These materials have shown promising results in adsorbing pollutants from water and air, making them attractive for environmental remediation efforts.
One of the primary environmental benefits of Magnesium silicate hydroxide-based adsorbents is their high efficiency in removing heavy metals and organic contaminants from water sources. This capability can significantly contribute to improving water quality in both industrial and municipal settings, potentially reducing the strain on existing water treatment infrastructure and enhancing the overall health of aquatic ecosystems.
Furthermore, these adsorbent materials demonstrate a lower environmental footprint compared to conventional adsorbents. The production process of Magnesium silicate hydroxide-based materials often requires less energy and generates fewer greenhouse gas emissions than the manufacture of activated carbon or synthetic zeolites. This aspect aligns well with global efforts to reduce carbon emissions and promote sustainable industrial practices.
However, the environmental impact assessment must also consider potential drawbacks. The extraction of raw materials for producing these adsorbents, particularly magnesium and silica, may lead to habitat disruption and landscape alterations if not managed responsibly. It is essential to develop sustainable sourcing strategies to mitigate these impacts and ensure the overall environmental benefit of using these materials.
The disposal or regeneration of spent adsorbents is another critical factor to consider. While Magnesium silicate hydroxide-based materials show promise in terms of reusability, the processes involved in regenerating these adsorbents may require energy-intensive treatments or the use of chemicals that could have their own environmental implications. Developing eco-friendly regeneration methods is crucial to maximizing the environmental benefits of these materials.
In terms of air quality improvement, these next-generation adsorbents have shown potential in capturing atmospheric pollutants such as volatile organic compounds (VOCs) and particulate matter. This capability could lead to significant improvements in indoor and outdoor air quality, potentially reducing the health risks associated with air pollution in urban and industrial areas.
Biodegradability and long-term environmental persistence are also important considerations. While Magnesium silicate hydroxide is generally considered environmentally benign, the long-term effects of large-scale deployment of these materials in various ecosystems need to be carefully studied. This includes assessing their potential impact on soil chemistry, aquatic life, and microbial communities.
In conclusion, the environmental impact assessment of next-generation adsorbent materials featuring Magnesium silicate hydroxide reveals a promising balance of benefits and challenges. Their high efficiency in pollutant removal and potentially lower carbon footprint offer significant environmental advantages. However, careful consideration must be given to sustainable sourcing, efficient regeneration processes, and long-term ecosystem effects to ensure that their implementation truly contributes to environmental protection and sustainability goals.
One of the primary environmental benefits of Magnesium silicate hydroxide-based adsorbents is their high efficiency in removing heavy metals and organic contaminants from water sources. This capability can significantly contribute to improving water quality in both industrial and municipal settings, potentially reducing the strain on existing water treatment infrastructure and enhancing the overall health of aquatic ecosystems.
Furthermore, these adsorbent materials demonstrate a lower environmental footprint compared to conventional adsorbents. The production process of Magnesium silicate hydroxide-based materials often requires less energy and generates fewer greenhouse gas emissions than the manufacture of activated carbon or synthetic zeolites. This aspect aligns well with global efforts to reduce carbon emissions and promote sustainable industrial practices.
However, the environmental impact assessment must also consider potential drawbacks. The extraction of raw materials for producing these adsorbents, particularly magnesium and silica, may lead to habitat disruption and landscape alterations if not managed responsibly. It is essential to develop sustainable sourcing strategies to mitigate these impacts and ensure the overall environmental benefit of using these materials.
The disposal or regeneration of spent adsorbents is another critical factor to consider. While Magnesium silicate hydroxide-based materials show promise in terms of reusability, the processes involved in regenerating these adsorbents may require energy-intensive treatments or the use of chemicals that could have their own environmental implications. Developing eco-friendly regeneration methods is crucial to maximizing the environmental benefits of these materials.
In terms of air quality improvement, these next-generation adsorbents have shown potential in capturing atmospheric pollutants such as volatile organic compounds (VOCs) and particulate matter. This capability could lead to significant improvements in indoor and outdoor air quality, potentially reducing the health risks associated with air pollution in urban and industrial areas.
Biodegradability and long-term environmental persistence are also important considerations. While Magnesium silicate hydroxide is generally considered environmentally benign, the long-term effects of large-scale deployment of these materials in various ecosystems need to be carefully studied. This includes assessing their potential impact on soil chemistry, aquatic life, and microbial communities.
In conclusion, the environmental impact assessment of next-generation adsorbent materials featuring Magnesium silicate hydroxide reveals a promising balance of benefits and challenges. Their high efficiency in pollutant removal and potentially lower carbon footprint offer significant environmental advantages. However, careful consideration must be given to sustainable sourcing, efficient regeneration processes, and long-term ecosystem effects to ensure that their implementation truly contributes to environmental protection and sustainability goals.
Scalability and Manufacturing Considerations
The scalability and manufacturing considerations for next-generation adsorbent materials featuring Magnesium silicate hydroxide (Mg2Si2O5(OH)4) are crucial for their successful implementation in industrial applications. One of the primary challenges is developing cost-effective and efficient production methods that can be scaled up to meet potential market demands.
Current manufacturing processes for Magnesium silicate hydroxide typically involve hydrothermal synthesis or sol-gel methods. These techniques, while effective for small-scale production, may face limitations when scaled up to industrial levels. Factors such as reaction time, temperature control, and uniform particle size distribution become increasingly challenging to manage in larger reactors.
To address these issues, continuous flow synthesis methods are being explored as a promising alternative. These methods offer better control over reaction parameters and can potentially reduce production costs through improved energy efficiency and reduced waste generation. However, optimizing these processes for consistent product quality across large-scale production runs remains an ongoing research focus.
Another critical aspect of scalability is the availability and sourcing of raw materials. Magnesium and silicon precursors must be obtained in sufficient quantities and at competitive prices to ensure the economic viability of large-scale production. This may involve developing strategic partnerships with suppliers or exploring alternative precursor materials that offer similar performance characteristics.
The environmental impact of manufacturing processes is also a key consideration. As production scales up, minimizing energy consumption, reducing waste, and implementing effective recycling strategies become increasingly important. This may involve the development of closed-loop systems or the integration of green chemistry principles into the manufacturing process.
Quality control and characterization techniques must also be adapted for large-scale production. Rapid, in-line monitoring methods for assessing particle size, surface area, and adsorption capacity are essential for maintaining consistent product quality. Advanced analytical techniques, such as X-ray diffraction and electron microscopy, may need to be integrated into the production line for real-time quality assurance.
Lastly, the development of efficient post-synthesis processing techniques, such as drying, shaping, and activation, is crucial for producing adsorbent materials in forms suitable for various applications. This may involve the use of spray drying, granulation, or extrusion techniques, which must be optimized for large-scale production while maintaining the desired material properties.
Current manufacturing processes for Magnesium silicate hydroxide typically involve hydrothermal synthesis or sol-gel methods. These techniques, while effective for small-scale production, may face limitations when scaled up to industrial levels. Factors such as reaction time, temperature control, and uniform particle size distribution become increasingly challenging to manage in larger reactors.
To address these issues, continuous flow synthesis methods are being explored as a promising alternative. These methods offer better control over reaction parameters and can potentially reduce production costs through improved energy efficiency and reduced waste generation. However, optimizing these processes for consistent product quality across large-scale production runs remains an ongoing research focus.
Another critical aspect of scalability is the availability and sourcing of raw materials. Magnesium and silicon precursors must be obtained in sufficient quantities and at competitive prices to ensure the economic viability of large-scale production. This may involve developing strategic partnerships with suppliers or exploring alternative precursor materials that offer similar performance characteristics.
The environmental impact of manufacturing processes is also a key consideration. As production scales up, minimizing energy consumption, reducing waste, and implementing effective recycling strategies become increasingly important. This may involve the development of closed-loop systems or the integration of green chemistry principles into the manufacturing process.
Quality control and characterization techniques must also be adapted for large-scale production. Rapid, in-line monitoring methods for assessing particle size, surface area, and adsorption capacity are essential for maintaining consistent product quality. Advanced analytical techniques, such as X-ray diffraction and electron microscopy, may need to be integrated into the production line for real-time quality assurance.
Lastly, the development of efficient post-synthesis processing techniques, such as drying, shaping, and activation, is crucial for producing adsorbent materials in forms suitable for various applications. This may involve the use of spray drying, granulation, or extrusion techniques, which must be optimized for large-scale production while maintaining the desired material properties.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!