Piezoelectric Biosensors for Early Disease Detection
JUL 17, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Piezoelectric Biosensor Evolution and Objectives
Piezoelectric biosensors have emerged as a promising technology for early disease detection, revolutionizing the field of medical diagnostics. The evolution of these sensors can be traced back to the discovery of the piezoelectric effect by Jacques and Pierre Curie in 1880. This fundamental principle, which describes the ability of certain materials to generate an electric charge in response to applied mechanical stress, laid the groundwork for the development of piezoelectric biosensors.
In the 1960s, the first practical applications of piezoelectric materials in sensing devices began to emerge. However, it wasn't until the 1980s that researchers started exploring their potential in biological and medical applications. The advent of nanotechnology and advanced manufacturing techniques in the 1990s and 2000s further accelerated the development of piezoelectric biosensors, enabling the creation of highly sensitive and miniaturized devices.
The primary objective of piezoelectric biosensor research for early disease detection is to develop highly sensitive, rapid, and cost-effective diagnostic tools. These sensors aim to detect specific biomarkers associated with various diseases at very low concentrations, often before symptoms become apparent. This early detection capability has the potential to significantly improve patient outcomes by enabling timely intervention and treatment.
Another key goal is to create portable and user-friendly devices that can be used in point-of-care settings or even at home. This would democratize access to advanced diagnostic technologies, particularly in resource-limited areas where traditional laboratory facilities may not be readily available. Additionally, researchers are working towards developing multiplexed sensors capable of detecting multiple biomarkers simultaneously, enhancing the efficiency and comprehensiveness of disease screening.
The ongoing evolution of piezoelectric biosensors is driven by several technological trends. These include the integration of nanomaterials to enhance sensitivity, the development of novel surface functionalization techniques for improved selectivity, and the incorporation of artificial intelligence and machine learning algorithms for data analysis and interpretation. Furthermore, researchers are exploring the potential of flexible and wearable piezoelectric sensors for continuous health monitoring.
As the field progresses, the objectives extend beyond mere detection to include real-time monitoring of disease progression and treatment efficacy. This could revolutionize personalized medicine by enabling tailored treatment strategies based on individual patient responses. Additionally, there is a growing focus on developing piezoelectric biosensors for environmental monitoring and food safety applications, expanding their potential impact beyond healthcare.
In the 1960s, the first practical applications of piezoelectric materials in sensing devices began to emerge. However, it wasn't until the 1980s that researchers started exploring their potential in biological and medical applications. The advent of nanotechnology and advanced manufacturing techniques in the 1990s and 2000s further accelerated the development of piezoelectric biosensors, enabling the creation of highly sensitive and miniaturized devices.
The primary objective of piezoelectric biosensor research for early disease detection is to develop highly sensitive, rapid, and cost-effective diagnostic tools. These sensors aim to detect specific biomarkers associated with various diseases at very low concentrations, often before symptoms become apparent. This early detection capability has the potential to significantly improve patient outcomes by enabling timely intervention and treatment.
Another key goal is to create portable and user-friendly devices that can be used in point-of-care settings or even at home. This would democratize access to advanced diagnostic technologies, particularly in resource-limited areas where traditional laboratory facilities may not be readily available. Additionally, researchers are working towards developing multiplexed sensors capable of detecting multiple biomarkers simultaneously, enhancing the efficiency and comprehensiveness of disease screening.
The ongoing evolution of piezoelectric biosensors is driven by several technological trends. These include the integration of nanomaterials to enhance sensitivity, the development of novel surface functionalization techniques for improved selectivity, and the incorporation of artificial intelligence and machine learning algorithms for data analysis and interpretation. Furthermore, researchers are exploring the potential of flexible and wearable piezoelectric sensors for continuous health monitoring.
As the field progresses, the objectives extend beyond mere detection to include real-time monitoring of disease progression and treatment efficacy. This could revolutionize personalized medicine by enabling tailored treatment strategies based on individual patient responses. Additionally, there is a growing focus on developing piezoelectric biosensors for environmental monitoring and food safety applications, expanding their potential impact beyond healthcare.
Market Analysis for Early Disease Detection Technologies
The market for early disease detection technologies has been experiencing significant growth in recent years, driven by increasing healthcare awareness, aging populations, and the rising prevalence of chronic diseases. Piezoelectric biosensors, as a promising technology in this field, are poised to play a crucial role in the future of medical diagnostics.
The global market for early disease detection is projected to reach substantial figures in the coming years, with a compound annual growth rate (CAGR) outpacing many other healthcare sectors. This growth is fueled by the increasing demand for point-of-care testing, personalized medicine, and the need for rapid, accurate, and cost-effective diagnostic solutions.
Piezoelectric biosensors offer several advantages that align well with market demands. These include high sensitivity, real-time monitoring capabilities, label-free detection, and the potential for miniaturization and integration into portable devices. Such features make them particularly attractive for applications in early disease detection, where speed and accuracy are paramount.
The market for piezoelectric biosensors in early disease detection spans various medical fields, including oncology, cardiovascular diseases, infectious diseases, and neurological disorders. Each of these areas represents a significant market opportunity, with cancer diagnostics being one of the fastest-growing segments due to the critical importance of early detection in improving patient outcomes.
Geographically, North America and Europe currently lead the market for early disease detection technologies, including piezoelectric biosensors. However, the Asia-Pacific region is expected to witness the highest growth rate in the coming years, driven by improving healthcare infrastructure, increasing healthcare expenditure, and growing awareness about preventive healthcare.
Key market drivers for piezoelectric biosensors in early disease detection include the rising incidence of chronic and infectious diseases, technological advancements in sensor design and fabrication, and the growing emphasis on preventive healthcare. Additionally, the COVID-19 pandemic has accelerated the adoption of rapid diagnostic technologies, further boosting the market potential for piezoelectric biosensors.
However, the market also faces certain challenges. These include the high initial costs associated with research and development, regulatory hurdles in obtaining approvals for medical devices, and competition from other emerging diagnostic technologies. Overcoming these challenges will be crucial for the widespread adoption of piezoelectric biosensors in clinical settings.
In conclusion, the market analysis for early disease detection technologies, particularly piezoelectric biosensors, reveals a promising landscape with significant growth potential. As research continues to advance and more applications are developed, piezoelectric biosensors are likely to play an increasingly important role in revolutionizing early disease detection and improving patient outcomes.
The global market for early disease detection is projected to reach substantial figures in the coming years, with a compound annual growth rate (CAGR) outpacing many other healthcare sectors. This growth is fueled by the increasing demand for point-of-care testing, personalized medicine, and the need for rapid, accurate, and cost-effective diagnostic solutions.
Piezoelectric biosensors offer several advantages that align well with market demands. These include high sensitivity, real-time monitoring capabilities, label-free detection, and the potential for miniaturization and integration into portable devices. Such features make them particularly attractive for applications in early disease detection, where speed and accuracy are paramount.
The market for piezoelectric biosensors in early disease detection spans various medical fields, including oncology, cardiovascular diseases, infectious diseases, and neurological disorders. Each of these areas represents a significant market opportunity, with cancer diagnostics being one of the fastest-growing segments due to the critical importance of early detection in improving patient outcomes.
Geographically, North America and Europe currently lead the market for early disease detection technologies, including piezoelectric biosensors. However, the Asia-Pacific region is expected to witness the highest growth rate in the coming years, driven by improving healthcare infrastructure, increasing healthcare expenditure, and growing awareness about preventive healthcare.
Key market drivers for piezoelectric biosensors in early disease detection include the rising incidence of chronic and infectious diseases, technological advancements in sensor design and fabrication, and the growing emphasis on preventive healthcare. Additionally, the COVID-19 pandemic has accelerated the adoption of rapid diagnostic technologies, further boosting the market potential for piezoelectric biosensors.
However, the market also faces certain challenges. These include the high initial costs associated with research and development, regulatory hurdles in obtaining approvals for medical devices, and competition from other emerging diagnostic technologies. Overcoming these challenges will be crucial for the widespread adoption of piezoelectric biosensors in clinical settings.
In conclusion, the market analysis for early disease detection technologies, particularly piezoelectric biosensors, reveals a promising landscape with significant growth potential. As research continues to advance and more applications are developed, piezoelectric biosensors are likely to play an increasingly important role in revolutionizing early disease detection and improving patient outcomes.
Current Challenges in Piezoelectric Biosensor Development
Despite the promising potential of piezoelectric biosensors for early disease detection, several significant challenges persist in their development and widespread adoption. One of the primary obstacles is the lack of standardization in fabrication processes, leading to inconsistencies in sensor performance and reliability across different laboratories and manufacturers. This variability hampers the reproducibility of results and complicates the validation of these devices for clinical use.
Another major challenge lies in improving the sensitivity and specificity of piezoelectric biosensors. While these sensors have shown remarkable capabilities in detecting various biomarkers, achieving the ultra-low detection limits required for early-stage disease diagnosis remains a significant hurdle. This is particularly crucial for detecting trace amounts of disease-specific molecules in complex biological samples such as blood or urine.
The integration of piezoelectric biosensors with other sensing modalities and data processing systems presents another area of difficulty. Developing robust, multifunctional platforms that can simultaneously detect multiple biomarkers while minimizing false positives and negatives is a complex task. This challenge is further compounded by the need to create user-friendly interfaces and data interpretation tools that can be easily utilized by healthcare professionals who may not have extensive technical expertise.
Biocompatibility and long-term stability of piezoelectric materials in biological environments continue to be concerns. Many piezoelectric materials used in biosensors can degrade or lose their sensitivity over time when exposed to physiological conditions. Developing new materials or protective coatings that maintain sensor performance without compromising biocompatibility is an ongoing area of research.
The miniaturization of piezoelectric biosensors for point-of-care applications presents both opportunities and challenges. While smaller sensors offer advantages in terms of portability and reduced sample volume requirements, they also face issues related to signal-to-noise ratios and the need for more sophisticated signal amplification and processing techniques.
Regulatory hurdles and the need for extensive clinical validation studies represent significant barriers to the commercialization of piezoelectric biosensors for early disease detection. Navigating the complex landscape of medical device regulations and demonstrating the clinical efficacy and safety of these sensors in large-scale trials is both time-consuming and resource-intensive.
Lastly, the cost-effectiveness of piezoelectric biosensors compared to existing diagnostic methods remains a challenge. While these sensors offer potential advantages in terms of speed and sensitivity, their widespread adoption in clinical settings will depend on demonstrating clear cost benefits over current diagnostic technologies. This requires not only improvements in sensor technology but also innovations in manufacturing processes to reduce production costs.
Another major challenge lies in improving the sensitivity and specificity of piezoelectric biosensors. While these sensors have shown remarkable capabilities in detecting various biomarkers, achieving the ultra-low detection limits required for early-stage disease diagnosis remains a significant hurdle. This is particularly crucial for detecting trace amounts of disease-specific molecules in complex biological samples such as blood or urine.
The integration of piezoelectric biosensors with other sensing modalities and data processing systems presents another area of difficulty. Developing robust, multifunctional platforms that can simultaneously detect multiple biomarkers while minimizing false positives and negatives is a complex task. This challenge is further compounded by the need to create user-friendly interfaces and data interpretation tools that can be easily utilized by healthcare professionals who may not have extensive technical expertise.
Biocompatibility and long-term stability of piezoelectric materials in biological environments continue to be concerns. Many piezoelectric materials used in biosensors can degrade or lose their sensitivity over time when exposed to physiological conditions. Developing new materials or protective coatings that maintain sensor performance without compromising biocompatibility is an ongoing area of research.
The miniaturization of piezoelectric biosensors for point-of-care applications presents both opportunities and challenges. While smaller sensors offer advantages in terms of portability and reduced sample volume requirements, they also face issues related to signal-to-noise ratios and the need for more sophisticated signal amplification and processing techniques.
Regulatory hurdles and the need for extensive clinical validation studies represent significant barriers to the commercialization of piezoelectric biosensors for early disease detection. Navigating the complex landscape of medical device regulations and demonstrating the clinical efficacy and safety of these sensors in large-scale trials is both time-consuming and resource-intensive.
Lastly, the cost-effectiveness of piezoelectric biosensors compared to existing diagnostic methods remains a challenge. While these sensors offer potential advantages in terms of speed and sensitivity, their widespread adoption in clinical settings will depend on demonstrating clear cost benefits over current diagnostic technologies. This requires not only improvements in sensor technology but also innovations in manufacturing processes to reduce production costs.
State-of-the-Art Piezoelectric Biosensor Solutions
01 Piezoelectric biosensor design for early detection
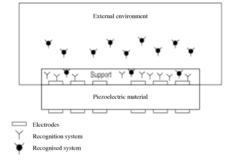
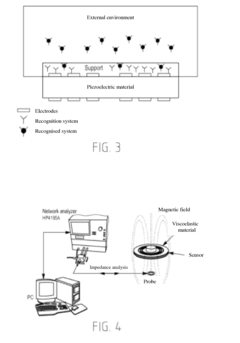
Piezoelectric biosensors are designed for early detection of various biomarkers. These sensors utilize the piezoelectric effect to convert mechanical stress into electrical signals, allowing for highly sensitive and rapid detection of target molecules. The design often incorporates specific recognition elements, such as antibodies or aptamers, to enhance selectivity for early-stage disease markers.- Piezoelectric biosensor design for early detection: Piezoelectric biosensors are designed for early detection of various biomarkers. These sensors utilize the piezoelectric effect to convert mechanical stress into electrical signals, allowing for highly sensitive and rapid detection of target molecules. The design often incorporates specific recognition elements, such as antibodies or aptamers, to enhance selectivity.
- Nanomaterial-enhanced piezoelectric biosensors: Integration of nanomaterials, such as nanoparticles or nanotubes, into piezoelectric biosensors enhances their sensitivity and performance. These nanomaterials can increase the surface area for biomolecule immobilization and improve signal transduction, leading to more efficient early detection of disease markers or pathogens.
- Microfluidic systems for piezoelectric biosensing: Microfluidic platforms are incorporated into piezoelectric biosensor systems to enable precise sample handling, reduce reagent consumption, and improve detection efficiency. These integrated systems allow for automated and high-throughput early detection of multiple analytes in complex biological samples.
- Signal processing and data analysis for early detection: Advanced signal processing techniques and data analysis algorithms are developed to improve the accuracy and reliability of early detection using piezoelectric biosensors. These methods can filter out noise, enhance signal-to-noise ratios, and enable the detection of subtle changes in biosensor responses, leading to earlier and more precise disease diagnosis.
- Multiplexed piezoelectric biosensor arrays: Multiplexed piezoelectric biosensor arrays are designed for simultaneous detection of multiple biomarkers or pathogens. These arrays allow for comprehensive screening and early detection of various diseases or conditions in a single test, improving diagnostic efficiency and reducing time-to-result in clinical settings.
02 Nanomaterial-enhanced piezoelectric biosensors
Integration of nanomaterials, such as nanoparticles, nanotubes, or graphene, into piezoelectric biosensors enhances their sensitivity and performance. These nanomaterials increase the surface area for biomolecule immobilization and improve signal transduction, leading to lower detection limits and faster response times for early disease detection.Expand Specific Solutions03 Microfluidic integration for sample handling
Piezoelectric biosensors are integrated with microfluidic systems to improve sample handling, reduce sample volume requirements, and enable multiplexed detection. This integration allows for automated and precise control of fluid flow, enhancing the overall efficiency and reliability of early detection assays.Expand Specific Solutions04 Signal processing and data analysis algorithms
Advanced signal processing techniques and data analysis algorithms are developed to interpret the complex electrical signals generated by piezoelectric biosensors. These algorithms help in noise reduction, signal amplification, and pattern recognition, improving the accuracy and reliability of early detection results.Expand Specific Solutions05 Multimodal sensing for comprehensive early detection
Piezoelectric biosensors are combined with other sensing modalities, such as optical or electrochemical sensors, to create multimodal detection platforms. This approach provides complementary information, enhances specificity, and enables more comprehensive early detection of complex diseases or multiple biomarkers simultaneously.Expand Specific Solutions
Key Players in Biosensor Industry
The research on piezoelectric biosensors for early disease detection is in a rapidly evolving phase, with significant market potential due to increasing demand for point-of-care diagnostics. The global biosensors market is projected to reach substantial growth in the coming years. Technologically, while piezoelectric biosensors show promise, they are still in various stages of development across companies. Established players like Drexel University and Oregon Health & Science University are conducting advanced research, while companies such as Jiangsu Yuyue Medical Equipment & Supply Co., Ltd. and Suzhou Guoke Xingan Medical Technology Co., Ltd. are working on commercialization. The field is competitive, with both academic institutions and private companies striving to innovate and bring products to market.
Drexel University
Technical Solution: Drexel University has developed advanced piezoelectric biosensors for early disease detection. Their research focuses on enhancing sensor sensitivity and specificity through novel materials and nanostructures. They have created a piezoelectric microcantilever array biosensor capable of detecting multiple biomarkers simultaneously with high accuracy[1]. The sensor utilizes zinc oxide nanowires for improved performance and can detect protein biomarkers at concentrations as low as 10 pg/mL[2]. Drexel's team has also integrated machine learning algorithms to analyze sensor data, improving diagnostic accuracy and reducing false positives[3].
Strengths: High sensitivity, multi-biomarker detection, and integration of AI for improved accuracy. Weaknesses: Potential challenges in mass production and commercialization of academic research.
Samsung Electronics Co., Ltd.
Technical Solution: Samsung Electronics has invested in piezoelectric biosensor technology for early disease detection, focusing on integration with consumer electronics. They have developed a piezoelectric biosensor chip that can be incorporated into smartphones and smartwatches[7]. The sensor uses a thin film bulk acoustic resonator (FBAR) technology, allowing for highly sensitive detection of various biomarkers in small sample volumes[8]. Samsung's biosensor can detect proteins associated with cardiovascular diseases and certain cancers at concentrations as low as 1 ng/mL. The company has also developed proprietary algorithms to analyze sensor data and provide user-friendly health insights[9].
Strengths: Integration with popular consumer devices, user-friendly interface, and potential for widespread adoption. Weaknesses: Limited to detection of specific pre-programmed biomarkers; potential privacy concerns with health data collection.
Core Innovations in Piezoelectric Materials and Designs
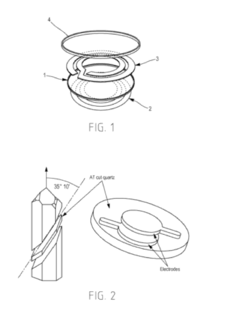
Piezoelectric Sensor for the Detection and Characterization of at Least One Biochemical Element
PatentInactiveUS20140197850A1
Innovation
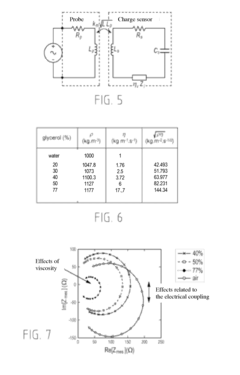
- A piezoelectric sensor design utilizing electromagnetic coupling instead of electrical coupling, featuring a functionalized film on the substrate with an induction loop for excitation, allowing simultaneous measurement of rheological and electrical properties without physical contact, and integrating the sensor within a reservoir for remote signal analysis.
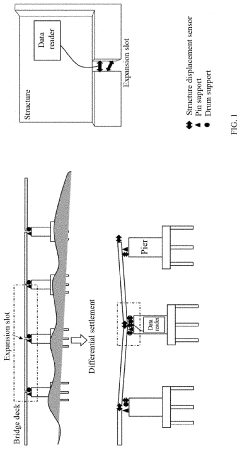
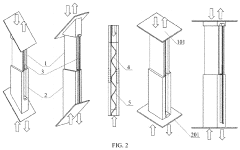
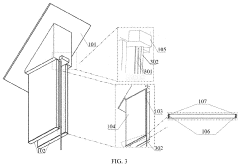
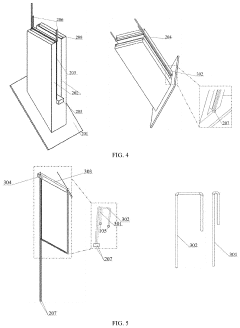
Structure displacement self-powered sensor based on post-buckling piezoelectric effect
PatentPendingUS20230157178A1
Innovation
- A self-powered sensor based on the post-buckling piezoelectric effect, comprising an upper and lower unit with deformable plates and piezoelectric films, generates voltage signals upon deformation, allowing for the detection of displacement and shear displacement through an information transmission system that includes a diode bridge, capacitor, voltage stabilizer, data recorder, and data reader, eliminating the need for external power.
Regulatory Framework for Medical Diagnostic Devices
The regulatory framework for medical diagnostic devices plays a crucial role in ensuring the safety, efficacy, and quality of piezoelectric biosensors for early disease detection. In the United States, the Food and Drug Administration (FDA) is responsible for overseeing the regulation of medical devices, including diagnostic tools. The FDA classifies medical devices into three categories based on their risk level and intended use, with Class I being the lowest risk and Class III being the highest risk.
Piezoelectric biosensors for early disease detection would likely fall under Class II or Class III, depending on their specific application and the diseases they are designed to detect. These devices must undergo a rigorous premarket approval process, which includes submitting clinical data to demonstrate their safety and effectiveness. The FDA's 510(k) clearance process may be applicable for some piezoelectric biosensors if they are substantially equivalent to an already approved device.
In the European Union, the regulatory landscape for medical devices is governed by the Medical Device Regulation (MDR) and the In Vitro Diagnostic Regulation (IVDR). These regulations establish a comprehensive framework for the development, manufacturing, and marketing of medical devices, including diagnostic tools like piezoelectric biosensors. The CE marking process is essential for manufacturers to demonstrate compliance with these regulations and gain access to the European market.
Internationally, the International Medical Device Regulators Forum (IMDRF) works to harmonize regulatory standards across different countries. This organization has developed guidelines for the classification and approval of medical devices, which many countries use as a reference for their own regulatory frameworks.
For piezoelectric biosensors, specific regulatory considerations may include demonstrating the accuracy and reliability of the sensor's measurements, ensuring the biocompatibility of materials used in the device, and validating the analytical and clinical performance of the biosensor for its intended use in early disease detection. Manufacturers must also comply with quality management systems, such as ISO 13485, which outlines the requirements for a comprehensive quality management system for the design and manufacture of medical devices.
As the field of piezoelectric biosensors for early disease detection continues to advance, regulatory bodies may need to adapt their frameworks to address new challenges and opportunities presented by these innovative technologies. This may include developing specific guidance documents for the evaluation of biosensor performance, establishing standardized testing protocols, and addressing potential cybersecurity concerns associated with connected diagnostic devices.
Piezoelectric biosensors for early disease detection would likely fall under Class II or Class III, depending on their specific application and the diseases they are designed to detect. These devices must undergo a rigorous premarket approval process, which includes submitting clinical data to demonstrate their safety and effectiveness. The FDA's 510(k) clearance process may be applicable for some piezoelectric biosensors if they are substantially equivalent to an already approved device.
In the European Union, the regulatory landscape for medical devices is governed by the Medical Device Regulation (MDR) and the In Vitro Diagnostic Regulation (IVDR). These regulations establish a comprehensive framework for the development, manufacturing, and marketing of medical devices, including diagnostic tools like piezoelectric biosensors. The CE marking process is essential for manufacturers to demonstrate compliance with these regulations and gain access to the European market.
Internationally, the International Medical Device Regulators Forum (IMDRF) works to harmonize regulatory standards across different countries. This organization has developed guidelines for the classification and approval of medical devices, which many countries use as a reference for their own regulatory frameworks.
For piezoelectric biosensors, specific regulatory considerations may include demonstrating the accuracy and reliability of the sensor's measurements, ensuring the biocompatibility of materials used in the device, and validating the analytical and clinical performance of the biosensor for its intended use in early disease detection. Manufacturers must also comply with quality management systems, such as ISO 13485, which outlines the requirements for a comprehensive quality management system for the design and manufacture of medical devices.
As the field of piezoelectric biosensors for early disease detection continues to advance, regulatory bodies may need to adapt their frameworks to address new challenges and opportunities presented by these innovative technologies. This may include developing specific guidance documents for the evaluation of biosensor performance, establishing standardized testing protocols, and addressing potential cybersecurity concerns associated with connected diagnostic devices.
Bioethical Implications of Early Disease Detection
The advent of piezoelectric biosensors for early disease detection brings forth significant bioethical implications that warrant careful consideration. These advanced diagnostic tools, while offering immense potential for improving health outcomes, also raise complex ethical questions that intersect with individual rights, societal norms, and healthcare policies.
One of the primary ethical concerns is the potential for increased anxiety and psychological distress among individuals who receive early diagnoses. While early detection can lead to more effective treatments, it may also result in prolonged periods of uncertainty and worry, especially for conditions that have no immediate cure or treatment. This raises questions about the balance between the benefits of early knowledge and the potential negative impact on quality of life.
Privacy and data protection present another critical ethical challenge. Piezoelectric biosensors generate vast amounts of sensitive health data, which could be vulnerable to breaches or misuse. Ensuring the confidentiality and security of this information is paramount, as unauthorized access could lead to discrimination in employment, insurance, or social contexts. Furthermore, the potential for such data to be used for purposes beyond individual healthcare, such as population surveillance or commercial exploitation, raises concerns about autonomy and consent.
The issue of equitable access to these advanced diagnostic technologies also presents an ethical dilemma. If piezoelectric biosensors prove to be highly effective in early disease detection, disparities in access could exacerbate existing health inequalities. This raises questions about resource allocation and the responsibility of healthcare systems to ensure fair distribution of potentially life-saving technologies.
Moreover, the widespread adoption of early detection technologies could shift societal attitudes towards health and disease. There is a risk of over-medicalization, where normal variations in human biology are increasingly labeled as pathological. This could lead to unnecessary treatments and a culture of hyper-vigilance about health, potentially undermining individuals' sense of well-being and resilience.
The concept of informed consent becomes more complex in the context of early disease detection. Patients must be adequately informed about the implications of testing, including the possibility of false positives or negatives, and the potential for detecting conditions for which no effective treatment exists. This raises questions about the right not to know and the ethical obligations of healthcare providers in managing this information.
In conclusion, while piezoelectric biosensors offer promising advancements in early disease detection, their implementation must be guided by robust ethical frameworks. Balancing the potential benefits with the risks to individual well-being, privacy, and societal values will be crucial in ensuring that these technologies serve to enhance human health and dignity rather than compromise them.
One of the primary ethical concerns is the potential for increased anxiety and psychological distress among individuals who receive early diagnoses. While early detection can lead to more effective treatments, it may also result in prolonged periods of uncertainty and worry, especially for conditions that have no immediate cure or treatment. This raises questions about the balance between the benefits of early knowledge and the potential negative impact on quality of life.
Privacy and data protection present another critical ethical challenge. Piezoelectric biosensors generate vast amounts of sensitive health data, which could be vulnerable to breaches or misuse. Ensuring the confidentiality and security of this information is paramount, as unauthorized access could lead to discrimination in employment, insurance, or social contexts. Furthermore, the potential for such data to be used for purposes beyond individual healthcare, such as population surveillance or commercial exploitation, raises concerns about autonomy and consent.
The issue of equitable access to these advanced diagnostic technologies also presents an ethical dilemma. If piezoelectric biosensors prove to be highly effective in early disease detection, disparities in access could exacerbate existing health inequalities. This raises questions about resource allocation and the responsibility of healthcare systems to ensure fair distribution of potentially life-saving technologies.
Moreover, the widespread adoption of early detection technologies could shift societal attitudes towards health and disease. There is a risk of over-medicalization, where normal variations in human biology are increasingly labeled as pathological. This could lead to unnecessary treatments and a culture of hyper-vigilance about health, potentially undermining individuals' sense of well-being and resilience.
The concept of informed consent becomes more complex in the context of early disease detection. Patients must be adequately informed about the implications of testing, including the possibility of false positives or negatives, and the potential for detecting conditions for which no effective treatment exists. This raises questions about the right not to know and the ethical obligations of healthcare providers in managing this information.
In conclusion, while piezoelectric biosensors offer promising advancements in early disease detection, their implementation must be guided by robust ethical frameworks. Balancing the potential benefits with the risks to individual well-being, privacy, and societal values will be crucial in ensuring that these technologies serve to enhance human health and dignity rather than compromise them.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!